Reliability and Validity of a Novel Wearable Device for Measuring Elbow Strength
Abstract
:1. Introduction
2. Methods
2.1. Participants and Raters
2.2. Experimental Design
2.3. Protocol
2.4. Data Analysis
2.4.1. Objective 1: Reliability of LSMD for Strength Assessment
2.4.2. Objective 2: Validity of the LSMD for Assessment
3. Results
3.1. Reliability Analysis
3.1.1. Intra-Rater Repeatability
3.1.2. Inter-Rater Reproducibility
3.1.3. Inter-Session Reproducibility
3.1.4. Post-Hoc Analysis of Reproducibility Results
3.2. Validity Analysis
3.2.1. Criterion Validity
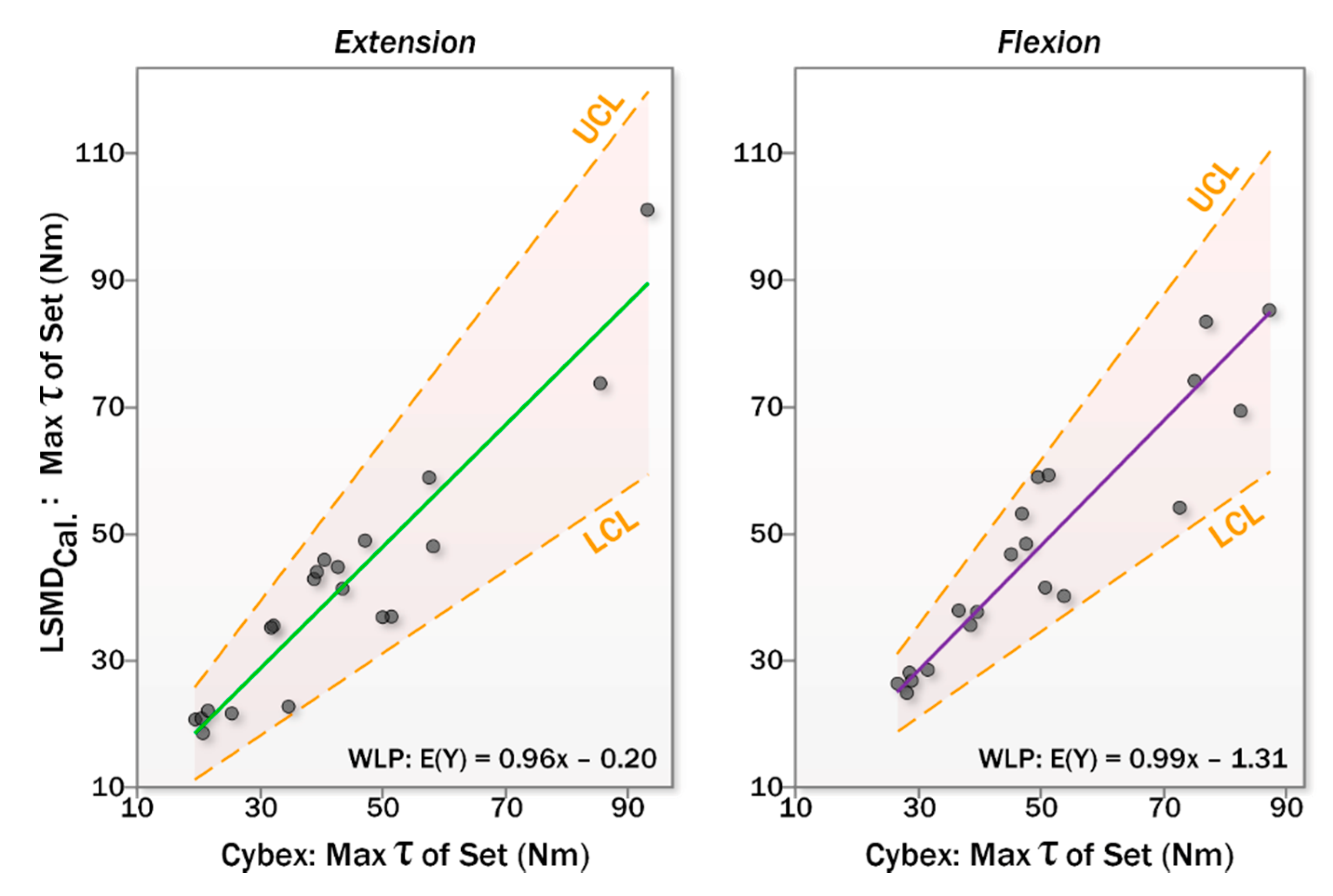
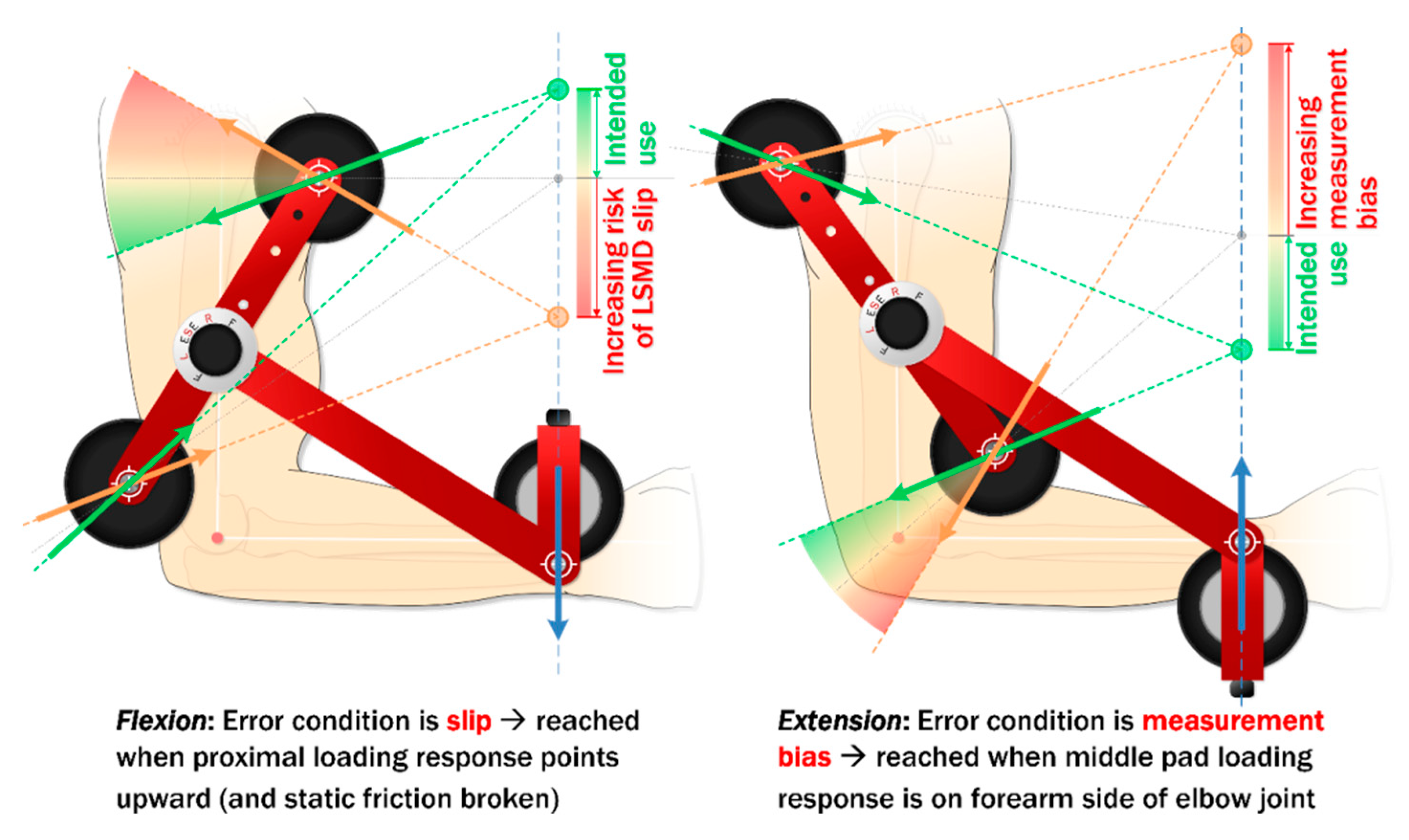
3.2.2. Calibration Using the Criterion Standard
4. Discussion
4.1. Reliability of the LSMD
4.2. Validity of the LSMD
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osternig, L.R. Isokinetic dynamometry: Implications for muscle testing and rehabilitation. Exerc. Sport Sci. Rev. 1986, 14, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Baltzopoulos, V.; Brodie, D. Isokinetic dynamometry. Applications and limitations. Sport. Med. 1989, 8, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Schrama, P.P.; Stenneberg, M.S.; Lucas, C.; van Trijffel, E. Intraexaminer reliability of hand-held dynamometry in the upper extremity: A systematic review. Arch. Phys. Med. Rehabil. 2014, 95, 2444–2469. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.N.; Opar, D.A.; Williams, M.D.; Shield, A.J. Eccentric knee flexor strength and risk of hamstring injuries in rugby union: A prospective study. Am. J. Sports Med. 2015, 43, 2663–2670. [Google Scholar] [CrossRef]
- Keating, J.L.; Matyas, T.A. The influence of subject and test design on dynamometric measurements of extremity muscles. Phys. Ther. 1996, 76, 866–889. [Google Scholar] [CrossRef] [Green Version]
- Marx, R.G.; Menezes, A.; Horovitz, L.; Jones, E.C.; Warren, R.F. A comparison of two time intervals for test-retest reliability of health status instruments. J. Clin. Epidemiol. 2003, 56, 730–735. [Google Scholar] [CrossRef]
- Harris-Love, M.; Benson, K.; Leasure, E.; Adams, B.; McIntosh, V. The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests. J. Funct. Morphol. Kinesiol. 2018, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.J.; McCarty, E.; Provencher, M.; Manske, R.C. ACL Return to Sport Guidelines and Criteria. Curr. Rev. Musculoskelet. Med. 2017, 10, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Considerations and Practical Options for Measuring Muscle Strength: A Narrative Review. Biomed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Bohannon, R.W. Hand-held dynamometry: Factors influencing reliability and validity. Clin. Rehabil. 1997, 11, 263–264. [Google Scholar]
- Wikholm, J.B.; Bohannon, R.W. Hand-held dynamometer measurements: Tester strength makes a difference. J. Orthop. Sports Phys. Ther. 1991, 13, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, C.A.; Bríd, N.; Lawlor, P.G.; Kenny, R.A. Hand-held dynamometry: Tester strength is paramount, even in frail populations. J. Rehabil. Med. 2011, 43, 808–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byl, N.N.; Richards, S.; Asturias, J. Intrarater and interrater reliability of strength measurements of the biceps and deltoid using a hand held dynamometer. J. Orthop. Sports Phys. Ther. 1988, 9, 395–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelln, B.M.; McKeon, P.O.; Gontkof, L.M.; Hertel, J. Hand-held dynamometry: Reliability of lower extremity muscle testing in healthy, physically active, young adults. J. Sport Rehabil. 2008, 17, 160–170. [Google Scholar] [CrossRef]
- Bohannon, R.W. Intertester reliability of hand-held dynamometry: A concise summary of published research. Percept. Mot. Skills 1999, 88, 899–902. [Google Scholar] [CrossRef]
- Brinkrnann, J.R. Comparison of a hand-held and fixed dynamometer in measuring strength of patients with neuromuscular disease. J Orthop Sport. Phys Ther. 1994, 19, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Thorborg, K.; Bandholm, T.; Hölmich, P. Hip- and knee-strength assessments using a hand-held dynamometer with external belt-fixation are inter-tester reliable. Knee Surg Sport. Traumatol Arthrosc. 2013, 21, 550–555. [Google Scholar] [CrossRef]
- Gagnon, D.; Nadeau, S.; Gravel, D.; Robert, J.; Belanger, D.; Hilsenrath, M. Reliability and validity of static knee strength measurements obtained with a chair-fixed dynamometer in subjects with hip or knee arthroplasty. Arch. Phys. Med. Rehabil. 2005, 86, 1998–2008. [Google Scholar] [CrossRef]
- Bandy, W.D.; McLaughlin, S. Intramachine and intermachine reliability for selected dynamic muscle performance tests. J. Orthop. Sports Phys. Ther. 1993, 18, 609–613. [Google Scholar] [CrossRef]
- Reed, R.L.; Hartog, R.D.; Yochum, K.; Pearlmutter, L.; Ruttinger, A.C.; Mooradian, A.D. A comparison of hand-held isometric strength measurement with isokinetic muscle strength measurement in the elderly. J. Am. Geriatr. Soc. 1993, 41, 53–56. [Google Scholar] [CrossRef]
- Rothstein, J.M.; Lamb, R.L.; Mayhew, T.P. Clinical uses of isokinetic measurements. Critical issues. Phys. Ther. 1987, 67, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, N.P.; Mercer, T.H. The utility of isokinetic dynamometry in the assessment of human muscle function. Sport. Med. 1996, 21, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Walker, B.; Phillips, J.K.; Fejer, R.; Beck, R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: A systematic review. PM&R 2011, 3, 472–479. [Google Scholar]
- Reinking, M.F.; Bockrath-Pugliese, K.; Worrell, T.; Kegerreis, R.L.; Miller-Sayers, K.; Farr, J. Assessment of quadriceps muscle performance by hand-held, isometric, and isokinetic dynamometry in patients with knee dysfunction. J. Orthop. Sport. Phys. Ther. 1996, 24, 154–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, E.; Hayes, P. Test-retest reliability of isokinetic concentric knee extension and flexion. Br. J. Sports Med. 2010, 44, i26. [Google Scholar] [CrossRef]
- Kannus, P. Isokinetic evaluation of muscular performance: Implications for muscle testing and rehabilitation. Int. J. Sports Med. 1994, 15, S11-8. [Google Scholar] [CrossRef]
- Lund, H.H.; Søndergaard, K.; Zachariassen, T.; Christensen, R.; Bülow, P.; Henriksen, M.; Bartels, E.M.; Danneskiold-Samsøe, B.; Bliddal, H. Learning effect of isokinetic measurements in healthy subjects, and reliability and comparability of Biodex and Lido dynamometers. Clin. Physiol. Funct. Imaging 2005, 25, 75–82. [Google Scholar] [CrossRef]
- Drouin, J.M.; Valovich-McLeod, T.C.; Shultz, S.J.; Gansneder, B.M.; Perrin, D.H. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur. J. Appl. Physiol. 2004, 91, 22–29. [Google Scholar]
- Landry, J.; Sexton, A.; Hughes, G.; McGibbon, C.A. Limb Strength Measurement Device. U.S. Patent US9,028,433, 12 May 2015. [Google Scholar]
- McGibbon, C.A.; Sexton, A.; Hughes, G.; Wilson, A.; Jones, M.; O’Connell, C.; Parker, K.; Adans-Dester, C.; O’Brien, A.; Bonato, P. Evaluation of a toolkit for standardizing clinical measures of muscle tone. Physiol. Meas. 2018, 39, 085001. [Google Scholar] [CrossRef]
- McGibbon, C.A.; Sexton, A.; Jones, M.; O’Connell, C.A.; O’Connell, C. Elbow spasticity during passive stretch-reflex: Clinical evaluation using a wearable sensor system. J. Neuroeng. Rehabil. 2013, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Kollock, R.O.; Onate, J.A.; Lunen, B. Van The reliability of portable fixed dynamometry during hip and knee strength assessments. J. Athl. Train. 2010, 45, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schless, S.H.; Desloovere, K.; Aertbeliën, E.; Molenaers, G.; Huenaerts, C.; Bar-On, L. The intra- and inter-rater reliability of an instrumented spasticity assessment in children with cerebral palsy. PLoS ONE 2015, 10, e0131011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.A.; Bond, E.Q.; Sisto, S.A.; Nadler, S.F. The intra- and interrater reliability of hip muscle strength assessments using a handheld versus a portable dynamometer anchoring station. Arch. Phys. Med. Rehabil. 2004, 85, 598–603. [Google Scholar] [CrossRef]
- Ford-Smith, C.D.; Wyman, J.F.; Elswick, R.K. Reliability of stationary dynamometer muscle strength testing in community-dwelling older adults. Arch. Phys. Med. Rehabil. 2001, 82, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.A.; Martins, J.C.; Teixeira-Salmela, L.F.; Lara, E.M.; Moura, J.B.; Aguiar, L.T.; de Morais Faria, C.D. Validity and reliability of the modified sphygmomanometer test to assess strength of the lower limbs and trunk muscles after stroke. J Rehabil Med. 2014, 46, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.W.; Hsu, H.C.; Chang, L.Y.; Chen, H.L. Enhancing the examiner’s resisting force improves the reliability of manual muscle strength measurements: Comparison of a new device with hand-held dynamometry. J. Rehabil. Med. 2007, 39, 679–684. [Google Scholar] [CrossRef] [Green Version]
- HUMAC NORM Brochure; Computer Sports Medicine Inc.: Stoughton, MA, USA, 2009.
- Hoggan Scientific Inc. Ergo Force Gauges, Medical Force Gauges & Sensors - microFET2. Available online: https://hogganscientific.com/product/microfet2-muscle-tester-digital-handheld-dynamometer/ (accessed on 17 June 2020).
- Bohannon, R.W. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys. Ther. 1986, 66, 206–209. [Google Scholar] [CrossRef]
- HUMAC/NORM Testing and Rehabilitation System: User Guide (Model 770); Computer Sports Medicine Inc.: Stoughton, MA, USA, 2009.
- Matheson, L.; Mooney, V.; Caiozzo, V.; Jarvis, G.; Pottinger, J.; DeBerry, C.; Backlund, K.; Klein, K.; Antoni, J. Effect of instructions on isokinetic trunk strength testing variability, reliability, absolute value, and predictive validity. Spine (Phila. Pa. 1976) 1992, 17, 914–921. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Ludbrook, J.J. Confidence in Altman-Bland plots: A critical review of the method of differences. Clin. Exp. Pharmacol. Physiol. 2010, 37, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, J.J. Comparing methods of measurements. Clin. Exp. Pharmacol. Physiol. 1997, 24, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Miczak, K.; Padova, J. Muscle Overactivity in the Upper Motor Neuron Syndrome: Assessment and Problem Solving for Complex Cases: The Role of Physical and Occupational Therapy. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 529–536. [Google Scholar] [CrossRef]
- Lohr, K.N.; Aaronson, N.K.; Alonso, J.; Audrey, B.M.; Patrick, D.L.; Perrin, E.B.; Roberts, J.S. Evaluating quality-of-life and health status instruments: Development of scientific review criteria. Clin. Ther. 1996, 18, 979–992. [Google Scholar] [CrossRef]
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef]
- van Trijffel, E.E.; van de Pol, R.J.; Oostendorp, R.A.; Lucas, C. Inter-rater reliability for measurement of passive physiological movements in lower extremity joints is generally low: A systematic review. J. Physiother. 2010, 56, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.; Mans, E.; de Visser, M.; van den Berg-Vos, R.M.; Franssen, H.; de Jong, J.M.; van den Berg, L.H.; Wokke, J.H.; de Haan, R.J. Comparison of maximal voluntary isometric contraction and hand-held dynamometry in measuring muscle strength of patients with progressive lower motor neuron syndrome. Neuromuscul. Disord. 2003, 13, 744–750. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch. Phys. Med. Rehabil. 1997, 78, 26–32. [Google Scholar] [CrossRef]
- Aufsesser, P.M.; Horvat, M.; Austin, R. The reliability of hand held muscle testers with individuals with spinal cord injury. Clin Kinesiol. 2003, 57, 71–75. [Google Scholar]
- Mathur, S.; Makrides, L.; Hernandez, P. Test-retest reliability of isometric and isokinetic torque in patients with chronic obstructive pulmonary disease. Physiother. Canada 2004, 56, 94–101. [Google Scholar] [CrossRef]
- Ekstrand, E.; Lexell, J.; Brogårdh, C. Isometric and isokinetic muscle strength in the upper extremity can be reliably measured in persons with chronic stroke. J. Rehabil. Med. 2015, 47, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratford, P.W.; Balsor, B.E. A comparison of make and break tests using a hand-held dynamometer and the Kin-Com. J. Orthop. Sports Phys. Ther. 1994, 19, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, J.; Brown, L.; Tufano, J. The reproducibility of isokinetic dynamometry data. Isokinet. Exerc. Sci. 2012, 20, 239–253. [Google Scholar] [CrossRef]
- Jensen, A.M. Estimating the prevalence of use of kinesiology-style manual muscle testing: A survey of educators. Adv. Integr. Med. 2015, 2, 96–102. [Google Scholar] [CrossRef]
- Wadsworth, C.; Nielsen, D.H.; Corcoran, D.S.; Phillips, C.E.; Sannes, T.L. Interrater reliability of hand-held dynamometry: Effects of rater gender, body weight, and grip strength. J. Orthop. Sports Phys. Ther. 1992, 16, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, D.D.; McCrory, M.A.; Wright, N.C.; Rosko, R.A.; Kim, H.R.; Aitkens, S.G. Hand-held dynamometry reliability in persons with neuropathic weakness. Arch. Phys. Med. Rehabil. 1997, 78, 1364–1368. [Google Scholar] [CrossRef]
- Hogrel, J.Y.; Payan, C.A.; Ollivier, G.; Tanant, V.; Attarian, S.; Couillandre, A.; Dupeyron, A.; Lacomblez, L.; Doppler, V.; Meininger, V.; et al. Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch. Phys. Med. Rehabil. 2007, 88, 1289–1297. [Google Scholar] [CrossRef]
- Whelan, A.; Sexton, A.; Jones, M.; O’Connell, C.; McGibbon, C.A. Predictive value of the pendulum test for assessing knee extensor spasticity. J. Neuroeng. Rehabil. 2018, 15, 68. [Google Scholar] [CrossRef]



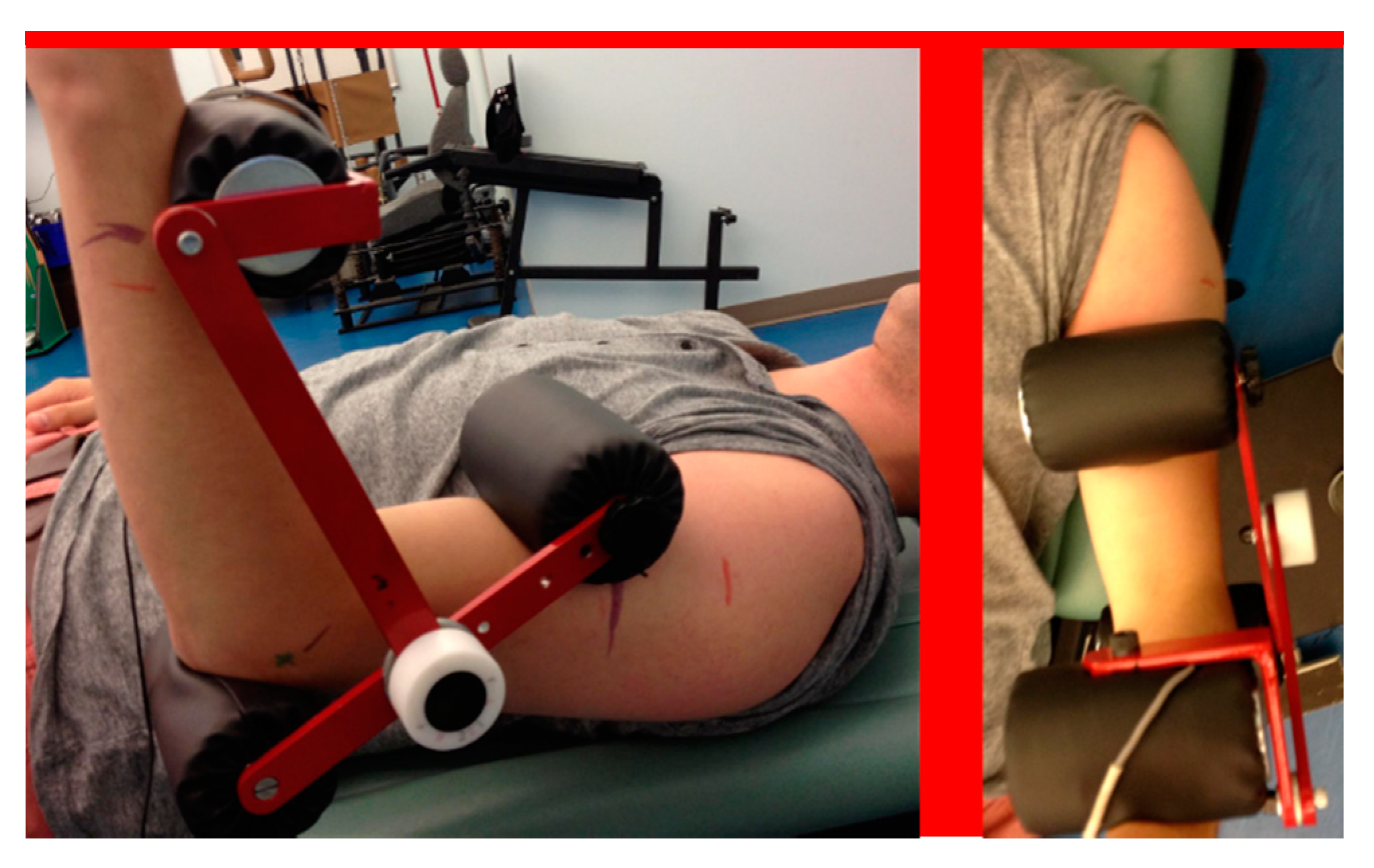

| Device | Dimensions (cm) | Mass (kg) | Sensor | Power | |
|---|---|---|---|---|---|
| LSMD | Arm | 43 × 17 × 10 † | 1.45 | Linear force: LC703–300 load cell (136 kg max capacity) | Internally powered: 9 V battery |
| Leg | 55 × 29 × 10 † | 2.64 | |||
| Cybex [38] | 302 × 234 × 152 | 318 | Axially aligned torque: Torque (678 Nm max) and angle (500°/s max) | Wall connection: Isolated, 20 A 220 VAC single-phase line | |
| MicroFET 2 [39] | 10 × 10 × 4 | 0.36 | Linear force: Internal load cell (136 kg max capacity) | Internally powered: 3.7 V battery (½ AA cell) | |
| Group 1 | Group 2 | Full Cohort | |
|---|---|---|---|
| Sex n Female/Male | 3 F/7 M | 5 F/5 M | 8 F/12 M |
| Hand dominance n Right/Left | 8 R/2 L | 8 R/2 L | 16 R/4 L |
| Age mean years (s; min–max) | 24 (3; 21–32) | 28 (10; 21–53) | 26 (8; 21–53) |
| Height mean cm (s; min–max) | 171 (11; 152–188) | 172 (11; 154–192) | 172 (11; 152–192) |
| Mass mean kg (s; min–max) | 73 (18; 48–101) | 71 (16; 55–108) | 72 (17; 48–108) |
| BMI mean BMI (s; min–max) | 25 (4; 19–33) | 24 (4; 19–33) | 24 (4; 19–33) |
| Peak Extension τ mean (s; max–min) | ||||
| Group A | Group B | Full Cohort | ||
| HHD | S I | 47.1 (20.3; 20.6–78.7) | 31.2 (7.9; 19.5–41.8) | 39.2 (17.0; 19.5–78.7) |
| S II | 50.0 (25.5; 20.6–94.9) | 40.0 (10.7; 25.0–56.0) | 45.0 (19.7; 20.6–94.9) | |
| LSMD | S I | 59.0 (34.9; 19.7–120.2) | 41.6 (17.7; 21.5–77.2) | 50.3 (28.8; 19.7–120.2) |
| S II | 62.8 (39.0; 19.2–143.9) | 43.4 (14.5; 23.7–65.1) | 53.1 (30.3; 19.2–143.9) | |
| IKD | S I | 46.1 (22.6; 16.5–76.6) | 31.8 (10.3; 18.3–54.5) | 39.0 (18.6; 16.5–76.6) |
| S II | 47.3 (26.1; 19.5–93.0) | 38.0 (10.4; 21.5–51.3) | 42.6 (19.9; 19.5–93.0) | |
| Peak Flexion τ mean (s; max–min) | ||||
| Group A | Group B | Full Cohort | ||
| HHD | S I | 44.4 (18.8; 16.6–75.5) | 40.6 (10.5; 26.8–59.8) | 42.5 (14.9; 16.6–75.5) |
| S II | 52.6 (23.1; 21.0–85.2) | 38.8 (8.7; 27.2–54.3) | 45.7 (18.4; 21.0–85.2) | |
| LSMD | S I | 45.0 (19.4; 20.8–68.8) | 35.7 (10.3; 19.8–51.3) | 40.3 (15.9; 19.8–68.8) |
| S II | 49.1 (21.1; 23.0–77.2) | 36.6 (10.2; 21.7–52.9) | 42.9 (17.4; 21.7–77.2) | |
| IKD | S I | 50.9 (23.2; 23.4–91.6) | 40.3 (11.3; 24.0–60.4) | 45.6 (18.6; 23.4–91.6) |
| S II | 55.3 (23.3; 26.7–87.2) | 44.5 (13.3; 28.1–72.6) | 49.9 (19.2; 26.7–87.2) | |
| Extension | Flexion | |||||
|---|---|---|---|---|---|---|
| CI95 | CI95 | |||||
| ICC | [LB, UB] | dCohen | ICC | [LB, UB] | dCohen | |
| Intra-rater Repeatability † | ||||||
| HHD | 0.975 | [0.948, 0.989] | 15.0 | 0.969 | [0.935, 0.986] | 12.9 |
| LSMD | 0.996 | [0.991, 0.998] | 34.6 | 0.965 | [0.924, 0.985] | 12.5 |
| IKD | 0.975 | [0.947, 0.990] | 15.0 | 0.915 | [0.829, 0.964] | 7.9 |
| Inter-rater Reproducibility ‡ | ||||||
| HHD | 0.575 | [–0.107, 0.891] | 2.7 | 0.875 | [0.597, 0.967] | 5.1 |
| LSMD | 0.897 | [0.653, 0.973] | 6.8 | 0.856 | [0.526, 0.962] | 5.6 |
| IKD | 0.654 | [0.040, 0.904] | 3.6 | 0.876 | [0.397, 0.971] | 6.9 |
| Inter-session Reproducibility ‡ | ||||||
| HHD | 0.909 | [0.695, 0.976] | 6.0 | 0.854 | [0.297, 0.966] | 5.2 |
| LSMD | 0.959 | [0.852, 0.989] | 11.3 | 0.938 | [0.725, 0.985] | 9.7 |
| IKD | 0.916 | [0.700, 0.978] | 7.5 | 0.933 | [0.738, 0.983] | 9.1 |
| Extension | Flexion | |||||
|---|---|---|---|---|---|---|
| n = 10 per Group | Fj† | Pj† | Fj† | pj† | ||
| Inter-session | S I v. S II | HHD | 0.9 | 0.372 | 8.4 | 0.017 * |
| ICCaa(2, 1) | LSMD | 1.3 | 0.283 | 4.5 | 0.064 | |
| [Group 1] | IKD | 0.1 | 0.721 | 3.3 | 0.104 | |
| Inter-rater | A v. B | HHD | 24.5 | 0.001 * | 1.4 | 0.260 |
| ICCaa(2, 1) | LSMD | 0.6 | 0.470 | 0.2 | 0.634 | |
| [Group 2] | IKD | 7.3 | 0.025 * | 7.4 | 0.023 * | |
| Comparison | r2 | a | CI95 for a’ | pa† | b | CI95 for b | pb† | ||
|---|---|---|---|---|---|---|---|---|---|
| Session I | |||||||||
| LSMD vs. IKD | Ext. | 0.810 | −9.022 | [−26.627, 8.583] | 0.296 | 1.513 | [0.870, 2.155] | <0.000 * | |
| Flex. | 0.884 | −1.459 | [−8.005, 5.087] | 0.645 | 0.922 | [0.736, 1.108] | <0.000 * | ||
| Session II | |||||||||
| LSMD vs. IKD | Measured | Ext. | 0.885 | −9.220 | [−21.973, 3.534] | 0.146 | 1.451 | [1.006, 1.895] | < 0.000 * |
| Flex. | 0.855 | −2.666 | [−10.856, 5.523] | 0.503 | 0.912 | [0.700, 1.124] | <0.000 * | ||
| Calibrated | Ext. | 0.885 | −0.203 | [−7.380, 6.974] | 0.953 | 0.964 | [0.732, 1.196] | <0.000 * | |
| Flex. | 0.855 | −1.315 | [−8.841, 6.212] | 0.718 | 0.990 | [0.806, 1.174] | <0.000 * | ||
| Muscle | τ (Nm) | ∆τ (IKD–LSMD) (Nm) | |||||
|---|---|---|---|---|---|---|---|
| Group | IKD | LSMD | Mean | |min| | |max| | s | |
| As Measured | Ext. | 42.6 | 53.1 | −10.4 | 1.5 | 50.9 | 13.4 |
| Flex. | 49.9 | 42.9 | 7.0 | 0.7 | 24.2 | 7.2 | |
| Calibrated | Ext. | 42.6 | 41.1 | 1.6 | 0.5 | 14.3 | 6.9 |
| Flex. | 49.9 | 48.1 | 1.8 | 0.2 | 18.5 | 7.2 | |
| Reliability | Recommended Cut-Off | Source(s) | |
|---|---|---|---|
| Intra-rater | ICCcon(3, 1) | >0.90 | [3,48,49] |
| Inter-rater | ICCaa(2, 1) | >0.75 | [3,48] |
| Inter-session | ICCaa(2, 1) | >0.75 | [3,48] |
| Overall reliability of the study | ≥75% of listed ICC values are >0.75 | [50] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brookshaw, M.; Sexton, A.; McGibbon, C.A. Reliability and Validity of a Novel Wearable Device for Measuring Elbow Strength. Sensors 2020, 20, 3412. https://doi.org/10.3390/s20123412
Brookshaw M, Sexton A, McGibbon CA. Reliability and Validity of a Novel Wearable Device for Measuring Elbow Strength. Sensors. 2020; 20(12):3412. https://doi.org/10.3390/s20123412
Chicago/Turabian StyleBrookshaw, Marcus, Andrew Sexton, and Chris A. McGibbon. 2020. "Reliability and Validity of a Novel Wearable Device for Measuring Elbow Strength" Sensors 20, no. 12: 3412. https://doi.org/10.3390/s20123412




