Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices
Abstract
1. Introduction
2. Continuous, Real-Time Electrochemical Biosensors: Towards Antibiofouling, Reagentless, No-Wash, Single-Step, Reusable, and Calibration-Free Devices
2.1. Electrochemical Biosensors with Antibiofouling Properties
2.2. Reagentless, No-Wash, Single-Step, Near Real-Time, and Reusable Electrochemical Biosensors
2.3. Calibration-Free Biosensors
3. Opportunities, Impact, Challenges, and Future Insights
- Able to achieve high sensitivity and selectivity when defied punctually in multicomponent and protein-rich samples or continuously in flowing undiluted samples.
- Capable of responding to ups and downs in analyte concentration within seconds or minutes in a reversible way and without batch processing or addition of exogenous reagents.
- Insensitive to biofouling and stable after storage for more than one week in room-temperature blood serum.
- In ingestible formats coupled to transitory commercial polymer coatings with remarkable prolonged resistance to complex media of denaturing pH values such as gastrointestinal fluids.
- Integrated into microfluidic systems to monitor cell secretions.
- Deployed on screen-printed electrodes to provide rapid and accurate determinations when challenged to in finger-prick-scale sample volumes, suitable for application in POCT circumstances.
- Able to minimize the variability of the sensors fabrication and baseline drift and provide the required accuracy when operating continuously in vivo without the need for calibration, invoking physical barriers or using active drift-correction algorithms, thus surpassing main limitations of the commercial continuous glucose monitors.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Chen, X. Nanotechnology and nanomaterials-based no-wash electrochemical biosensors: From design to application. Nanoscale 2019, 11, 19105–19118. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Dai, J.; Li, C.; Zhu, M.; Li, H.; Lou, X.; Xia, F.; Plaxco, K.W. High frequency, calibration-free molecular measurements in situ in the living body. Chem. Sci. 2019, 10, 10843–10848. [Google Scholar] [CrossRef]
- Idili, A.; Parolo, C.; Ortega, G.; Plaxco, K.W. Calibration-free measurement of phenylalanine levels in the blood using an electrochemical aptamer-based sensor suitable for point-of-care applications. ACS Sens. 2019, 4, 3227–3233. [Google Scholar] [CrossRef]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef]
- Qiu, W.; Baryeh, K.; Takalkar, S.; Chen, W.; Liu, G. Carbon nanotube-based lateral flow immunoassay for ultrasensitive detection of proteins: Application to the determination of IgG. Microchim. Acta 2019, 186, 436. [Google Scholar] [CrossRef]
- Li, H.; Somerson, J.; Xia, F.; Plaxco, K.W. Electrochemical DNA-based sensors for molecular quality control: Continuous, real-time melamine detection in flowing whole milk. Anal. Chem. 2018, 90, 10641–10645. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Ortega, G.; Plaxco, K.W. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J. Am. Chem. Soc. 2017, 139, 11207–11213. [Google Scholar] [CrossRef]
- Li, C.; Hu, X.; Lu, J.; Mao, X.; Xiang, Y.; Shu, Y.; Li, G. Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem. Sci. 2018, 9, 979–984. [Google Scholar] [CrossRef]
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-based sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Somerson, J.; Vieira, P.A.; Ploense, K.L.; Kippine, T.E.; Plaxco, K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 2017, 114, 645–650. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Ortega, G.; Copp, D.A.; Ploense, K.L.; Plaxco, Z.A.; Kippin, T.E.; Hespanha, J.P.; Plaxco, K.W. High-precision control of plasma drug levels using feedback-controlled dosing. ACS Pharmacol. Transl. Sci. 2018, 1, 110–118. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Arroyo-Currás, N.; Tran, C.H.; Vieira, P.A.; Li, S.; Shin, C.; Somerson, J.; Kippin, T.E.; Plaxco, K.W. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors. Angew. Chem. Int. Ed. 2017, 56, 1–5. [Google Scholar]
- Bissonnette, S.; del Grosso, E.; Simon, A.J.; Plaxco, K.W.; Ricci, F.; Vallée-Bélisle, A. Optimizing the specificity window of biomolecular receptors using structure-switching and allostery. ACS Sens. 2020, in press. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Hubble, L.J.; Wang, J. Sensing at Your Fingertips: Glove-based wearable chemical sensors. Electroanalysis 2019, 31, 428–436. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2019, 1906713. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Esteban-Fernández de Ávila, B.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable chemical sensors: Emerging systems for on-body analytical chemistry. Anal. Chem. 2020, 92, 378–396. [Google Scholar] [CrossRef]
- Campuzano, S.; Kuralay, F.; Lobo-Castañón, M.J.; Bartošik, M.; Vyavahare, K.; Paleček, E.; Haake, D.A.; Wang, J. Ternary monolayers as DNA recognition interfaces for direct and sensitive electrochemical detection in untreated clinical samples. Biosens. Bioelectron. 2011, 26, 3577–3583. [Google Scholar] [CrossRef]
- Kuralay, F.; Campuzano, S.; Haake, D.A.; Wang, J. Highly sensitive disposable nucleic acid biosensors for direct bioelectronic detection in raw biological samples. Talanta 2011, 85, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Esteban-Fernández de Ávila, B.; Whitworth, A.; Campuzano, S.; Pingarrón, J.M.; Wang, J. Delayed sensor activation based on transient coatings: Biofouling protection in complex biofluids. J. Am. Chem. Soc. 2018, 140, 14050–14053. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Campuzano, S.; Pingarrón, J.M.; Esteban-Fernández de Ávila, B.; Wang, J. Direct electrochemical biosensing in gastrointestinal fluids. Anal. Bioanal. Chem. 2019, 411, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, M.; Ding, C.; Luo, X. Designed three-in-one peptides with anchoring, antifouling and recognizing capabilities for highly sensitive and low fouling electrochemical sensing in complex biological media. Anal. Chem. 2020, 92, 5795–5802. [Google Scholar] [CrossRef]
- Parolo, C.; Greenwood, A.S.; Ogden, N.E.; Kang, D.; Hawes, C.; Ortega, G.; Arroyo-Currás, N.; Plaxco, K.W. E-DNA scaffold sensors and the reagentless, single step, measurement of HIV-diagnostic antibodies in human serum. Microsyst. Nanoeng. 2020, 6, 13. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Matharu, Z.; Rahimian, A.; Revzin, A. Detecting multiple cell-secreted cytokines from the same aptamer functionalized electrode. Biosens. Bioelectron. 2015, 64, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhad, H.R.L.Z.; Lai, R.Y. Application of calcium-binding motif of E-cadherin for electrochemical detection of Pb(II). Anal. Chem. 2018, 90, 6519–6525. [Google Scholar] [CrossRef]
- Zhad, H.R.L.Z.; Lai, R.Y. A Hg(II)-mediated ‘‘signal-on’’ electrochemical glutathione sensor. Chem. Commun. 2014, 50, 8385–8387. [Google Scholar] [CrossRef][Green Version]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (bio)materials for electrochemical (bio)sensing. Int. J. Mol. Sci. 2019, 20, 423. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Jiao, M.; Jayachandran, S.; Wu, Y.; Fan, X.; Luo, X. Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef]
- Wang, G.; Han, R.; Sua, X.; Lia, Y.; Xua, G.; Luo, X. Zwitterionic peptide anchored to conducting polymer PEDOT for the development of antifouling and ultrasensitive electrochemical DNA sensor. Biosens. Bioelectron. 2017, 92, 396–401. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Anal. Bioanal. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Henry, O.Y.F.; Acero Sanchez, J.L.; O’Sullivan, C.K. Bipodal PEGylated alkanethiol for the enhanced electrochemical detection of genetic markers involved in breast cancer. Biosens. Bioelectron. 2010, 26, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Dharuman, V.; Chang, B.-Y.; Park, S.-M.; Hahn, J.H. Ternary mixed monolayers for simultaneous DNA orientation control and surface passivation for label free DNA hybridization electrochemical sensing. Biosens. Bioelectron. 2010, 25, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Dharuman, V.; Vijayaraj, K.; Radhakrishnan, S.; Dinakaran, T.; Narayanan, J.S.; Bhuvana, M.; Wilson, J. Sensitive label-free electrochemical DNA hybridization detection in the presence of 11-mercaptoundecanoic acid on the thiolated single strand DNA and mercaptohexanol binary mixed monolayer surface. Electrochim. Acta 2011, 56, 8147–8155. [Google Scholar] [CrossRef]
- Campuzano, S.; Kuralay, F.; Wang, J. Ternary monolayer interfaces for ultrasensitive and direct bioelectronics detection of nucleic acids in complex matrices. Electroanalysis 2012, 24, 483–493. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef]
- Josephs, E.A.; Ye, T. A Single-Molecule View of conformational switching of DNA tethered to a gold electrode. J. Am. Chem. Soc. 2012, 134, 10021–10030. [Google Scholar] [CrossRef]
- Halford, C.; Gonzalez, R.; Campuzano, S.; Hu, B.; Babbitt, J.T.; Liu, J.; Wang, J.; Churchill, B.M.; Haake, D.A. Rapid antimicrobial susceptibility testing by sensitive detection of precursor rRNA using a novel electrochemical biosensing platform. Antimicrob. Agents Chemother. 2013, 57, 936–943. [Google Scholar] [CrossRef]
- Goda, T.; Tabata, M.; Sanjoh, M.; Uchimura, M.; Iwasaki, Y.; Miyahara, Y. Thiolated 2-methacryloyloxyethyl phosphorylcholine for an antifouling biosensor platform. Chem. Commun. 2013, 49, 8683–8685. [Google Scholar] [CrossRef]
- McQuistan, A.; Zaitouna, A.J.; Echeverria, E.; Lai, R.Y. Use of thiolated oligonucleotides as anti-fouling diluents in electrochemical peptide-based sensors. Chem. Commun. 2014, 50, 4690–4692. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Formisano, N.; Tkáč, J.; Kasák, P.; Frost, C.G.; Estrela, P. Label-free impedimetric aptasensor with antifouling surface chemistry: A prostate specific antigen case study. Sens. Actuators B Chem. 2015, 209, 306–312. [Google Scholar] [CrossRef]
- González-Fernández, E.; Avlonitis, N.; Murray, A.F.; Mount, A.R.; Bradley, M. Methylene blue not ferrocene: Optimal reporters for electrochemical detection of protease activity. Biosens. Bioelectron. 2016, 84, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liang, B.; Fang, L.; Ma, G.; Yang, G.; Zhu, Q.; Chen, S.; Ye, X. Antifouling Zwitterionic Coating via Electrochemically Mediated Atom Transfer Radical Polymerization on Enzyme-Based Glucose Sensors for Long-Time Stability in 37 °C Serum. Langmuir 2016, 32, 11763–11770. [Google Scholar] [CrossRef]
- Gao, J.; Jeffries, L.; Mach, K.E.; Craft, D.W.; Thomas, N.J.; Gau, V.; Liao, J.C.; Wong, P.K. A multiplex electrochemical biosensor for bloodstream infection diagnosis. SLAS Technol. 2017, 22, 466–474. [Google Scholar] [CrossRef]
- Altobelli, E.; Mohan, R.; Mach, K.E.; Sin, M.L.Y.; Anikst, V.; Buscarini, M.; Wong, P.K.; Gau, V.; Banaei, N.; Liao, J.C. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus 2017, 3, 293–299. [Google Scholar] [CrossRef]
- Ding, S.; Mosher, C.; Lee, X.Y.; Das, S.R.; Cargill, A.A.; Tang, X.; Chen, B.; McLamore, E.S.; Gomes, C.; Hostetter, J.M.; et al. Rapid and label-free detection of interferon gamma via an electrochemical aptasensor comprising a ternary surface monolayer on a gold interdigitated electrode array. ACS Sens. 2017, 2, 210–217. [Google Scholar] [CrossRef]
- Miranda-Castro, R.; Sánchez-Salcedo, R.; Suárez-Álvarez, B.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Thioaromatic DNA monolayers for target-amplification-free electrochemical sensing of environmental pathogenic bacteria. Biosens. Bioelectron. 2017, 92, 162–170. [Google Scholar] [CrossRef]
- Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Understanding the factors affecting the analytical performance of sandwich-hybridization genosensors on gold electrodes. Electroanalysis 2018, 30, 1229–1240. [Google Scholar] [CrossRef]
- González-Fernández, E.; Staderini, M.; Yussof, A.; Scholefield, E.; Murray, A.F.; Mount, A.R.; Bradley, M. Electrochemical sensing of human neutrophil elastase and polymorphonuclear neutrophil activity. Biosens. Bioelectron. 2018, 119, 209–214. [Google Scholar] [CrossRef]
- González-Fernández, E.; Staderini, M.; Avlonitis, N.; Murray, A.F.; Mount, A.R.; Bradley, M. Effect of spacer length on the performance of peptide-based electrochemical biosensors for protease detection. Sens. Actuators B Chem. 2018, 255, 3040–3046. [Google Scholar] [CrossRef]
- Boozer, C.; Chen, S.F.; Jiang, S.Y. Controlling DNA orientation on mixed ssDNA/OEG SAMs. Langmuir 2006, 22, 4694–4698. [Google Scholar] [CrossRef] [PubMed]
- Dharuman, V.; Hahn, J.H. Label-free electrochemical DNA hybridization discrimination effects at the binary and ternary mixed monolayers of single stranded DNA/diluent/s in presence of cationic intercalators. Biosens. Bioelectron. 2008, 23, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Campuzano, S.; Halford, C.; Haake, D.A.; Wang, J. Ternary surface monolayers for ultrasensitive (zeptomole) amperometric detection of nucleic acid hybridization without signal amplification. Anal. Chem. 2010, 82, 8830–8837. [Google Scholar] [CrossRef]
- Goodman, R.P.; Berry, R.M.; Turberfield, A.J. The single-step synthesis of a DNA tetrahedron. Chem. Commun. 2004, 12, 1372–1373. [Google Scholar] [CrossRef]
- Pei, H.; Lu, N.; Wen, Y.; Song, S.; Liu, Y.; Yan, H.; Fan, C. A DNA Nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv. Mater. 2010, 22, 4754–4758. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Ji, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA biosensor based on a tetrahedral nanostructure probe for the detection of Avian Influenza A (H7N9) virus. ACS Appl. Mater. Interfaces 2015, 5, 8834–8842. [Google Scholar] [CrossRef]
- Lubin, A.A.; Plaxco, K.W. Folding-based electrochemical biosensors: The case for responsive nucleic acid architectures. Acc. Chem. Res. 2010, 43, 496–505. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Reagentless and reusable electrochemical affinity biosensors for near real-time and/or continuous operation. Advances and prospects. Curr. Opin. Electrochem. 2019, 16, 35–41. [Google Scholar] [CrossRef]
- Plaxco, K.W.; Soh, H.T. Switch-based biosensors: A new approach towards real-time, in vivo molecular detection. Trends Biotechnol. 2011, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Currás, N.; Dauphin-Ducharme, P.; Scida, K.; Chávez, J.L. From the beaker to the body: Translational challenges for electrochemical, aptamer-based sensors. Anal. Methods 2020, 12, 1288–1310. [Google Scholar]
- Ranallo, S.; Rossetti, M.; Plaxco, K.W.; Vallée-Bélisle, A.; Ricci, F. A modular, DNA-based beacon for single-step fluorescence detection of antibodies and other proteins. Angew. Chem. Int. Ed. 2015, 54, 13214–13218. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Kallewaard, H.M.; Hsieh, W.; Patterson, A.S.; Kasehagen, J.B.; Cash, K.J.; Uzawa, T.; Soh, H.T.; Plaxco, K.W. Wash-free, electrochemical platform for the quantitative, multiplexed detection of specific antibodies. Anal. Chem. 2012, 84, 1098–1103. [Google Scholar] [CrossRef][Green Version]
- Ferguson, B.S.; Hoggarth, D.A.; Maliniak, D.; Ploense, K.; White, R.J.; Woodward, N.; Hsieh, K.; Bonham, A.J.; Eisenstein, M.; Kippin, T.E.; et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci. Transl. Med. 2013, 5, 213ra165. [Google Scholar] [CrossRef]
- Kang, D.; Sun, S.; Kurnik, M.; Morales, D.; Dahlquist, F.W.; Plaxco, K.W. New architecture for reagentless, protein-based electrochemical biosensors. J. Am. Chem. Soc. 2017, 139, 12113–12116. [Google Scholar] [CrossRef]
- Xiao, Y.; Lubin, A.A.; Baker, B.R.; Plaxco, K.W.; Heeger, A.J. Single-step electronic detection of femtomolar DNA by target-induced strand displacement in an electrode-bound duplex. Proc. Natl. Acad. Sci. USA 2006, 103, 16677–16680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, F.; Ni, J.; Liao, X.; Zhang, X.; Lin, Z. Facile construction of a highly sensitive DNA biosensor by in-situ assembly of electro-active tags on hairpin-structured probe fragment. Sci. Rep. 2016, 6, 22441. [Google Scholar] [CrossRef] [PubMed]
- Zaitouna, A.J.; Lai, R.Y. An electrochemical peptide-based Ara h 2 antibody sensor fabricated on a nickel(II)-nitriloacetic acid self-assembled monolayer using a His-tagged peptide. Anal. Chim. Acta 2014, 828, 85–91. [Google Scholar] [CrossRef]
- Mayer, M.D.; Lai, R.Y. Effects of redox label location on the performance of an electrochemical aptamer-based tumor necrosis factor-alpha sensor. Talanta 2018, 189, 585–591. [Google Scholar] [CrossRef]
- Lai, R.Y.; Seferos, D.S.; Heeger, A.J.; Bazan, G.C.; Plaxco, K.W. Comparison of the signaling and stability of electrochemical DNA sensors fabricated from 6- or 11-carbon self-assembled monolayers. Langmuir 2016, 22, 10796–10800. [Google Scholar] [CrossRef]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological Fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef] [PubMed]
- Parate, K.; Karunakaran, C.; Claussen, J.C. Electrochemical cotinine sensing with a molecularly imprinted polymer on a graphene-platinum nanoparticle modified carbon electrode towards cigarette smoke exposure monitoring. Sens. Actuators B Chem. 2019, 287, 165–172. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Shen, C.; Cheong, L.Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@MOF nanofibersbased biosensor. Talanta 2019, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lim, B.J.; Li, B.; Jiang, Y.S.; Sessler, J.L.; Ellington, A.D. Reagentless, ratiometric electrochemical DNA sensors with improved robustness and reproducibility. Anal. Chem. 2014, 86, 8010–8016. [Google Scholar] [CrossRef] [PubMed]





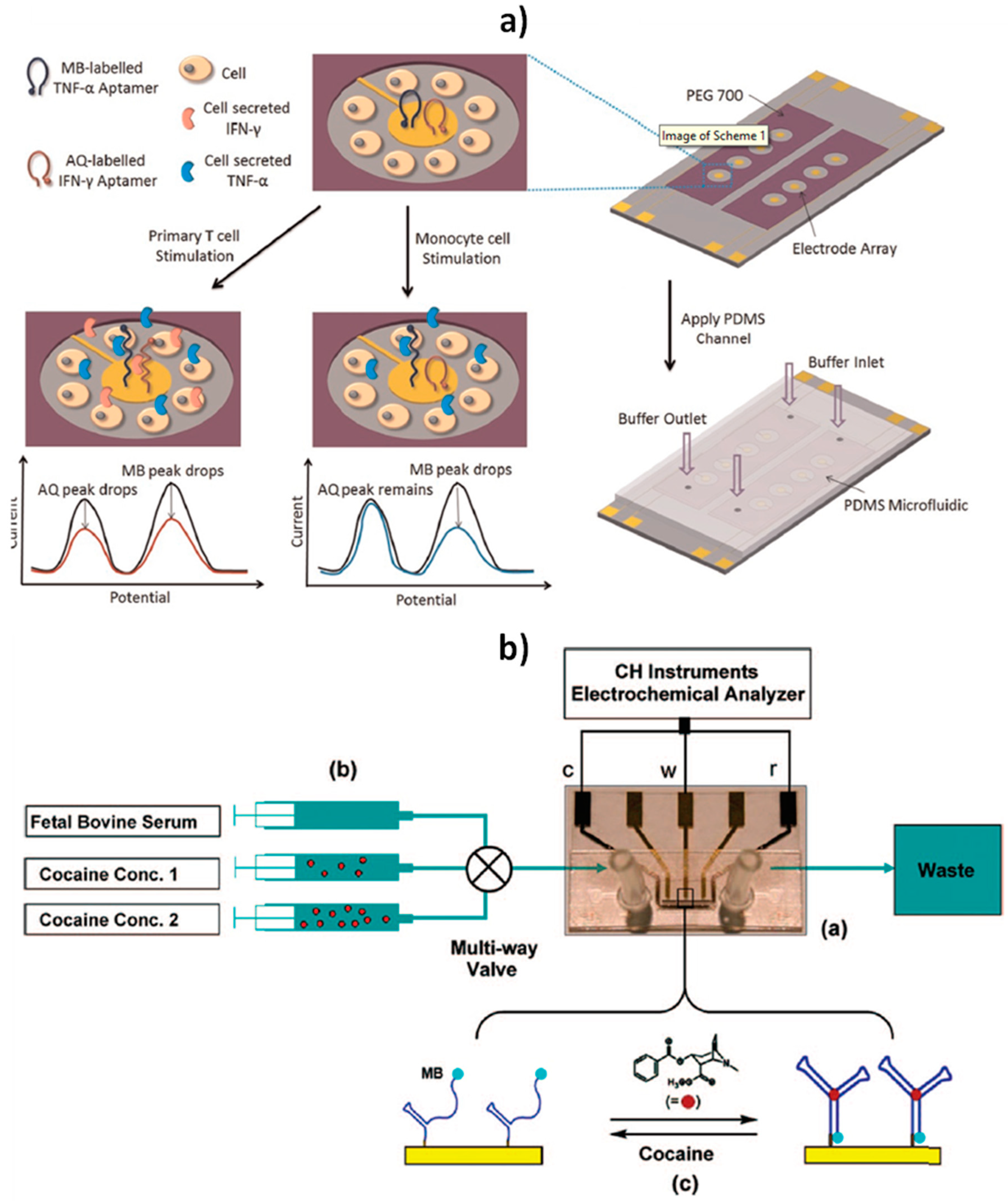
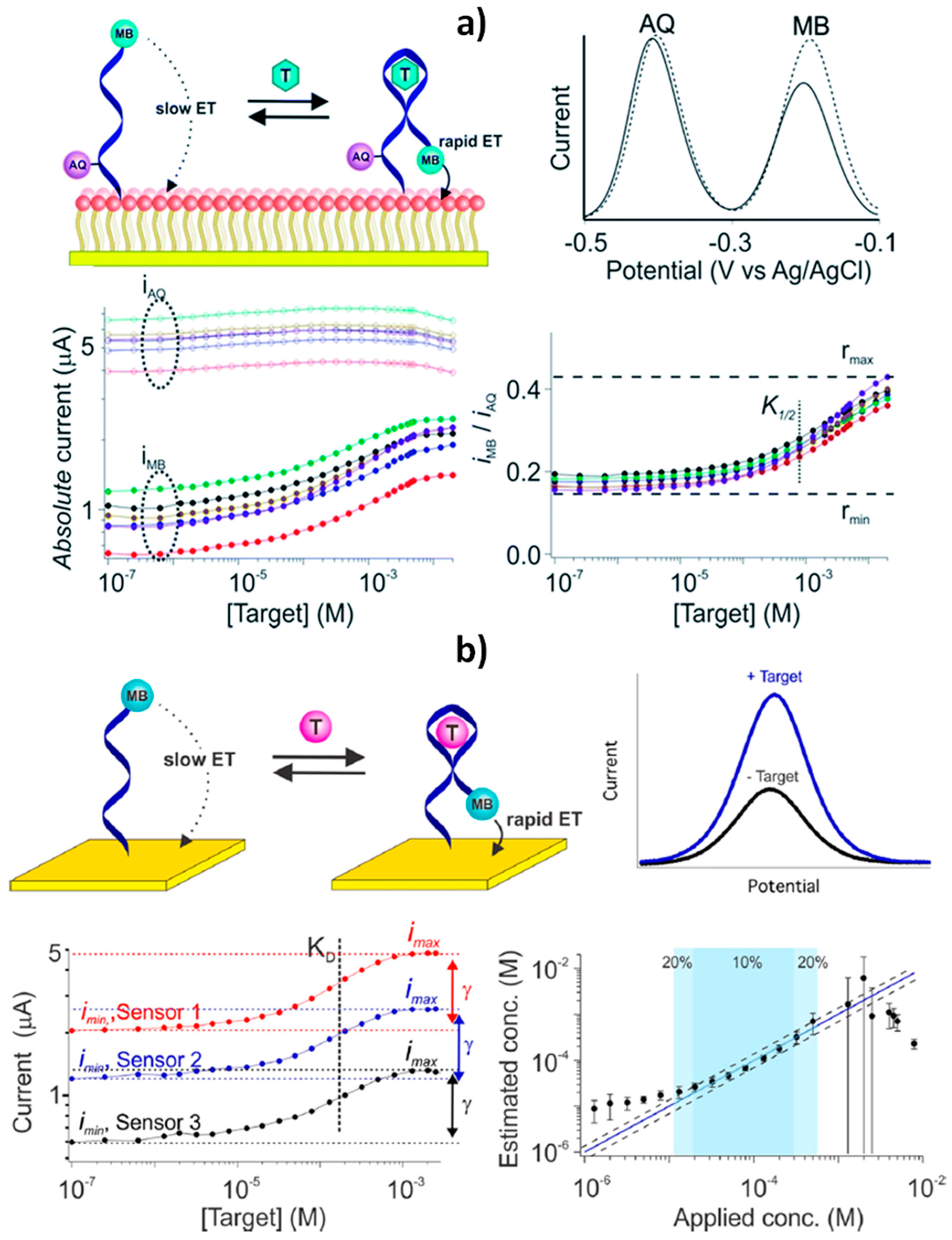
| Electrode | Sensor Fundamentals | Transduction Technique | Attribute (used Approach) | Additional Features | Molecular Target Tested | L.R./LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 16× Au electrode arrays prepared by photolithography | Sandwich hybridization assay at arrays modified with SHCP/HDT+MCH | Chrono-amperometry (TMB/H2O2) | Antibiofouling (thiolated ternary monolayer) | — | Synthetic target DNA (characteristic region of E. coli 16S rRNA) | LOD: 7 pM and 17 pM in spiked undiluted human serum and urine | Raw undiluted human serum and urine | [20] |
| Au/SPEs | Sandwich hybridization assay at arrays modified with SHCP/HDT+MCH | Chrono-amperometry (TMB/H2O2) | Antibiofouling (thiolated ternary monolayer) | — | Synthetic target DNA and E. coli 16S rRNA | LOD: 25 pM and 100 pM in spiked undiluted human serum and urine and 16S rRNA E. coli corresponding to 3000 CFU mL−1 in raw cell lysate samples | Untreated raw serum, urine, and crude bacterial lysate solutions | [21] |
| AuE | E-AB: Aptamer dually modified with a thiol and a redox reporter + PC-terminated SAM | SWV (MB) | Antibiofouling (PC-terminated SAM) | Continuous operation label-free | Kanamycin, doxorubicin | — | Flowing whole blood, both in vitro and in vivo (sensors placed in the jugular veins of live rats) | [12] |
| GOx-PB-graphite SPEs | Electrode modified with Eudragit® L100 | CV ([Fe(CN)6]4−/3−) and Chrono-amperometry (PB/H2O2) | Antibiofouling (pH-sensitive transient polymer coating) | Continuous operation | Glucose | — | Raw undiluted blood and saliva | [22] |
| Edible carbon paste GOx biosensors | Electrodes coated with Eudragit® E PO (pH < 5.0) or Eudragit® L100 (pH > 6.0) | Chrono-amperometry (PB/H2O2) | Antibiofouling (pH-sensitive transient polymer coating) | Continuous operation Biocatalytic activity preservation at media with denaturing pH values | Glucose | L.R.: 2−10 mM | GI fluids | [23] |
| PEDOT-citrate film-modified GCE | Covalent immobilization using EDC/NHS chemistry of a peptide with anchoring, antifouling, and recognizing capabilities onto GCE/PEDOT-citrate | DPV ([Fe(CN)6]4−/3−) | Antibiofouling (multifunctional peptide) | — | APN, HepG2 cells | L.R.: 1 ng mL−1−15 μg mL−1 (APN) and 50–5 × 105 cells mL−1 (HepG2 cells) LOD: 0.4 ng mL−1 (APN) and 20 cells mL−1 (HepG2 cells) | Human urine | [24] |
| Au disk | E-DNA: DNA probe dually modified with a thiol and a redox reporter + MCH SAM | SWV (MB) | Continuous and real-time operation (Folded-biosensor) | Reagentless and single-step | Melamine | LOD: 150 μM (∼19 ppm) in buffered solutions and 20 μM (∼2.5 ppm) in whole milk | Flowing undiluted whole milk | [6] |
| Au | E-DNA: TDNs with two functional DNA/aptamer strands, one of them modified with MB | SWV (MB) | Continuous and real-time operation (Folded-biosensor) | Reagentless and single-step Antibiofouling Reusability | Target DNA, ATP | LOD: 300 fM (target DNA), 5 nM (ATP) | Flowing whole blood | [8] |
| AuE | E-DNA: nucleic acid “scaffold” attached on one end to an electrode and presenting both a redox reporter and a specific epitope on the other | SWV (MB) | Reagentless and single-step (Folded-biosensor) | — | Three types of HIV-diagnostic antibodies | — | Human serum | [25] |
| Microfabricated Au onto MECAS chip | E-AB: Aptamer dually modified with a thiol and a redox reporter + MCH SAM | ACV (MB) | Continuous and real-time operation (Folded-biosensor) | Reagentless and single-step Reusability | Cocaine | — | Flowing undiluted blood serum | [9] |
| 100 nm Au layer sputtered on glass slides | E-AB: Hairpin structure aptamer dually modified with a thiol and a redox reporter (MB or AQ) + MCH SAM | SWV (MB, AQ) | Continuous operation (Folded-biosensor) | Antibiofouling Reagentless and single-step | IFN-γ + TNF-α | LOD: 6.35 ng mL−1 (IFN-γ), 5.46 ng mL−1 (TNF-α) | Integrated into microfluidic devices to dynamically monitoring of cytokine release from immune cells (2.5 h) | [26] |
| Au wire | E-AB: Aptamer dually modified with a thiol and a redox reporter + MCH SAM | SWV (MB) | Continuous and real-time and in vivo operation (Folded-biosensor) | Reagentless and single-step | Doxorubicin, Kanamycin, Gentamycin, and Tobramycin | — | Bloodstream awake, ambulatory rats | [10] |
| Au disk, Au-plated SPCEs | E-PB: Peptide dually modified with a thiol and a redox reporter + MCH SAM | ACV, CV (MB) | Real-time operation (Folded-biosensor) | Reagentless and single-step | Pb2+ | LOD: 5 μM (ACV) | Diluted tap water, saliva, and urine samples | [27] |
| Au disk | E-ION: T-rich ssDNA dually modified with thiol and redox reporter + Hg2+ + MCH SAM | ACV (MB) | Real-time operation (Folded-biosensor) | Reagentless and single-step Reusable | GSH (displaces Hg2+ by chelation) | LOD: 5 nM | 50% synthetic human saliva | [28] |
| AuE | E-AB: Aptamer dually modified with a thiol and a redox reporter + MCH SAM | SWV (MB) | Calibration-free (“dual-frequency”) | Continuous and real-time operation Reagentless and single-step | Cocaine, doxorubicin | — | Continuous measurement in flowing, undiluted whole blood | [7] |
| Au-SPE | E-AB: Aptamer dually modified with a thiol and a redox reporter + MCH | SWV (MB) | Calibration-free (“dual-frequency”) | Reagentless and single-step | Phenylalanine | L.R.: 90 nM−7 μM | Whole blood (diluted 1000-fold to match the sensor’s dynamic range) | [3] |
| AuE | E-AB: Aptamer modified with a thiol and two different redox reporters + PC-terminated SAM | SWV (MB and AQ) | Calibration-free, (“dual reporter”) and in vivo operation | Continuous operation Antibiofouling Reagentless and single-step | Cocaine, ATP, kanamycin | — | Flowing whole blood, both in vitro and in vivo (sensors placed in the jugular veins of live rats) | [2] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, S.; Pedrero, M.; Gamella, M.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors 2020, 20, 3376. https://doi.org/10.3390/s20123376
Campuzano S, Pedrero M, Gamella M, Serafín V, Yáñez-Sedeño P, Pingarrón JM. Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors. 2020; 20(12):3376. https://doi.org/10.3390/s20123376
Chicago/Turabian StyleCampuzano, Susana, María Pedrero, Maria Gamella, Verónica Serafín, Paloma Yáñez-Sedeño, and José Manuel Pingarrón. 2020. "Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices" Sensors 20, no. 12: 3376. https://doi.org/10.3390/s20123376
APA StyleCampuzano, S., Pedrero, M., Gamella, M., Serafín, V., Yáñez-Sedeño, P., & Pingarrón, J. M. (2020). Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors, 20(12), 3376. https://doi.org/10.3390/s20123376







