Low-Cost Hyperspectral Imaging System: Design and Testing for Laboratory-Based Environmental Applications
Abstract
:1. Introduction
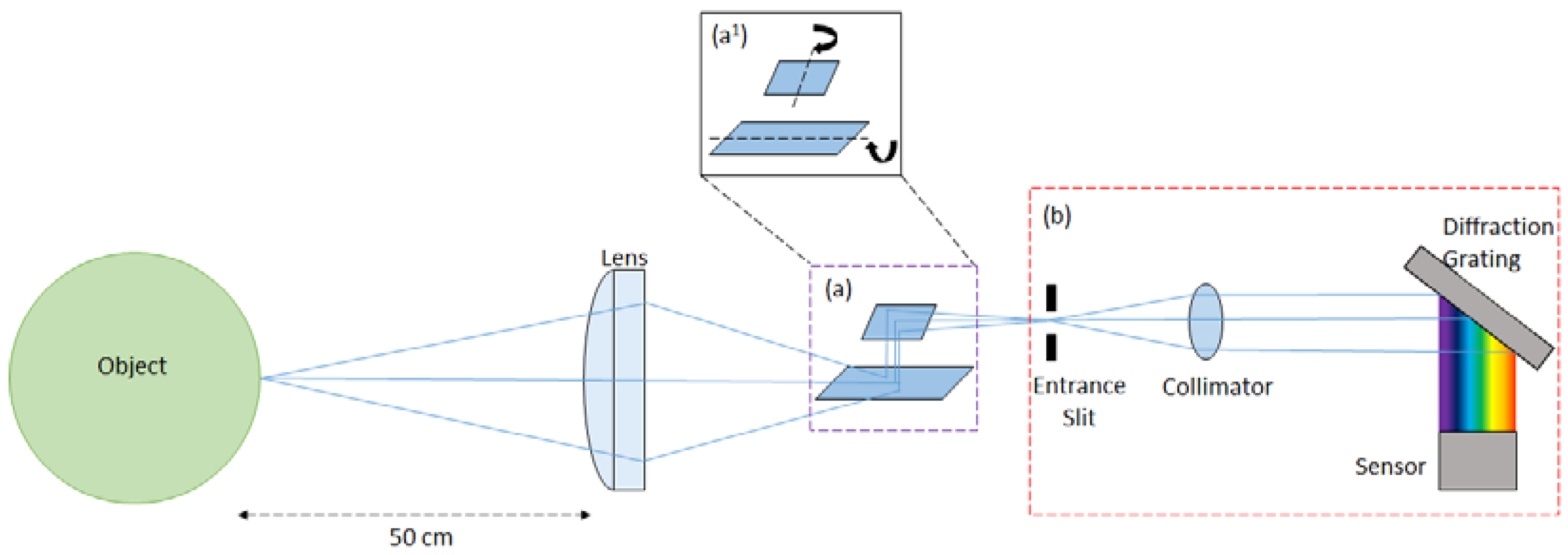
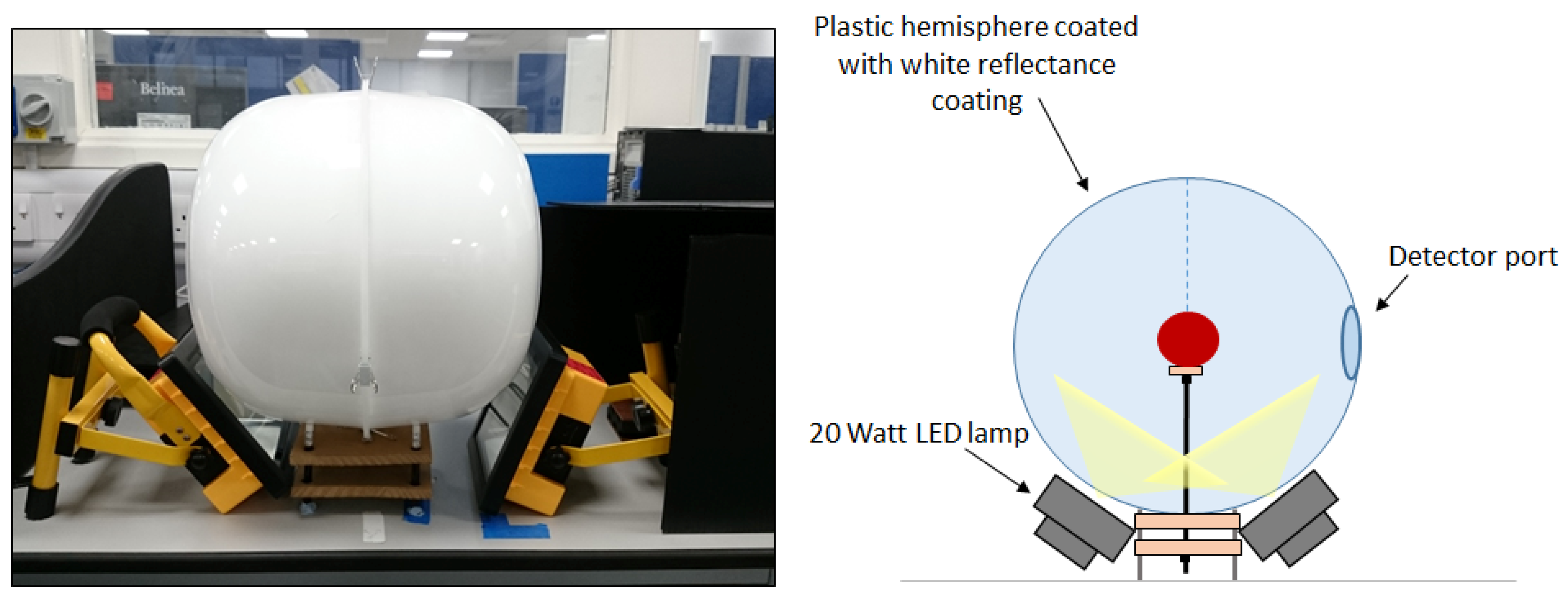
2. Materials and Methods
3. Results and Discussion
3.1. Environmental Applications
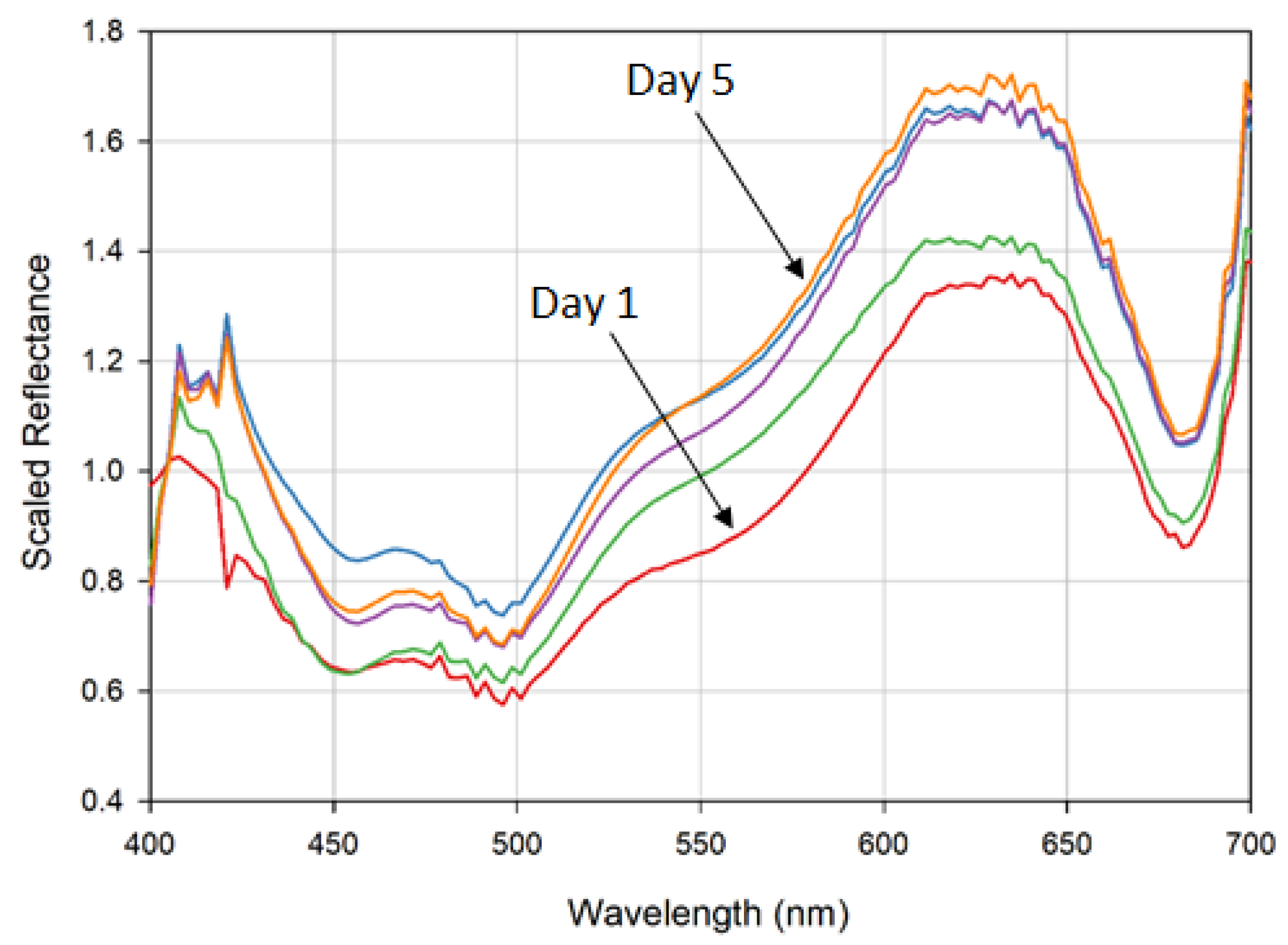
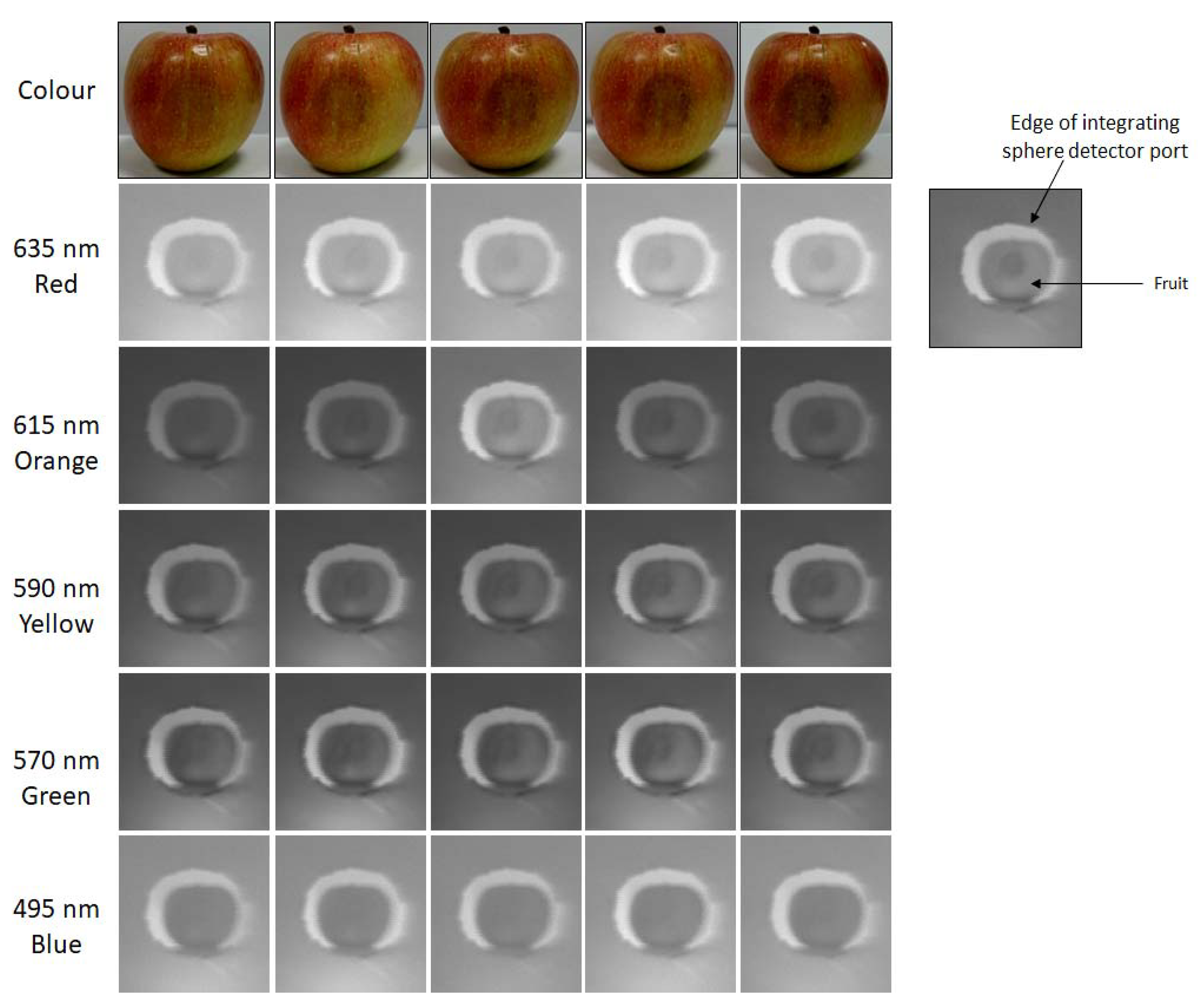
3.1.1. Fruit Quality Identification
3.1.2. Volcanic Rock Mineralogy
3.1.3. Tooth Shade Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habib, A.; Zhou, T.; Masjedi, A.; Zhang, Z.; Flatt, J.E.; Crawford, M. Boresight calibration of GNSS/INSassisted push-broom hyperspectral scanners on UAV platforms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1734–1749. [Google Scholar] [CrossRef]
- Jaud, M.; Dantec, N.L.; Ammann, J.; Grandjean, P.; Constantin, D.; Akhtman, Y.; Barbieux, K.; Allemand, P.; Delacourt, C.; Merminod, B. Direct georeferencing of a pushbroom, lightweight hyperspectral system for mini-UAV applications. Remote Sens. 2018, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Stuart, M.B.; McGonigle, A.J.S.; Willmott, J.R. Hyperspectral Imaging in Environmental Monitoring: A Review of Recent Developments and Technological Advances in Compact Field Deployable Systems. Sensors 2019, 19, 3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigernes, F.; Syrjӓsuo, M.; Strovold, R.; Fortuna, J.; Grøtte, M.E.; Johansen, T.A. Do it yourself hyperspectral imager for handheld to airborne operations. Opt. Express 2018, 26, 6021–6035. [Google Scholar] [CrossRef]
- Aasen, H.; Burkart, A.; Bolten, A.; Bareth, G. Generating 3D hyperspectral information with lightweight UAV snapshot cameras for vegetation monitoring: From camera calibration to quality assurance. ISPRS J. Photogramm. Remote Sens. 2015, 108, 245–259. [Google Scholar] [CrossRef]
- Honkavaara, E.; Heikki, S.; Kaivosoja, J.; Pӧlӧnen, I.; Hakala, T.; Litkey, P.; Mӓkynen, J.; Pesonen, L. Processing and assessment of spectrometric stereoscopic imagery collected using a lightweight UAV spectral camera for precision agriculture. Remote Sens. 2013, 5, 5006–5039. [Google Scholar] [CrossRef] [Green Version]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Vanbrabant, Y.; Tits, L.; Delalieux, S.; Pauly, K.; Verjans, W.; Somers, B. Multitemporal chlorophyll mapping in pome fruit orchards from remotely piloted aircraft systems. Remote Sens. 2019, 11, 1468. [Google Scholar] [CrossRef] [Green Version]
- Garzonio, R.; Di Mauro, B.; Colombo, R.; Cogliati, S. Surface reflectance and sun-induced fluorescence spectroscopy measurements using a small hyperspectral UAS. Remote Sens. 2017, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Hobbs, M.J.; Masters, R.C.; Rodenburg, C.; Willmott, J.R. An accurate device for apparent emissivity characterization in controlled atmospheric conditions up to 1423 K. IEEE Trans. Instrum. Meas. 2019. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Rapid and non-invasive detection of fish microbial spoilage by visible and near infrared hyperspectral imaging and multivariate analysis. LWT-Food Sci. Technol. 2015, 62, 1060–1068. [Google Scholar] [CrossRef]
- Pu, H.; Liu, D.; Wang, L.; Sun, D.W. Soluble solids content and pH prediction and maturity discrimination of lychee fruits using visible and near infrared hyperspectral imaging. Food Anal. Method 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Jarolmasjed, S.; Hkot, L.R.; Sankaran, S. Hyperspectral imaging and spectrometry-derived spectral features for bitter pit detection in storage apples. Sensors 2018, 18, 1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Chen, J.; Fan, Y.; Qiu, Z.; He, Y. SeeFruits: Design and evaluation of a cloud-based ultra-portable NIRS system for sweet cherry quality detection. Comput. Electron. Agric. 2018, 152, 302–313. [Google Scholar] [CrossRef]
- Li, J.; Luo, W.; Wang, Z.; Fan, S. Early detection of decay on apples using hyperspectral reflectance imaging combining both principal component analysis and improved watershed segmentation method. Postharvest Biol. Tec. 2019, 149, 235–246. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Inagaki, T.; Yang, H.; Tsuchikawa, S. Noncontact evaluation of soluble solids content in apples by near-infrared hyperspectral imaging. J. Food Eng. 2018, 224, 53–61. [Google Scholar] [CrossRef]
- Che, W.; Sun, L.; Zhang, Q.; Tan, W.; Ye, D.; Zhang, D.; Liu, Y. Pixel based bruise region extraction of apple using Vis-NIR hyperspectral imaging. Comput. Electron. Agric. 2018, 146, 12–21. [Google Scholar] [CrossRef]
- Xing, J.; Bravo, C.; Jancsók, P.T.; Ramon, H.; De Baerdemaeker, J. Detecting bruises on ‘Golden Delicious’ apples using hyperspectral imaging with multiple wavebands. Biosyst. Eng. 2005, 90, 27–36. [Google Scholar] [CrossRef]
- Dale, L.M.; Thewis, A.; Boudry, C.; Rotar, I.; Dardenne, P.; Baeten, V.; Pierna, J.A.F. Hyperspectral imaging applications in agriculture and agro-food production quality and safety control: A review. Appl. Spectrosc. Rev. 2013, 48, 142–159. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Sun, D.W. Innovative nondestructive imaging techniques for ripening and maturity of fruits—A review of recent applications. Trends. Food Sci. Technol. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Hossain, A.; Canning, J.; Cook, K.; Jamalipour, A. Optical fiber smartphone spectrometer. Opt. Lett. 2016, 41, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- Beghi, R.; Spinardi, A.; Bodria, L.; Mignani, I.; Guidetti, R. Apples nutraceutic properties evaluation through a visible and near-infrared portable system. Food Bioprocess Technol. 2013, 6, 2547–2554. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Das, A.J.; Wahi, A.; Kothari, I.; Raskar, R. Ultra-portable, wireless smartphone spectrometer for rapid non-destructive testing of fruit ripeness. Sci. Rep. 2016, 6, 32504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonigle, A.J.S.; Wilkes, T.C.; Pering, T.D.; Willmott, J.R.; Cook, J.M.; Mims, F.M.; Parisi, A.V. Smartphone spectrometers. Sensors 2018, 18, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chivkunova, O.B.; Solovchenko, A.E.; Sokolova, S.G.; Merzlyak, M.N.; Reshetnikova, I.V.; Gitelson, A.A. Reflectance spectral features and detection of superficial scald-induced browning in storing apple fruit. J. Russ. Phytopathol. Soc. 2001, 2, 73–77. [Google Scholar]
- Solovchenko, A.E.; Chivkunova, O.B.; Gitelson, A.A.; Merzlyak, M.N. Non-destructive estimation pigment content, ripening, quality and damage in apple fruit with spectral reflectance in the visible range. Fresh Prod. 2010, 4, 91–102. [Google Scholar]
- Gutiérrez, S.; Wendel, A.; Underwood, J. Spectral filter design based on in-field hyperspectral imaging and machine learning for mango ripeness estimation. Comput. Electron. Agric. 2019, 164, 104890. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Bai, S.H.; Wang, Y.; Boyds, S.; Zhou, J.; Esmaeilani, R.; Xu, Z. Using laboratory-based hyperspectral imaging method to determine carbon functional group distributions in decomposing forest litterfall. Catena 2018, 167, 18–27. [Google Scholar] [CrossRef]
- Wang, N.; El Masry, G. Bruise detection of apples using hyperspectral imaging. In Hyperspectral Imaging for Food Quality Analysis and Control; Academic Press: Cambridge, MA, USA, 2010; pp. 295–320. [Google Scholar]
- Kim, M.S.; Chen, Y.R.; Mehl, P.M. Hyperspectral reflectance and fluorescence imaging system for food quality and safety. Trans. Am. Soc. Agric. Eng. 2001, 44, 721–729. [Google Scholar]
- Aufaristama, M.; Hoskuldsson, A.; Ulfarsson, M.O.; Jonsdottir, I.; Thordarson, T. The 2014–2015 lava flow field at Hluhraun, Iceland: Using airborne hyperspectral remote sensing for discriminating the lava surface. Remote Sens. 2019, 11, 476. [Google Scholar] [CrossRef] [Green Version]
- Abrams, M.; Abbott, E.; Kahle, A. Combined use of vsible, reflected infrared, and thermal infrared images for mapping Hawaiian lava flows. J. Geophys. Res. 1991, 96, 475–484. [Google Scholar] [CrossRef]
- Li, L.; Solana, C.; Canters, F.; Chan, J.C.-W.; Kervyn, M. Impact of environmental factors on the spectral characteristics of lava surfaces: Field spectrometry of basaltic lava flows on Tenerife, Canary Islands, Spain. Remote Sens. 2015, 7, 16986–17012. [Google Scholar] [CrossRef] [Green Version]
- Aufaristama, M.; Hӧskuldsson, Á.; Jónsdóttir, I.; Ólafsdóttir, R. Mapping and assessing surface morphology of Holocene lava field in Krafla (NE Iceland) using hyperspectral remote sensing. Int. Symp. Geophys. Issues 2016. [Google Scholar] [CrossRef] [Green Version]
- Amici, S.; Piscini, A.; Neri, M. Reflectance spectra measurements of Mt. Etna: A comparison with multispectral/hyperspectral satellites. Adv. Remote Sens. 2014, 3, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Burbine, T.H.; McCoy, T.J.; Cloutis, E.A. Reflectance spectra of Aubrites, Sulfides, and E Asteroids: Possible implications for Mercury. In Mercury: Space Environment, Surface, and Interior; Lunar and Planetary Institute: Houston, TX, USA, 2001. [Google Scholar]
- Clark, R.N. Spectroscopy of rocks and minerals, and principle of spectroscopy. In Manual of Remote Sensing, 3rd ed.; Rencz, A.N., Ed.; John Wiley and Sons: New York, NY, USA, 1999; pp. 3–58. [Google Scholar]
- Capaccioni, F.; Bellucci, G.; Orosei, R.; Amici, S.; Bianchi, R.; Blecka, M.; Capria, M.T.; Coradini, A.; Erard, S.; Fonti, S.; et al. MARS-IRMA: In-Situ Infrared Microscope Analysis of Martian Soil and Rock Sample. Adv. Space Res. 2001, 28, 1219–1224. [Google Scholar] [CrossRef]
- Sgavetti, M.; Pompilio, L.; Roveria, M.; Manzia, V.; Valentino, G.M.; Lugli, S.; Carli, C.; Amici, S.; Marchese, F.; Lacava, T. Two Geologic Systems Providing Terrestrial Analogues for the Exploration of Sulfate Deposits on Mars: Initial Spectral Characterization. Planet Space Sci. 2009, 57, 614–627. [Google Scholar] [CrossRef]
- Amici, S.; Piscini, A.; Buongiorno, M.F.; Pieri, D. Geological Classification of Volcano Teide by Hyper-spectral and Multispectral Satellite Data. Int. J. Remote Sens. 2012, 34, 3356–3375. [Google Scholar] [CrossRef]
- Vallittu, P.; Vallittu, A.; Lassila, V. Dental aesthetics—A survey of attitudes in different groups of patients. J. Dent. 1996, 24, 335–338. [Google Scholar] [CrossRef]
- Belser, U.; Grütter, L.; Vailati, F.; Bornstein, M.; Weber, H.; Buser, D. Outcome Evaluation of Early Placed Maxillary Anterior Single-Tooth Implants Using Objective Esthetic Criteria: A Cross-Sectional, Retrospective Study in 45 Patients With a 2- to 4-Year Follow-Up Using Pink and White Esthetic Scores. J. Periodontol. 2009, 80, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.; Addy, M. Tooth discolouration and staining: A review of the literature. Brit. Dent. J. 2001, 190, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hill, A. How we see colour. In Colour Physics for Industry; McDonald, R., Ed.; H. Charlesworth & Co., Ltd.: Huddersfield, UK, 1987; pp. 211–281. [Google Scholar]
- Okubo, S.; Kanawati, A.; Richards, M.; Childress, S. Evaluation of visual and instrument shade matching. J. Prosthet. Dent. 1998, 80, 642–648. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Dong, X.; Qian, J.; He, J.; Qu, X.; Lu, E. A systematic review of visual and instrumental measurements for tooth shade matching. Quintessence Int. 2012, 43, 649–659. [Google Scholar] [PubMed]
- Lehmann, K.; Devigus, A.; Wentaschek, S.; Igiel, C.; Scheller, H.; Paravina, R. Comparison of visual shade matching and electronic color measurement device. Int. J. Esthet. Dent. 2017, 12, 396–404. [Google Scholar] [PubMed]
- Raghunathan, J.; Ramesh, A.; Prabhu, K.; Gayathri, R. A systematic review of efficacy of shade matching in prosthodontics. Int. J. Recent Sci. Res. 2016, 7, 9949–9954. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuart, M.B.; Stanger, L.R.; Hobbs, M.J.; Pering, T.D.; Thio, D.; McGonigle, A.J.S.; Willmott, J.R. Low-Cost Hyperspectral Imaging System: Design and Testing for Laboratory-Based Environmental Applications. Sensors 2020, 20, 3293. https://doi.org/10.3390/s20113293
Stuart MB, Stanger LR, Hobbs MJ, Pering TD, Thio D, McGonigle AJS, Willmott JR. Low-Cost Hyperspectral Imaging System: Design and Testing for Laboratory-Based Environmental Applications. Sensors. 2020; 20(11):3293. https://doi.org/10.3390/s20113293
Chicago/Turabian StyleStuart, Mary B., Leigh R. Stanger, Matthew J. Hobbs, Tom D. Pering, Daniel Thio, Andrew J.S. McGonigle, and Jon R. Willmott. 2020. "Low-Cost Hyperspectral Imaging System: Design and Testing for Laboratory-Based Environmental Applications" Sensors 20, no. 11: 3293. https://doi.org/10.3390/s20113293
APA StyleStuart, M. B., Stanger, L. R., Hobbs, M. J., Pering, T. D., Thio, D., McGonigle, A. J. S., & Willmott, J. R. (2020). Low-Cost Hyperspectral Imaging System: Design and Testing for Laboratory-Based Environmental Applications. Sensors, 20(11), 3293. https://doi.org/10.3390/s20113293





