Porous Graphene Oxide Decorated Ion Selective Electrode for Observing Across-Cytomembrane Ion Transport
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Principle of I−-Ion Selective Electrode
2.3. Synthesis of Porous Graphene Oxide
2.4. ISE’s Modification
2.5. Electrical Measurements
2.6. Instruments
3. Results
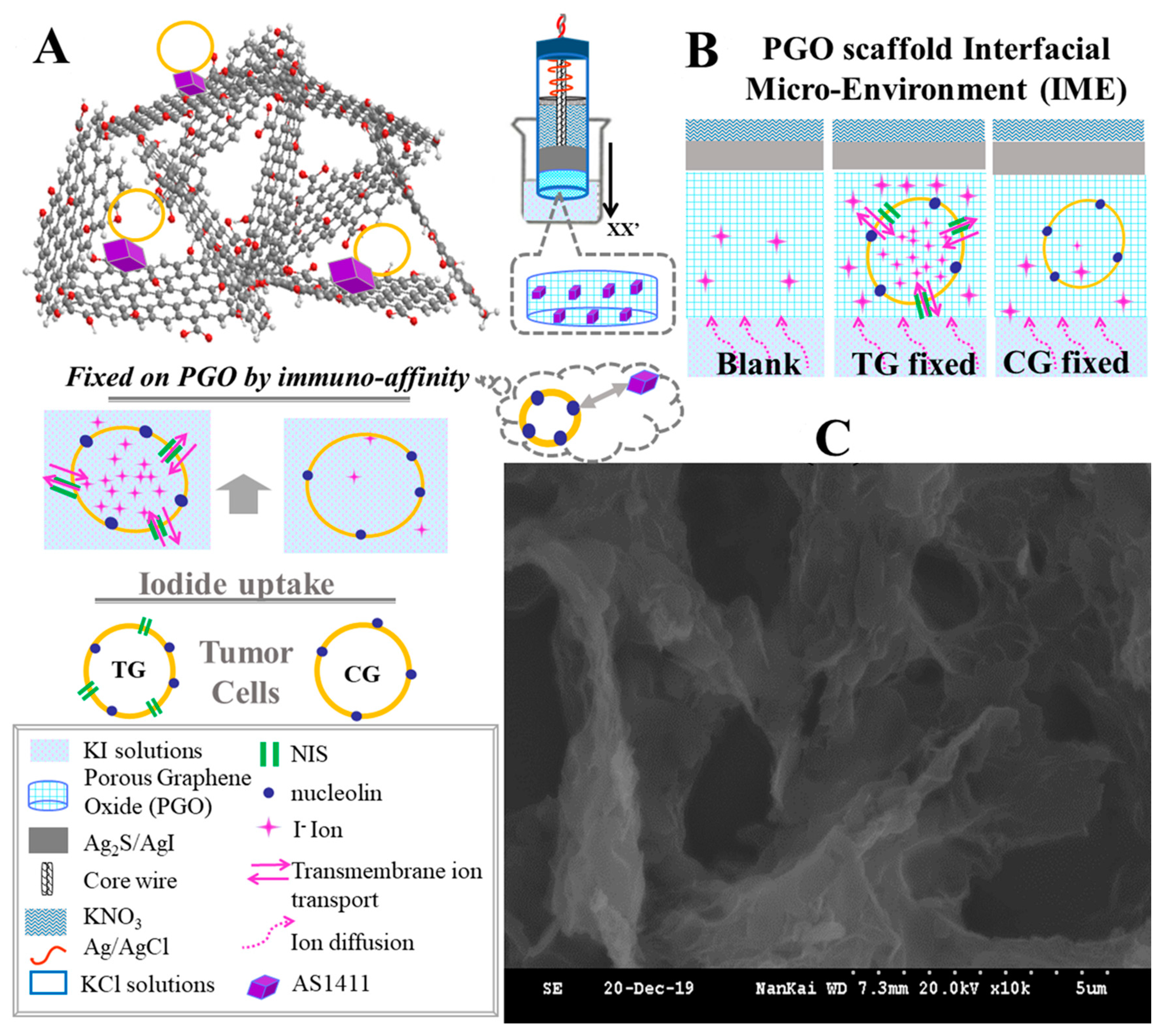
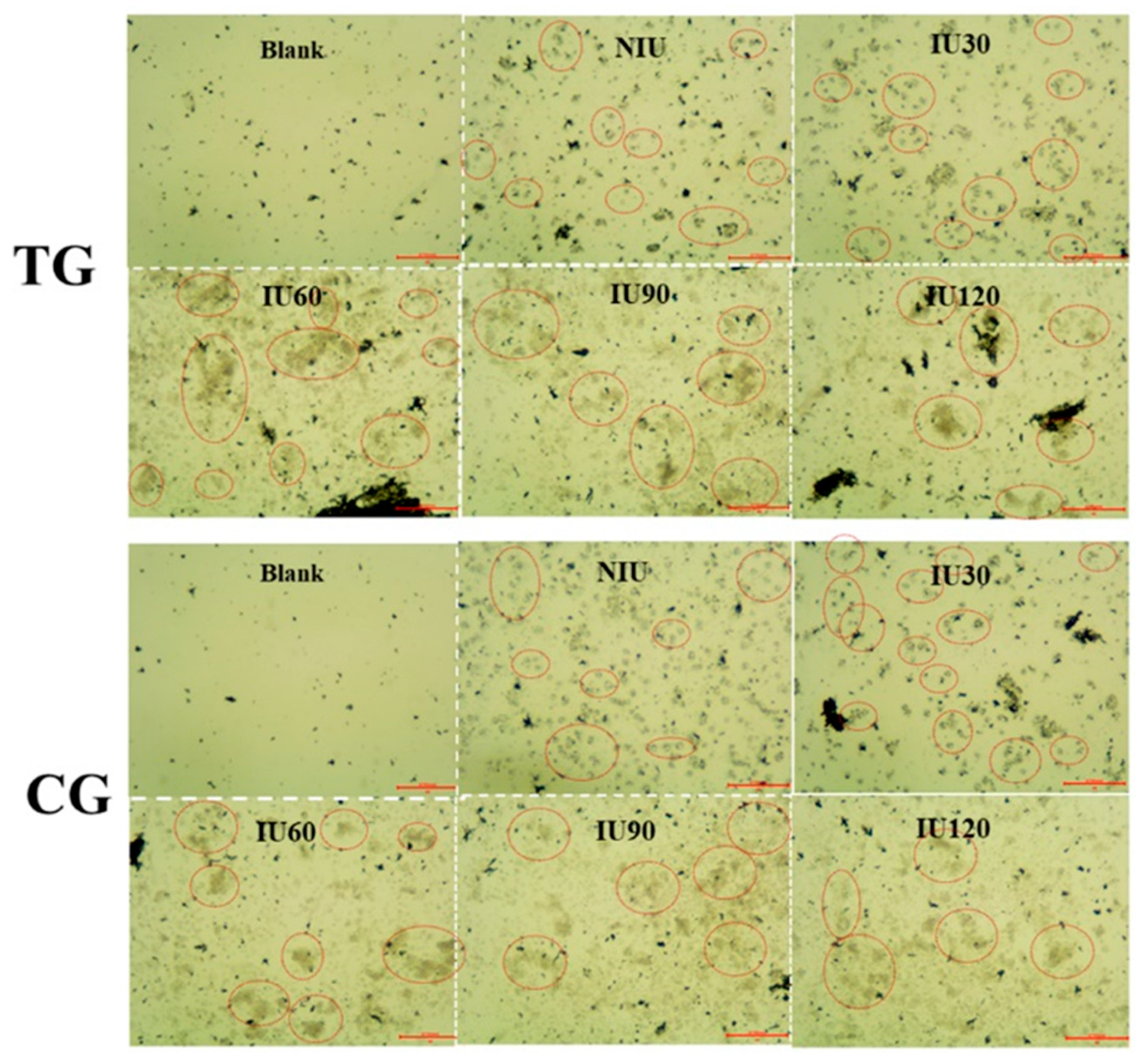
3.1. Microscopy of PGO and Immobilized Cells on It
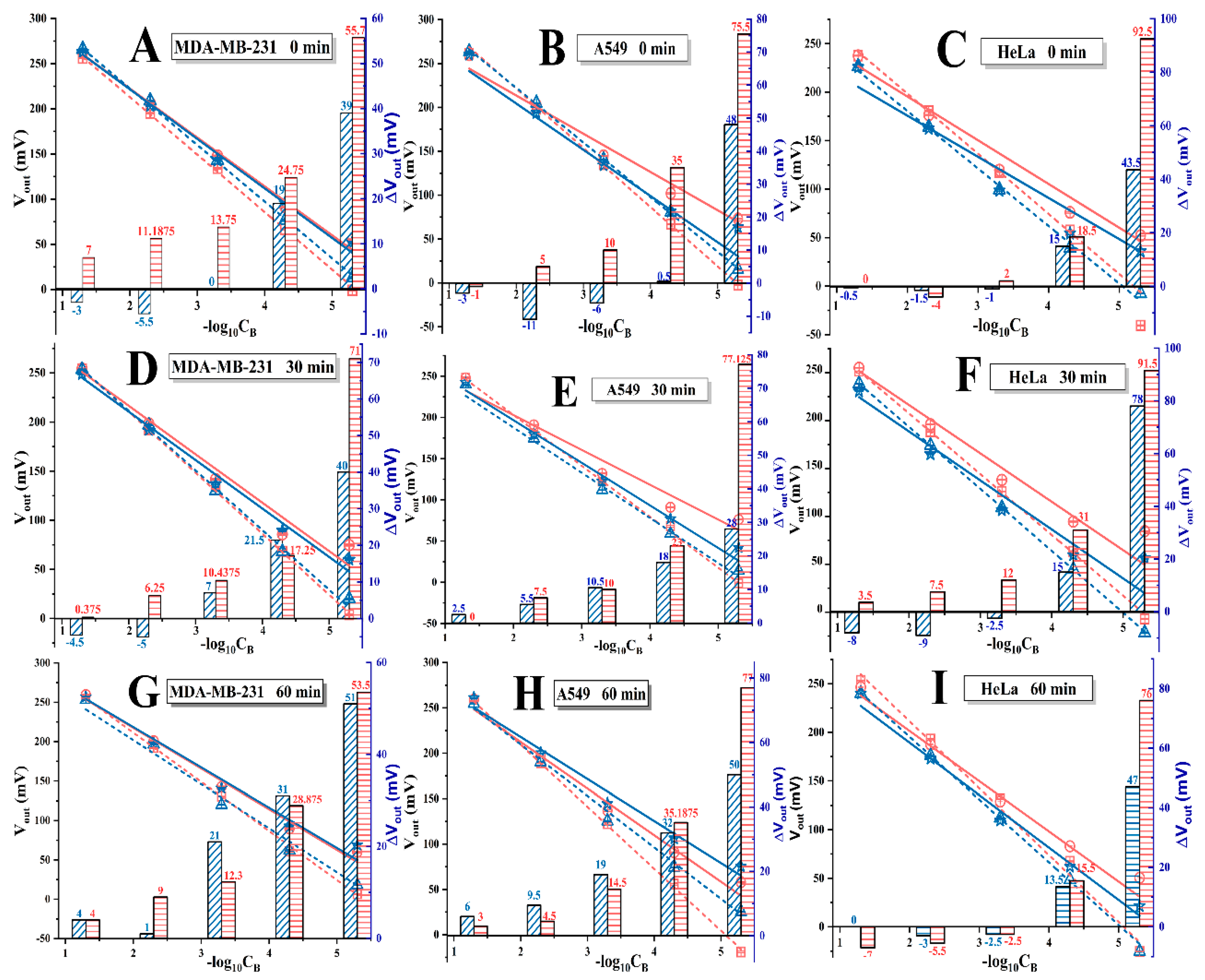
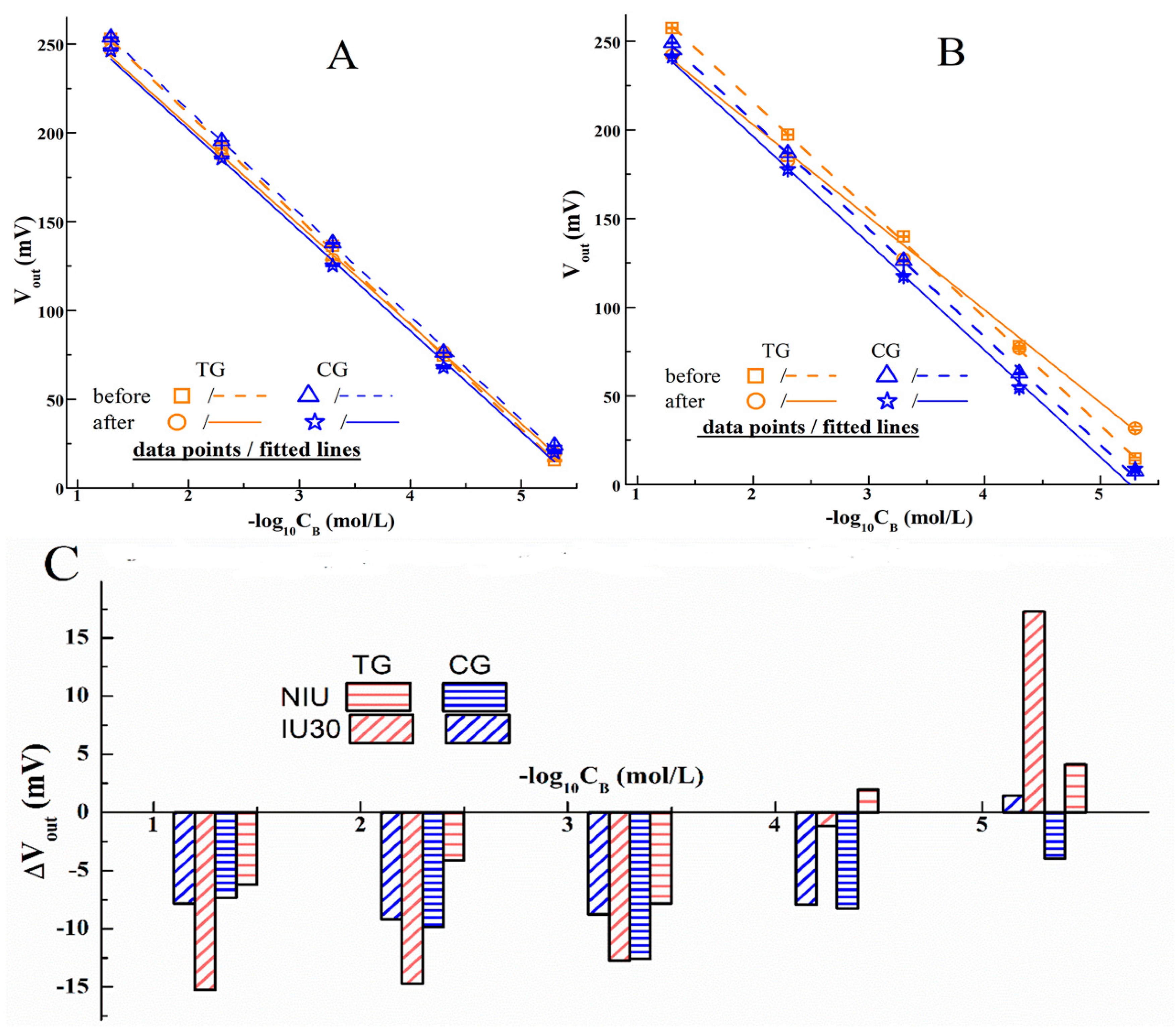
3.2. I− Responses of PGO-ISEs and Cells’ Immobilized Ones
3.3. Identification of PGO’s Function on ISE
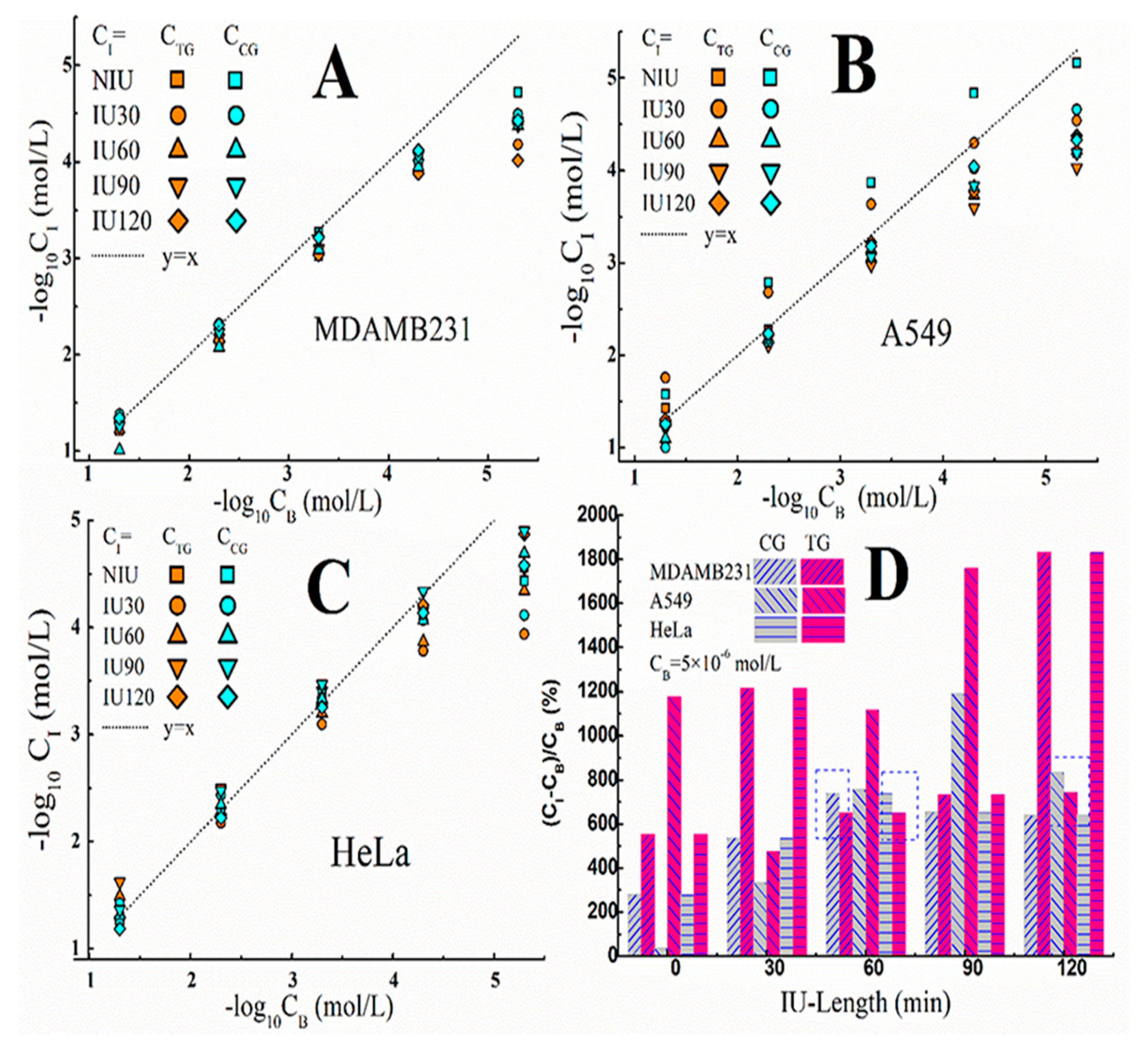
3.4. Variations of I− Concentration in IME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Djamgoz, M.B.A.; Coombes, R.C.; Schwab, A. Ion transport and cancer: From initiation to metastasis Introduction. Philos. Trans. R. Soc. B-Biol. Sci. 2014, 369, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, L.; Xu, Y.; Zhang, H.; Ye, W.; Mao, J.; Chen, L.; Wang, L. Uncoupling of K+ and Cl− transport across the cell membrane in the process of regulatory volume decrease. Biochem. Pharmacol. 2012, 84, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.A.; Fraser, S.P.; Stephens, G.J.; Downing, J.E.; Laniado, M.E.; Foster, C.S.; Abel, P.D.; Djamgoz, M.B. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: Contribu-tion to invasiveness in vitro. FEBS Lett. 1995, 369, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Boland, A.; Ricard, M.; Opolon, P.; Bidart, J.M.; Yeh, P.; Filetti, S.; Schlumberger, M.; Perricaudet, M. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radio-therapy. Cancer Res. 2000, 60, 3484–3492. [Google Scholar]
- Wan, S.S.; Zhang, L.; Zhang, X.Z. An ATP-Regulated Ion Transport Nanosystem for Homeostatic Perturbation Therapy and Sensitizing Photodynamic Therapy by Autophagy Inhibition of Tumors. ACS Central Sci. 2019, 5, 327–340. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [Green Version]
- Kornreich, B.G. The patch clamp technique: Principles and technical considerations. J. Vet. Cardio. 2007, 9, 25–37. [Google Scholar] [CrossRef]
- Sakmann, B.; Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 1984, 46, 455–472. [Google Scholar] [CrossRef]
- Matteson, D.R.; Deutsch, C. K channels in T lymphocytes: A patch clamp study using monoclonal antibody adhesion. Nature 1984, 307, 468–471. [Google Scholar] [CrossRef]
- Gray, R.; Johnston, D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J. Neurophysiol. 1985, 54, 134–142. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Pohl, P. Coupling of proton source and sink via H+-migration along the membrane surface as revealed by double patch-clamp experiments. FEBS Lett. 1998, 429, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Grimm, C.; Vierock, J.; Hegemann, P.; Wietek, J. Whole-cell Patch-clamp Recordings for Electrophysiological Determination of Ion Selectivity in Channelrhodopsins. J. Vis. Exp. 2017, 123. [Google Scholar] [CrossRef]
- Kusch, J.; Zifarelli, G. Patch-Clamp Fluorometry: Electrophysiology meets Fluorescence. Biophys. J. 2014, 106, 1250–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, M.; Forster, I.C. Correlating Charge Movements with Local Conformational Changes of a Na+-Coupled Cotransporter. Biophys. J. 2014, 106, 1618–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja, R.; Nagarah, J.M.; Starace, D.M.; Melosh, N.A.; Blunck, R.; Bezanilla, F.; Heath, J.R. Silicon chip-based patch-clamp electrodes integrated with PDMS microfluidics. Biosens. Bioelectron. 2004, 20, 509–517. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tu, T.-Y.; Jong, D.-S.; Wo, A.M. Ion Channel Electrophysiology via Integrated Planar Patch-Clamp Chip with On-Demand Drug Exchange. Biotechnol. Bioeng. 2011, 108, 1395–1403. [Google Scholar] [CrossRef]
- Smith, P.J.S.; Hammar, K.; Porterfield, D.M.; Sanger, R.H.; Trimarchi, J.R. Self-referencing, n.o.n.-i.n.v.a.s.i.v.e., ion selective electrode for single cell detection of transplasma membrane calcium flux. Microsc. Res. Tech. 1999, 46, 398–417. [Google Scholar] [CrossRef]
- Yao, C.; Xie, C.; Lin, P.; Yan, F.; Huang, P.; Hsing, I.M. Organic Electrochemical Transistor Array for Recording Transepithelial Ion Transport of Human Airway Epithelial Cells. Adv. Mater. 2013, 25, 6575–6580. [Google Scholar] [CrossRef]
- Walsh, K.B.; DeRoller, N.; Zhu, Y.; Koley, G. Application of ion-sensitive field effect transistors for ion channel screening. Biosens. Bioelectron. 2014, 54, 448–454. [Google Scholar] [CrossRef] [Green Version]
- ToczyłOwska-Mamińska, R.; Lewenstam, A.; Dołowy, K. Multielectrode Bisensor System for Time-Resolved Monitoring of Ion Transport Across an Epithelial Cell Layer. Anal. Chem. 2014, 86, 390–394. [Google Scholar] [CrossRef]
- Zajac, M.; Lewenstam, A.; Stobiecka, M.; Dolowy, K. New ISE-Based Apparatus for Na+, K+, Cl−, pH and Transepithelial Potential Difference Real-Time Simultaneous Measurements of Ion Transport across Epithelial Cells Monolayer-Advantages and Pitfalls. Sensors 2019, 19, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacAllister, I.E.; Jakoby, M.G.; Geryk, B.; Schneider, R.L.; Cropek, D.M. Use of the thyrocyte sodium iodide symporter as the basis for a perchlorate cell-based assay. Analyst 2009, 134, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Wu, D.; Daly, H.C.; Busschaert, N.; Morgunova, M.; Simpson, J.C.; Scholz, D.; Gale, P.A.; O’Shea, D.F. Real-Time Recording of the Cellular Effects of the Anion Transporter Prodigiosin. Chem 2018, 4, 879–895. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Gu, Y.; Wang, Z.; Li, Y.; Fan, Q.; Jia, Y. Aptamer cell sensor based on porous graphene oxide decorated ion-selective-electrode: Double sensing platform for cell and ion. Biosens. Bioelectron. 2018, 117, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Zhou, R.; Zhang, Y.; Hao, L.; Cai, X.; Zhu, B. AS1411 aptamer modified carbon dots via polyethylenimine-assisted strategy for efficient targeted cancer cell imaging. Cell Prolif. 2019. [Google Scholar] [CrossRef]
- Dohan, O.; De la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.A.; Wang, R.; Kreman, T.M.; Sheffield, V.C.; Karniski, L.P. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nature Genet. 1999, 21, 440–443. [Google Scholar] [CrossRef]
- Levy, O.; Carrasco, N. Structure and function of the thyroid iodide transporter and its implications for thyroid disease. Curr. Opin. Endocrinol. 1997, 4, 364–370. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Zhang, R.; Jia, Y. Porous Graphene Oxide Decorated Ion Selective Electrode for Observing Across-Cytomembrane Ion Transport. Sensors 2020, 20, 3500. https://doi.org/10.3390/s20123500
Hu S, Zhang R, Jia Y. Porous Graphene Oxide Decorated Ion Selective Electrode for Observing Across-Cytomembrane Ion Transport. Sensors. 2020; 20(12):3500. https://doi.org/10.3390/s20123500
Chicago/Turabian StyleHu, Shihui, Rong Zhang, and Yunfang Jia. 2020. "Porous Graphene Oxide Decorated Ion Selective Electrode for Observing Across-Cytomembrane Ion Transport" Sensors 20, no. 12: 3500. https://doi.org/10.3390/s20123500
APA StyleHu, S., Zhang, R., & Jia, Y. (2020). Porous Graphene Oxide Decorated Ion Selective Electrode for Observing Across-Cytomembrane Ion Transport. Sensors, 20(12), 3500. https://doi.org/10.3390/s20123500




