Enzyme-Based Biosensors: Tackling Electron Transfer Issues
Abstract
:1. Introduction
2. Theoretical Aspects of Electron Transfer (ET) Processes
2.1. Marcus Theory
2.2. Other Theoretical Aspects
3. Why Glucose Oxidase (GOx) Cannot Undergo DET?
4. Methods to Investigate DET Issues
4.1. Biochemical Methods
4.2. Electrochemical Methods
5. Different Approaches to Tackle DET Issues
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Willner, I.; Katz, E. Integration of layered redox proteins and conductive supports for bioelectronic applications. Angew. Chem. Int. Ed. 2000, 39, 1180–1218. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Yates, N.D.; Fascione, M.A.; Parkin, A. Methodologies for “wiring” redox proteins/enzymes to electrode surfaces. Chem. Eur. J. 2018, 24, 12164–12182. [Google Scholar] [CrossRef] [Green Version]
- Willner, I.; Katz, E.; Willner, B. Electrical contact of redox enzyme layers associated with electrodes: Routes to amperometric biosensors. Electroanalysis 1997, 9, 965–977. [Google Scholar] [CrossRef]
- Davidson, V.L. Electron transfer in quinoproteins. Arch. Biochem. Biophys. 2004, 428, 32–40. [Google Scholar] [CrossRef]
- Kennedy, M.L.; Gibney, B.R. Metalloprotein and redox protein design. Curr. Opin. Struct. Biol. 2001, 11, 485–490. [Google Scholar] [CrossRef]
- Mewies, M.; McIntire, W.S.; Scrutton, N.S. Covalent attachment of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) to enzymes: The current state of affairs. Protein Sci. 1998, 7, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorton, L. Carbon paste electrodes modified with enzymes, tissues, and cells. Electroanalysis 1995, 7, 23–45. [Google Scholar] [CrossRef]
- Schulz, C.; Kittl, R.; Ludwig, R.; Gorton, L. Direct electron transfer from the FAD cofactor of cellobiose dehydrogenase to electrodes. ACS Catal. 2016, 6, 555–563. [Google Scholar] [CrossRef]
- Hunt, J.; Massey, V.; Dunham, W.R.; Sands, R.H. Redox potentials of milk xanthine dehydrogenase. Room temperature measurement of the FAD and 2Fe/2S center potentials. J. Biol. Chem. 1993, 268, 18685–18691. [Google Scholar]
- Kawai, S.; Yakushi, T.; Matsushita, K.; Kitazumi, Y.; Shirai, O.; Kano, K. The electron transfer pathway in direct electrochemical communication of fructose dehydrogenase with electrodes. Electrochem. Commun. 2014, 38, 28–31. [Google Scholar] [CrossRef]
- Duine, J.A. Quinoproteins: Enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur. J. Biochem. 1991, 200, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Tkac, J.; Svitel, J.; Vostiar, I.; Navratil, M.; Gemeiner, P. Membrane-bound dehydrogenases from Gluconobacter sp.: Interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemistry 2009, 76, 53–62. [Google Scholar] [CrossRef]
- Anthony, C. Quinoprotein-catalysed reactions. Biochem. J. 1996, 320, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Oubrie, A. Structure and mechanism of soluble glucose dehydrogenase and other PQQ-dependent enzymes. Biochim. Biophys. Acta 2003, 1647, 143–151. [Google Scholar] [CrossRef]
- Léger, C.; Bertrand, P. Direct electrochemistry of redox enzymes as a tool for mechanistic studies. Chem. Rev. 2008, 108, 2379–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusling, J.F. (Ed.) Biomolecular Films: Design, Function, and Applications; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Ghindilis, A.L.; Atanasov, P.; Wilkins, E. Enzyme-catalyzed direct electron transfer: Fundamentals and analytical applications. Electroanalysis 1997, 9, 661–674. [Google Scholar] [CrossRef]
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F.D.; Ruzgas, T.; Gazaryan, I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta 1999, 400, 91–108. [Google Scholar] [CrossRef]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal electrodes. I. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- Rutherford, A.W.; Osyczka, A.; Rappaport, F. Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O2. FEBS Lett. 2012, 586, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, R.J.; Sweeney, M.D.; Leggio, L.L.; Otten, H.; Poulsen, J.-C.N.; Johansen, K.S.; Krogh, K.B.; Jørgensen, C.I.; Tovborg, M.; Anthonsen, A. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.M.; Beeson, W.T.; Cate, J.H.; Marletta, M.A. Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 2011, 6, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Nakashimada, Y. Recent development of anaerobic digestion processes for energy recovery from wastes. J. Biosci. Bioeng. 2007, 103, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Maesen, M.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour. Technol. 2014, 165, 350–354. [Google Scholar] [CrossRef]
- Schuhmann, W. Amperometric enzyme biosensors based on optimised electron-transfer pathways and non-manual immobilisation procedures. Rev. Mol. Biotechnol. 2002, 82, 425–441. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Calvo, E.J.; Danilowicz, C. Amperometric enzyme electrodes. J. Braz. Chem. Soc. 1997, 8, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S. Biosensors based on direct electron transfer in redox proteins. Microchim. Acta 2007, 159, 1–17. [Google Scholar] [CrossRef]
- Murphy, L. Biosensors and bioelectrochemistry. Curr. Opin. Chem. Biol. 2006, 10, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L.; Antiochia, R. Direct electron transfer of dehydrogenases for development of 3rd generation biosensors and enzymatic fuel cells. Sensors 2018, 18, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habermüller, K.; Mosbach, M.; Schuhmann, W. Electron-transfer mechanisms in amperometric biosensors. Fresenius J. Anal. Chem. 2000, 366, 560–568. [Google Scholar] [CrossRef]
- Lötzbeyer, T.; Schuhmann, W.; Schmidt, H.-L. Electron transfer principles in amperometric biosensors: Direct electron transfer between enzymes and electrode surface. Sens. Actuators B 1996, 33, 50–54. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian blue-based first-generation biosensor. A sensitive amperometric electrode for glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Scheller, F.W.; Schubert, F.; Neumann, B.; Pfeiffer, D.; Hintsche, R.; Dransfeld, I.; Wollenberger, U.; Renneberg, R.; Warsinke, A.; Johansson, G. Second generation biosensors. Biosens. Bioelectron. 1991, 6, 245–253. [Google Scholar] [CrossRef]
- Katz, E.; Lötzbeyer, T.; Schlereth, D.D.; Schuhmann, W.; Schmidt, H.-L. Electrocatalytic oxidation of reduced nicotinamide coenzymes at gold and platinum electrode surfaces modified with a monolayer of pyrroloquinoline quinone. Effect of Ca2+ cations. J. Electroanal. Chem. 1994, 373, 189–200. [Google Scholar] [CrossRef]
- Gorton, L.; Domínguez, E. Electrocatalytic oxidation of NAD(P)H at mediator-modified electrodes. Rev. Mol. Biotechnol. 2002, 82, 371–392. [Google Scholar] [CrossRef]
- Gorton, L. Chemically modified electrodes for the electrocatalytic oxidation of nicotinamide coenzymes. J. Chem. Soc. Faraday Trans. 1 1986, 82, 1245–1258. [Google Scholar] [CrossRef]
- Katz, E.; Lioubashevsky, O.; Willner, I. Electromechanics of a redox-active rotaxane in a monolayer assembly on an electrode. J. Am. Chem. Soc. 2004, 126, 15520–15532. [Google Scholar] [CrossRef]
- Heller, A. Electrical connection of enzyme redox centers to electrodes. J. Phys. Chem. 1992, 96, 3579–3587. [Google Scholar] [CrossRef]
- Heller, A. Electrical wiring of redox enzymes. Acc. Chem. Res. 1990, 23, 128–134. [Google Scholar] [CrossRef]
- Schuhmann, W.; Ohara, T.J.; Schmidt, H.-L.; Heller, A. Electron transfer between glucose oxidase and electrodes via redox mediators bound with flexible chains to the enzyme surface. J. Am. Chem. Soc. 1991, 113, 1394–1397. [Google Scholar] [CrossRef]
- Suzuki, N.; Lee, J.; Loew, N.; Takahashi-Inose, Y.; Okuda-Shimazaki, J.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Engineered glucose oxidase capable of quasi-direct electron transfer after a quick-and-easy modification with a mediator. Int. J. Mol. Sci. 2020, 21, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, I.; Riklin, A.; Shoham, B.; Rivenzon, D.; Katz, E. Development of novel biosensor enzyme electrodes: Glucose oxidase multilayer arrays immobilized onto self-assembled monolayers on electrodes. Adv. Mater. 1993, 5, 912–915. [Google Scholar] [CrossRef]
- Willner, I.; Katz, E.; Riklin, A.; Kasher, R. Mediated electron transfer in glutathione reductase organized in self-assembled monolayers on Au electrodes. J. Am. Chem. Soc. 1992, 114, 10965–10966. [Google Scholar] [CrossRef]
- Willner, I.; Lapidot, N.; Riklin, A.; Kasher, R.; Zahavy, E.; Katz, E. Electron transfer communication in glutathione reductase assemblies: Electrocatalytic, photocatalytic and catalytic systems for the reduction of oxidized glutathione. J. Am. Chem. Soc. 1994, 116, 1428–1441. [Google Scholar] [CrossRef]
- Freire, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct electron transfer: An approach for electrochemical biosensors with higher selectivity and sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, P.N. (Ed.) Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; Wiley: Chichester, UK, 2008. [Google Scholar]
- Frew, J.E.; Hill, H.A.O. Electron-transfer biosensors. Philos. Trans. R. Soc. B 1987, 316, 95–106. [Google Scholar]
- Frew, J.E.; Hill, H.A.O. Direct and indirect electron transfer between electrodes and redox proteins. Eur. J. Biochem. 1988, 172, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, F.A.; Hill, H.A.O.; Walton, N.J. Reactions of electron-transfer proteins at electrodes. Q. Rev. Biophys. 1985, 18, 261–322. [Google Scholar] [CrossRef] [PubMed]
- Léger, C.; Jones, A.K.; Albracht, S.P.; Armstrong, F.A. Effect of a dispersion of interfacial electron transfer rates on steady state catalytic electron transport in [NiFe]-hydrogenase and other enzymes. J. Phys. Chem. B 2002, 106, 13058–13063. [Google Scholar] [CrossRef]
- Lopez, R.J.; Babanova, S.; Ulyanova, Y.; Singhal, S.; Atanassov, P. Improved interfacial electron transfer in modified bilirubin oxidase biocathodes. ChemElectroChem 2014, 1, 241–248. [Google Scholar] [CrossRef]
- Hill, H.A.O.; Walton, N.J. Investigation of some intermolecular electron transfer reactions of cytochrome c by electrochemical methods. J. Am. Chem. Soc. 1982, 104, 6515–6519. [Google Scholar] [CrossRef]
- Schulz, C.; Ludwig, R.; Micheelsen, P.O.; Silow, M.; Toscano, M.D.; Gorton, L. Enhancement of enzymatic activity and catalytic current of cellobiose dehydrogenase by calcium ions. Electrochem. Commun. 2012, 17, 71–74. [Google Scholar] [CrossRef]
- Kracher, D.; Zahma, K.; Schulz, C.; Sygmund, C.; Gorton, L.; Ludwig, R. Inter-domain electron transfer in cellobiose dehydrogenase: Modulation by pH and divalent cations. FEBS J. 2015, 282, 3136–3148. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, R.; Ortiz, R.; Schulz, C.; Harreither, W.; Sygmund, C.; Gorton, L. Cellobiose dehydrogenase modified electrodes: Advances by materials science and biochemical engineering. Anal. Bioanal. Chem. 2013, 405, 3637–3658. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, R.; Harreither, W.; Tasca, F.; Gorton, L. Cellobiose dehydrogenase: A versatile catalyst for electrochemical applications. ChemPhysChem 2010, 11, 2674–2697. [Google Scholar] [CrossRef]
- Bollella, P.; Ludwig, R.; Gorton, L. Cellobiose dehydrogenase: Insights on the nanostructuration of electrodes for improved development of biosensors and biofuel cells. Appl. Mater. Today 2017, 9, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. The influence of pH and divalent/monovalent cations on the internal electron transfer (IET), enzymatic activity, and structure of fructose dehydrogenase. Anal. Bioanal. Chem. 2018, 410, 3253–3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, I.; Toyosawa, K.; Yamaguchi, H.; Yasukouchi, K. Reversible electrochemical reduction and oxidation of cytochrome c at a bis(4-pyridyl) disulphide-modified gold electrode. J. Chem. Soc. Chem. Commun. 1982, 18, 1032–1033. [Google Scholar] [CrossRef]
- Armstrong, F.A.; Hill, H.A.O.; Walton, N.J. Direct electrochemistry of redox proteins. Acc. Chem. Res. 1988, 21, 407–413. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Electron transfer in proteins. Annu. Rev. Biochem. 1996, 65, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.R.; Malmström, B.G.; Gray, H.B. Rapid electron injection into multisite metalloproteins: Intramolecular electron transfer in cytochrome oxidase. Biophys. Chem. 1995, 54, 199–209. [Google Scholar] [CrossRef]
- Winkler, J.R.; Gray, H.B. Long-range electron tunneling. J. Am. Chem. Soc. 2014, 136, 2930–2939. [Google Scholar] [CrossRef]
- Guo, L.-H.; Hill, H.A.O. Direct electrochemistry of proteins and enzymes. Adv. Inorg. Chem. 1991, 36, 341–375. [Google Scholar]
- Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature 1999, 402, 47–52. [Google Scholar] [CrossRef]
- Wilson, G.S. Native glucose oxidase does not undergo direct electron transfer. Biosens. Bioelectron. 2016, 82, Vii–Viii. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry: Theory and experiment (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Adams, D.M.; Brus, L.; Chidsey, C.E.; Creager, S.; Creutz, C.; Kagan, C.R.; Kamat, P.V.; Lieberman, M.; Lindsay, S.; Marcus, R.A. Charge transfer on the nanoscale: Current status. J. Phys. Chem. B 2003, 107, 6668–6697. [Google Scholar] [CrossRef]
- Bendall, D.S. (Ed.) Protein Electron Transfer; BIOS Scientific Pub.: Oxford, UK, 1996. [Google Scholar]
- Katz, E.; Shipway, A.N.; Willner, I. The electrochemical and photochemical activation of redox-enzymes. In Electron Transfer in Chemistry. Volume 4: Heterogeneous Systems, Solid State Systems, Gas Phase Systems. Section 1: Catalysis of Electron Transfer; Balzani, V., Piotrowiak, P., Rodgers, M.A.J., Eds.; Wiley-VCH: Weinheim, Germany, 2001; pp. 127–201. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Laviron, E.; Roullier, L. General expression of the linear potential sweep voltammogram for a surface redox reaction with interactions between the adsorbed molecules: Applications to modified electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1980, 115, 65–74. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar]
- Falk, M.; Blum, Z.; Shleev, S. Direct electron transfer based enzymatic fuel cells. Electrochim. Acta 2012, 82, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Barton, C.S.; Gallaway, J.; Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, B.A.; Heller, A. Cross-linked redox gels containing glucose oxidase for amperometric biosensor applications. Anal. Chem. 1990, 62, 258–263. [Google Scholar] [CrossRef]
- Quinn, C.A.; Connor, R.E.; Heller, A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials 1997, 18, 1665–1670. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L.; Ludwig, R.; Antiochia, R. A third generation glucose biosensor based on cellobiose dehydrogenase immobilized on a glassy carbon electrode decorated with electrodeposited gold nanoparticles: Characterization and application in human saliva. Sensors 2017, 17, 1912. [Google Scholar] [CrossRef] [Green Version]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Tasca, F.; Antiochia, R. Minimally invasive glucose monitoring using a highly porous gold microneedles-based biosensor: Characterization and application in artificial interstitial fluid. Catalysts 2019, 9, 580. [Google Scholar] [CrossRef] [Green Version]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive microneedle-based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Mao, F.; Mano, N.; Heller, A. Long tethers binding redox centers to polymer backbones enhance electron transport in enzyme “wiring” hydrogels. J. Am. Chem. Soc. 2003, 125, 4951–4957. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors–sense and sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Minteer, S.D. Direct enzymatic bioelectrocatalysis: Differentiating between myth and reality. J. R. Soc. Interface 2017, 14, 20170253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, P.N.; Al-Lolage, F.A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. 2018, 819, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—an overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Leskovac, V.; Trivić, S.; Wohlfahrt, G.; Kandrač, J.; Peričin, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.J.; Schomburg, D.; Kalisz, H.; Schmid, R.D. The 3D structure of glucose oxidase from Aspergillus niger. Implications for the use of GOD as a biosensor enzyme. Biosens. Bioelectron. 1993, 8, 197–203. [Google Scholar] [CrossRef]
- Hecht, H.J.; Kalisz, H.M.; Hendle, J.; Schmid, R.D.; Schomburg, D. Crystal structure of glucose oxidase from Aspergillus niger refined at 2.3 Å resolution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar] [CrossRef]
- Alvarez-Icaza, M.; Kalisz, H.M.; Hecht, H.J.; Aumann, K.-D.; Schomburg, D.; Schmid, R.D. The design of enzyme sensors based on the enzyme structure. Biosens. Bioelectron. 1995, 10, 735–742. [Google Scholar] [CrossRef]
- Pazur, J.H.; Kleppe, K.; Ball, E.M. The glycoprotein nature of some functional carbohydrases. Arch. Biochem. Biophys. 1963, 103, 515–516. [Google Scholar] [CrossRef]
- Swoboda, B.E.; Massey, V. Purification and properties of the glucose oxidase from Aspergillus niger. J. Biol. Chem. 1965, 240, 2209–2215. [Google Scholar] [PubMed]
- Kalisz, H.M.; Hecht, H.-J.; Schomburg, D.; Schmid, R.D. Effects of carbohydrate depletion on the structure, stability and activity of glucose oxidase from Aspergillus niger. Biochim. Biophys. Acta 1991, 1080, 138–142. [Google Scholar] [CrossRef]
- Raba, J.; Mottola, H.A. Glucose oxidase as an analytical reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, F.; Favero, G.; Bollella, P.; Tortolini, C.; Mannina, L.; Conti, M.E.; Antiochia, R. Recent trends in electrochemical nanobiosensors for environmental analysis. Int. J. Environ. Health 2015, 7, 267–291. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Pingarron, J.M. Gold nanoparticle-based electrochemical biosensors. Anal. Bioanal. Chem. 2005, 382, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Pingarrón, J.M.; Yanez-Sedeno, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Sánchez-Obrero, G.; Cano, M.; Ávila, J.L.; Mayén, M.; Mena, M.L.; Pingarrón, J.M.; Rodríguez-Amaro, R. A gold nanoparticle-modified PVC/TTF-TCNQ composite amperometric biosensor for glucose determination. J. Electroanal. Chem. 2009, 634, 59–63. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Pingarrón, J.M.; Riu, J.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. TrAC Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Bollella, P. Porous gold: A new frontier for enzyme-based electrodes. Nanomaterials 2020, 10, 722. [Google Scholar] [CrossRef] [Green Version]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry: A review. Anal. Chim. Acta 2008, 622, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Anderson, M.R. Electrochemical glucose sensors—developments using electrostatic assembly and carbon nanotubes for biosensor construction. Sensors 2010, 10, 8248–8274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luong, J.H.; Glennon, J.D.; Gedanken, A.; Vashist, S.K. Achievement and assessment of direct electron transfer of glucose oxidase in electrochemical biosensing using carbon nanotubes, graphene, and their nanocomposites. Microchim. Acta 2017, 184, 369–388. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Bioelectrocatalysis at carbon nanotubes. Methods Enzymol. 2020, 630, 215–247. [Google Scholar]
- Byers, J.C.; Güell, A.G.; Unwin, P.R. Nanoscale electrocatalysis: Visualizing oxygen reduction at pristine, kinked, and oxidized sites on individual carbon nanotubes. J. Am. Chem. Soc. 2014, 136, 11252–11255. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Residual metallic impurities within carbon nanotubes play a dominant role in supposedly “metal-free” oxygen reduction reactions. Chem. Commun. 2014, 50, 12662–12664. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Mello, P.A.; Rodrigues, L.F.; Nunes, M.A.; Mattos, J.C.P.; Müller, E.I.; Dressler, V.L.; Flores, E.M. Determination of metal impurities in carbon nanotubes by direct solid sampling electrothermal atomic absorption spectrometry. J. Braz. Chem. Soc. 2011, 22, 1040–1049. [Google Scholar] [CrossRef]
- Eddowes, M.J.; Hill, H.A.O. Investigation of electron-transfer reactions of proteins by electrochemical methods. Biosci. Rep. 1981, 1, 521–532. [Google Scholar] [CrossRef]
- Léger, C.; Elliott, S.J.; Hoke, K.R.; Jeuken, L.J.; Jones, A.K.; Armstrong, F.A. Enzyme electrokinetics: Using protein film voltammetry to investigate redox enzymes and their mechanisms. Biochemistry 2003, 42, 8653–8662. [Google Scholar] [CrossRef] [PubMed]
- Duca, M.; Weeks, J.R.; Fedor, J.G.; Weiner, J.H.; Vincent, K.A. Combining noble metals and enzymes for relay cascade electrocatalysis of nitrate reduction to ammonia at neutral pH. ChemElectroChem 2015, 2, 1086–1089. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of molybdenum nitrogenase. Chem. Rev. 1996, 96, 2983–3012. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Abdellaoui, S.; Khadka, N.; Dean, D.R.; Leech, D.; Seefeldt, L.C.; Minteer, S.D. Nitrogenase bioelectrocatalysis: Heterogeneous ammonia and hydrogen production by MoFe protein. Energy Environ. Sci. 2016, 9, 2550–2554. [Google Scholar] [CrossRef] [Green Version]
- Holade, Y.; Yuan, M.; Milton, R.D.; Hickey, D.P.; Sugawara, A.; Peterbauer, C.K.; Haltrich, D.; Minteer, S.D. Rational combination of promiscuous enzymes yields a versatile enzymatic fuel cell with improved coulombic efficiency. J. Electrochem. Soc. 2016, 164, H3073. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993, 94, 19–33. [Google Scholar] [CrossRef]
- Tortolini, C.; Bollella, P.; Antiochia, R.; Favero, G.; Mazzei, F. Inhibition-based biosensor for atrazine detection. Sens. Actuators B 2016, 224, 552–558. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Kjaergaard, C.H.; Rouhana, J.; Mazurenko, I.; Infossi, P.; Gounel, S.; Gadiou, R.; Giudici-Orticoni, M.T.; Solomon, E.I.; Mano, N. Mechanism of chloride inhibition of bilirubin oxidases and its dependence on potential and pH. ACS Catal. 2017, 7, 3916–3923. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Antiochia, R.; Favero, G.; Mazzei, F. Inhibition-based first-generation electrochemical biosensors: Theoretical aspects and application to 2, 4-dichlorophenoxy acetic acid detection. Anal. Bioanal. Chem. 2016, 408, 3203–3211. [Google Scholar] [CrossRef] [Green Version]
- Lo, K.K.-W.; Wong, L.-L.; Hill, H.A.O. Surface-modified mutants of cytochrome P450cam: Enzymatic properties and electrochemistry. FEBS Lett. 1999, 451, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Campàs, M.; Prieto-Simón, B.; Marty, J.-L. A review of the use of genetically engineered enzymes in electrochemical biosensors. In Proceedings of the Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3–9. [Google Scholar]
- Dementin, S.; Belle, V.; Bertrand, P.; Guigliarelli, B.; Adryanczyk-Perrier, G.; De Lacey, A.L.; Fernandez, V.M.; Rousset, M.; Léger, C. Changing the ligation of the distal [4Fe4S] cluster in NiFe hydrogenase impairs inter- and intramolecular electron transfers. J. Am. Chem. Soc. 2006, 128, 5209–5218. [Google Scholar] [CrossRef]
- Armstrong, F.A. Recent developments in dynamic electrochemical studies of adsorbed enzymes and their active sites. Curr. Opin. Chem. Biol. 2005, 9, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, F.A. Protein film voltammetry: Revealing the mechanisms of biological oxidation and reduction. Russ. J. Electrochem. 2002, 38, 49–62. [Google Scholar] [CrossRef]
- Armstrong, F.A. Insights from protein film voltammetry into mechanisms of complex biological electron-transfer reactions. J. Chem. Soc. Dalton Trans. 2002, 661–671. [Google Scholar] [CrossRef]
- Gulaboski, R.; Mirčeski, V.; Bogeski, I.; Hoth, M. Protein film voltammetry: Electrochemical enzymatic spectroscopy. A review on recent progress. J. Solid State Electrochem. 2012, 16, 2315–2328. [Google Scholar] [CrossRef] [Green Version]
- Hirst, J. Elucidating the mechanisms of coupled electron transfer and catalytic reactions by protein film voltammetry. Biochim. Biophys. Acta 2006, 1757, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Angove, H.C.; Cole, J.A.; Richardson, D.J.; Butt, J.N. Protein film voltammetry reveals distinctive fingerprints of nitrite and hydroxylamine reduction by a cytochrome c nitrite reductase. J. Biol. Chem. 2002, 277, 23374–23381. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, F.A.; Heering, H.A.; Hirst, J. Reaction of complex metalloproteins studied by protein-film voltammetry. Chem. Soc. Rev. 1997, 26, 169–179. [Google Scholar] [CrossRef]
- Wijma, H.J.; Jeuken, L.J.; Verbeet, M.P.; Armstrong, F.A.; Canters, G.W. Protein film voltammetry of copper-containing nitrite reductase reveals reversible inactivation. J. Am. Chem. Soc. 2007, 129, 8557–8565. [Google Scholar] [CrossRef]
- Best, S.P. Spectroelectrochemistry of hydrogenase enzymes and related compounds. Coord. Chem. Rev. 2005, 249, 1536–1554. [Google Scholar] [CrossRef]
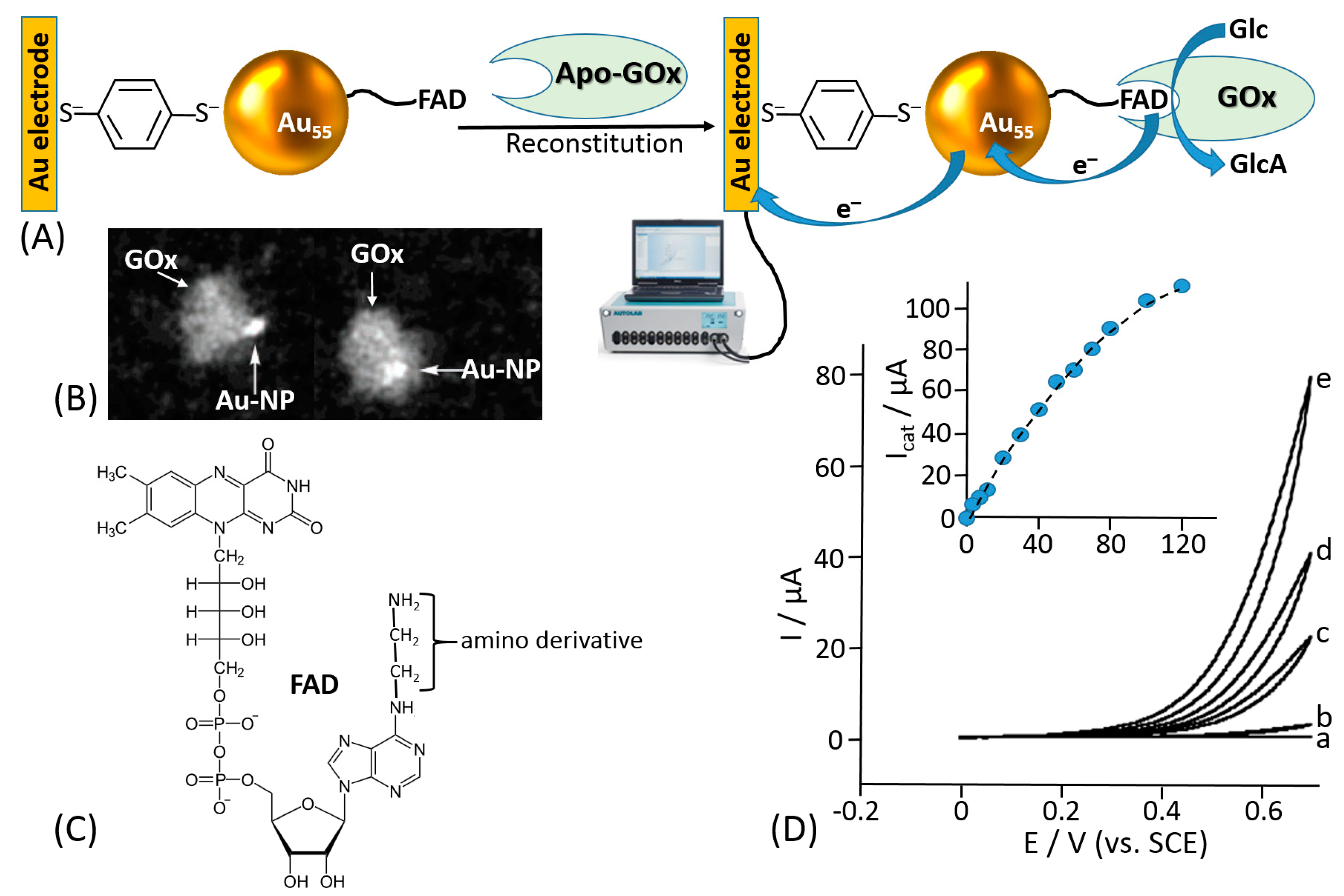
- Willner, I.; Blonder, R.; Katz, E.; Stocker, A.; Bückmann, A.F. Reconstitution of apo-glucose oxidase with a nitrospiropyran-modified FAD cofactor yields a photoswitchable biocatalyst for amperometric transduction of recorded optical signals. J. Am. Chem. Soc. 1996, 118, 5310–5311. [Google Scholar] [CrossRef]
- Katz, E.; Riklin, A.; Heleg-Shabtai, V.; Willner, I.; Bückmann, A.F. Glucose oxidase electrodes via reconstitution of the apo-enzyme: Tailoring of novel glucose biosensors. Anal. Chim. Acta 1999, 385, 45–58. [Google Scholar] [CrossRef]
- Zayats, M.; Katz, E.; Willner, I. Electrical contacting of glucose oxidase by surface-reconstitution of the apo-protein on a relay-boronic acid-FAD cofactor monolayer. J. Am. Chem. Soc. 2002, 124, 2120–2121. [Google Scholar] [CrossRef] [PubMed]
- Gorton, L.; Jönsson-Pettersson, G.; Csöregi, E.; Johansson, K.; Domínguez, E.; Marko-Varga, G. Amperometric biosensors based on an apparent direct electron transfer between electrodes and immobilized peroxidases. Plenary lecture. Analyst 1992, 117, 1235–1241. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G. Third-generation biosensors based on the direct electron transfer of proteins. Anal. Sci. 2004, 20, 603–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bistolas, N.; Wollenberger, U.; Jung, C.; Scheller, F.W. Cytochrome P450 biosensors—A review. Biosens. Bioelectron. 2005, 20, 2408–2423. [Google Scholar] [CrossRef]
- Bollella, P.; Mazzei, F.; Favero, G.; Fusco, G.; Ludwig, R.; Gorton, L.; Antiochia, R. Improved DET communication between cellobiose dehydrogenase and a gold electrode modified with a rigid self-assembled monolayer and green metal nanoparticles: The role of an ordered nanostructuration. Biosens. Bioelectron. 2017, 88, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Masci, G.; Sadun, C.; Tortolini, C.; Antonelli, M.L.; Bollella, P. Evaluation of new cholinium-amino acids based room temperature ionic liquids (RTILs) as immobilization matrix for electrochemical biosensor development: Proof-of-concept with Trametes Versicolor laccase. Microchem. J. 2018, 141, 346–352. [Google Scholar] [CrossRef]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
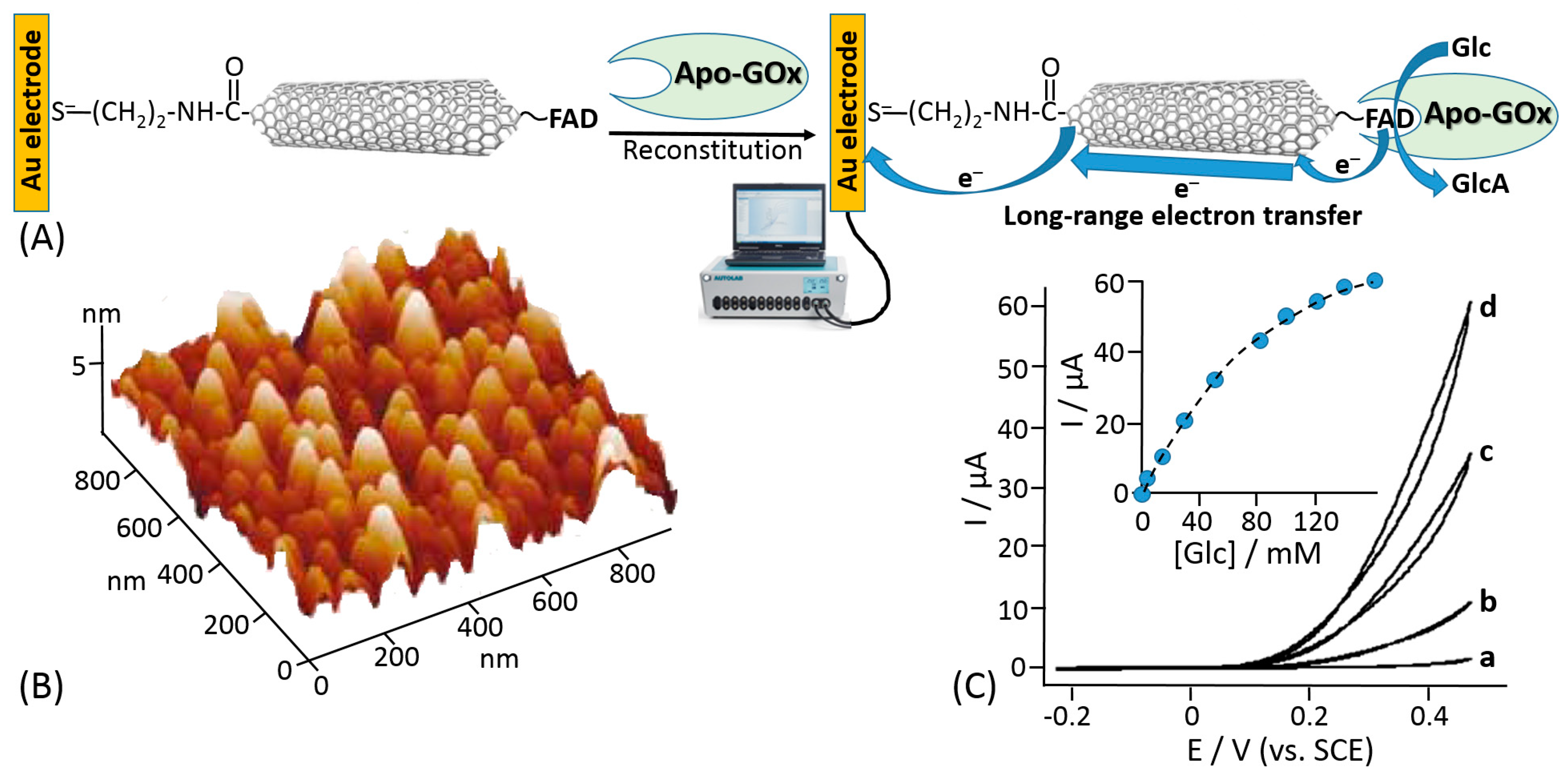
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.T.; Lau, C.; Brozik, S.; Atanassov, P.; Banta, S. Engineering of glucose oxidase for direct electron transfer via site-specific gold nanoparticle conjugation. J. Am. Chem. Soc. 2011, 133, 19262–19265. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Matsumura, H.; Tasca, F.; Zahma, K.; Samejima, M.; Igarashi, K.; Ludwig, R.; Gorton, L. Effect of deglycosylation of cellobiose dehydrogenases on the enhancement of direct electron transfer with electrodes. Anal. Chem. 2012, 84, 10315–10323. [Google Scholar] [CrossRef] [PubMed]
- Courjean, O.; Gao, F.; Mano, N. Deglycosylation of glucose oxidase for direct and efficient glucose electrooxidation on a glassy carbon electrode. Angew. Chem. Int. Ed. 2009, 48, 5897–5899. [Google Scholar] [CrossRef] [PubMed]
- Killyéni, A.; Yakovleva, M.E.; MacAodha, D.; Conghaile, P.Ó.; Gonaus, C.; Ortiz, R.; Leech, D.; Popescu, I.C.; Peterbauer, C.K.; Gorton, L. Effect of deglycosylation on the mediated electrocatalytic activity of recombinantly expressed Agaricus meleagris pyranose dehydrogenase wired by osmium redox polymer. Electrochim. Acta 2014, 126, 61–67. [Google Scholar] [CrossRef]
- Yakovleva, M.E.; Killyéni, A.; Ortiz, R.; Schulz, C.; MacAodha, D.; Conghaile, P.Ó.; Leech, D.; Popescu, I.C.; Gonaus, C.; Peterbauer, C.K. Recombinant pyranose dehydrogenase—A versatile enzyme possessing both mediated and direct electron transfer. Electrochem. Commun. 2012, 24, 120–122. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Wang, C.; Feng, B. Research progress on site-oriented and three-dimensional immobilization of protein. Mol. Biol. 2015, 49, 1–20. [Google Scholar] [CrossRef]
- Al-Lolage, F.A.; Bartlett, P.N.; Gounel, S.; Staigre, P.; Mano, N. Site-Directed Immobilization of Bilirubin Oxidase for Electrocatalytic Oxygen Reduction. ACS Catal. 2019, 9, 2068–2078. [Google Scholar] [CrossRef]
- Hitaishi, V.P.; Clement, R.; Bourassin, N.; Baaden, M.; De Poulpiquet, A.; Sacquin-Mora, S.; Ciaccafava, A.; Lojou, E. Controlling redox enzyme orientation at planar electrodes. Catalysts 2018, 8, 192. [Google Scholar] [CrossRef] [Green Version]
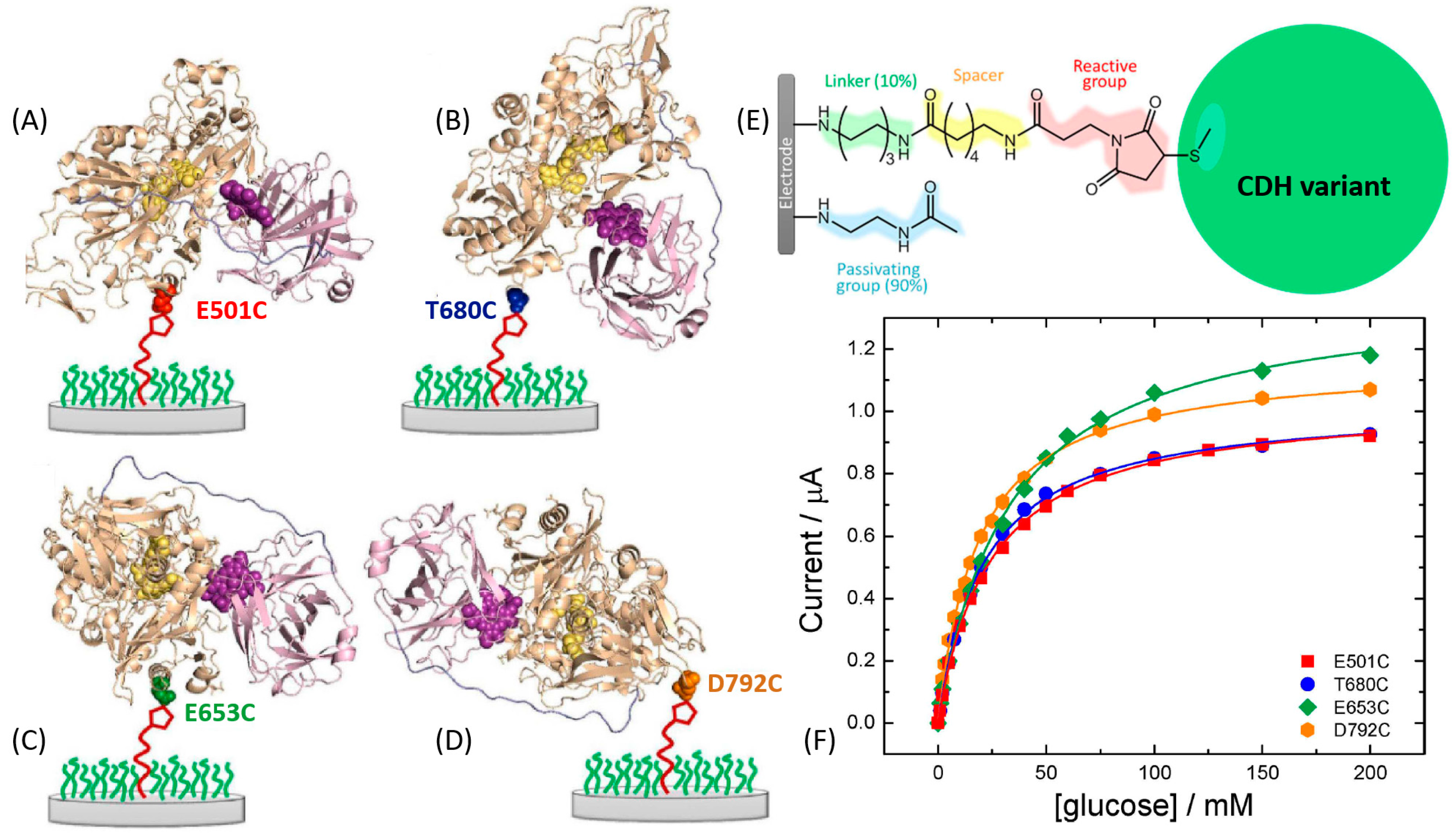
- Al-Lolage, F.A.; Meneghello, M.; Ma, S.; Ludwig, R.; Bartlett, P.N. A flexible method for the stable, covalent immobilization of enzymes at electrode surfaces. ChemElectroChem 2017, 4, 1528–1534. [Google Scholar] [CrossRef] [Green Version]
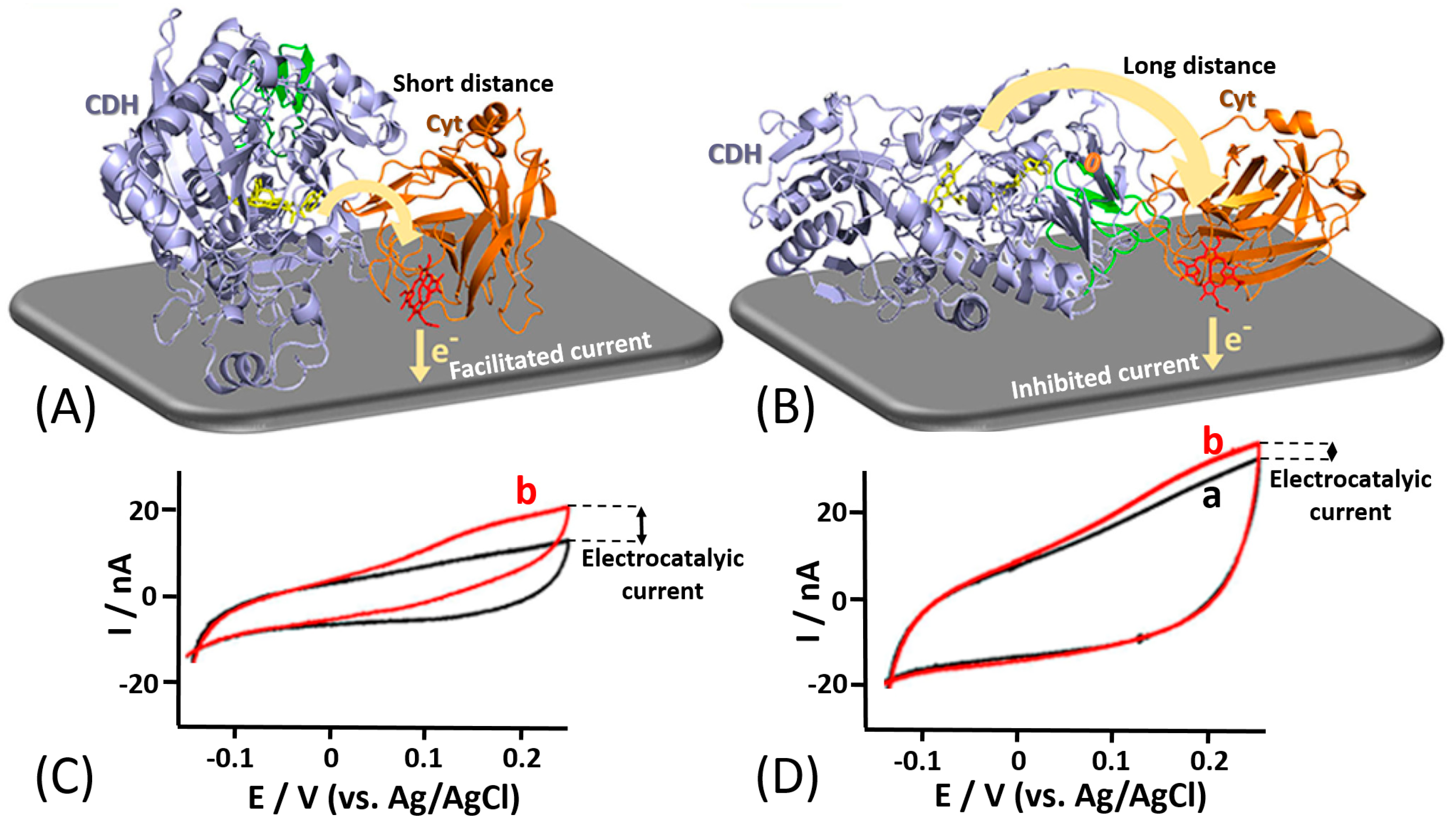
- Meneghello, M.; Al-Lolage, F.A.; Ma, S.; Ludwig, R.; Bartlett, P.N. Studying direct electron transfer by site-directed immobilization of cellobiose dehydrogenase. ChemElectroChem 2019, 6, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Laurent, C.V.; Meneghello, M.; Tuoriniemi, J.; Oostenbrink, C.; Gorton, L.; Bartlett, P.N.; Ludwig, R. Direct electron-transfer anisotropy of a site-specifically immobilized cellobiose dehydrogenase. ACS Catal. 2019, 9, 7607–7615. [Google Scholar] [CrossRef]
- Katz, E. Application of bifunctional reagents for immobilization of proteins on a carbon electrode surface: Oriented immobilization of photosynthetic reaction centers. J. Electroanal. Chem. 1994, 365, 157–164. [Google Scholar] [CrossRef]
- Blanford, C.F.; Heath, R.S.; Armstrong, F.A. A stable electrode for high-potential, electrocatalytic O2 reduction based on rational attachment of a blue copper oxidase to a graphite surface. Chem. Commun. 2007, 17, 1710–1712. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Solomon, E.I.; Augustine, A.J.; Yoon, J. O2 Reduction to H2O by the multicopper oxidases. Dalton Trans. 2008, 30, 3921–3932. [Google Scholar] [CrossRef]
- Gentil, S.; Rousselot-Pailley, P.; Sancho, F.; Robert, V.; Mekmouche, Y.; Guallar, V.; Tron, T.; Le Goff, A. Efficiency of site-specific clicked laccase-carbon nanotubes biocathodes towards O2 reduction. Chem. Eur. J. 2020, 26, 4798–4804. [Google Scholar] [CrossRef]
- Neto, S.A.; Da Silva, R.G.; Milton, R.D.; Minteer, S.D.; De Andrade, A.R. Hybrid bioelectrocatalytic reduction of oxygen at anthracene-modified multi-walled carbon nanotubes decorated with Ni90Pd10 nanoparticles. Electrochim. Acta 2017, 251, 195–202. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Milton, R.D.; Abdellaoui, S.; Hickey, D.P.; Minteer, S.D. Laccase inhibition by arsenite/arsenate: Determination of inhibition mechanism and preliminary application to a self-powered biosensor. Anal. Chem. 2016, 88, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Fusco, G.; Stevar, D.; Gorton, L.; Ludwig, R.; Ma, S.; Boer, H.; Koivula, A.; Tortolini, C.; Favero, G. A glucose/oxygen enzymatic fuel cell based on gold nanoparticles modified graphene screen-printed electrode. Proof-of-concept in human saliva. Sens. Actuators B 2018, 256, 921–930. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Enhanced direct electron transfer of fructose dehydrogenase rationally immobilized on a 2-aminoanthracene diazonium cation grafted single-walled carbon nanotube based electrode. ACS Catal. 2018, 8, 10279–10289. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly sensitive membraneless fructose biosensor based on fructose dehydrogenase immobilized onto aryl thiol modified highly porous gold electrode: Characterization and application in food samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Conejo-Valverde, P.; Soto-Cruz, J.; Bergueiro, J.; Calderón, M.; Rojas-Carrillo, O.; Kano, K.; Gorton, L. The influence of the shape of Au nanoparticles on the catalytic current of fructose dehydrogenase. Anal. Bioanal. Chem. 2019, 411, 7645–7657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calitri, G.; Bollella, P.; Ciogli, L.; Tortolini, C.; Mazzei, F.; Antiochia, R.; Favero, G. Evaluation of different storage processes of passion fruit (Passiflora edulis Sims) using a new dual biosensor platform based on a conducting polymer. Microchem. J. 2020, 154, 104573. [Google Scholar] [CrossRef]
- Bollella, P.; Schulz, C.; Favero, G.; Mazzei, F.; Ludwig, R.; Gorton, L.; Antiochia, R. Green synthesis and characterization of gold and silver nanoparticles and their application for development of a third generation lactose biosensor. Electroanalysis 2017, 29, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Carbon nanostructures for tagging in electrochemical biosensing: A review. J. Carbon Res. 2017, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Taurino, I.; Sanzò, G.; Antiochia, R.; Tortolini, C.; Mazzei, F.; Favero, G.; De Micheli, G.; Carrara, S. Recent advances in third generation biosensors based on Au and Pt nanostructured electrodes. Trends Anal. Chem. 2016, 79, 151–159. [Google Scholar] [CrossRef]
- Brdička, R. Etudes polarographiques des proteines du serum et leur signification pour le diagnostic du cancer. C. R. Soc. Biol. 1938, 128, 54–56. [Google Scholar]
- Müller, O.H. Polarographic analysis of proteins, amino acids, and other compounds by means of the Brdička reaction. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons: Easton, PA, USA, 1963; pp. 329–403. [Google Scholar]
- Brabec, V. Polarography of cytochrome c in ammoniacal buffers containing cobalt ions. The effect of the protein conformation. Gen. Physiol. Biophys. 1985, 4, 609–623. [Google Scholar] [PubMed]
- Blanford, C.F. The birth of protein electrochemistry. Chem. Commun. 2013, 49, 11130–11132. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Tabrizi, M.A. Direct electron transfer from glucose oxidase immobilized on a nano-porous glassy carbon electrode. Electrohim. Acta 2011, 56, 10101–10106. [Google Scholar] [CrossRef]
- Bai, Y.F.; Xu, T.B.; Luong, J.H.; Cui, H.F. Direct electron transfer of glucose oxidase-boron doped diamond interface: A new solution for a classical problem. Anal. Chem. 2014, 86, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ye, B.; Zhou, X. Direct electron transfer reaction of glucose oxidase at bare silver electrodes and its application in analysis. Electroanalysis 1997, 9, 641–644. [Google Scholar] [CrossRef]
- Ghica, M.E.; Pauliukaite, R.; Fatibello-Filho, O.; Brett, C.M.A. Application of functionalised carbon nanotubes immobilised into chitosan films in amperometric enzyme biosensors. Sens. Actuators B 2009, 142, 308–315. [Google Scholar] [CrossRef]
- Wu, F.; Yu, P.; Mao, L. Bioelectrochemistry for in vivo analysis: Interface engineering toward implantable electrochemical biosensors. Curr. Opin. Electrochem. 2017, 5, 152–157. [Google Scholar] [CrossRef]
- Desmet, C.; Marquette, C.A.; Blum, L.J.; Doumèche, B. Paper electrodes for bioelectrochemistry: Biosensors and biofuel cells. Biosens. Bioelectron. 2016, 76, 145–163. [Google Scholar] [CrossRef]
- Ruff, A. Redox polymers in bioelectrochemistry: Common playgrounds and novel concepts. Curr. Opin. Electrochem. 2017, 5, 66–73. [Google Scholar] [CrossRef]
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Gooding, J.J.; Gonçales, V.R. Recent advances in the molecular level modification of electrodes for bioelectrochemistry. Curr. Opin. Electrochem. 2017, 5, 203–210. [Google Scholar] [CrossRef]
- Pandey, C.M.; Malhotra, B.D. Biosensors: Fundamentals and Applications; Walter de Gruyter: Shawbury, UK, 2019. [Google Scholar]
- Tiwari, A.; Turner, A.P.F. (Eds.) Biosensors Nanotechnology; Wiley-VCH: Hoboken, NJ, USA, 2014. [Google Scholar]
- Luckarift, H.R.; Atanassov, P.; Johnson, G.R. Enzymatic Fuel Cells–From Fundamentals to Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Davis, F.; Higson, S.P.J. Biofuel cells-Recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luz, R.A.S.; Pereira, A.R.; de Souza, J.C.P.; Sales, F.C.P.F.; Crespilho, F.N. Enzyme biofuel cells: Thermodynamics, kinetics and challenges in applicability. ChemElectroChem 2014, 1, 1751–1777. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K. Enzymatic biofuel cells–Fabrication of enzyme electrodes. Energies 2010, 3, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Shleev, S. Quo vadis, implanted fuel cell? ChemPlusChem 2017, 82, 522–539. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Katz, E. (Ed.) Biomolecular Information Processing: From Logic Systems to Smart Sensors and Actuators; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Katz, E. Enzyme-Based Computing Systems; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]




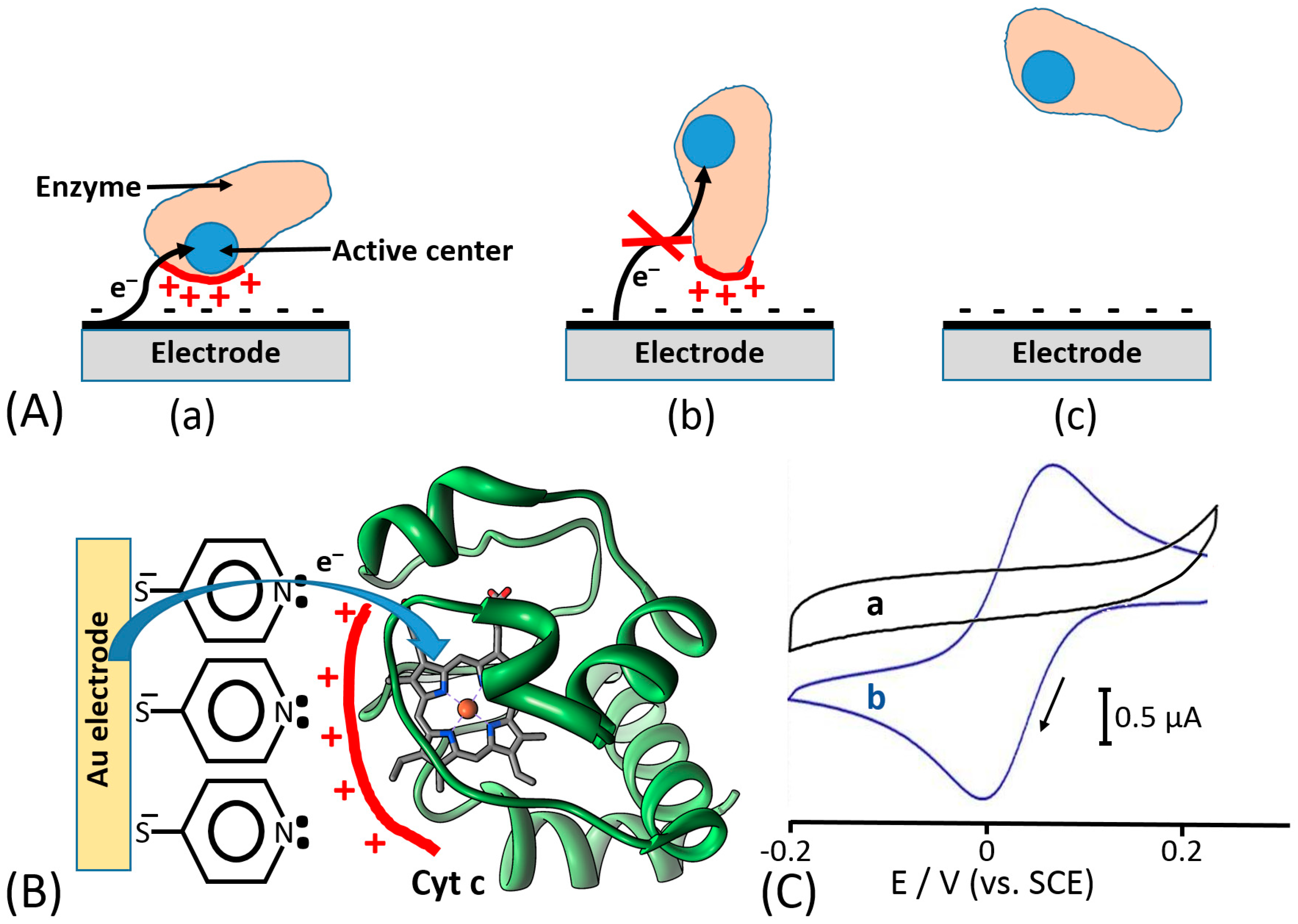
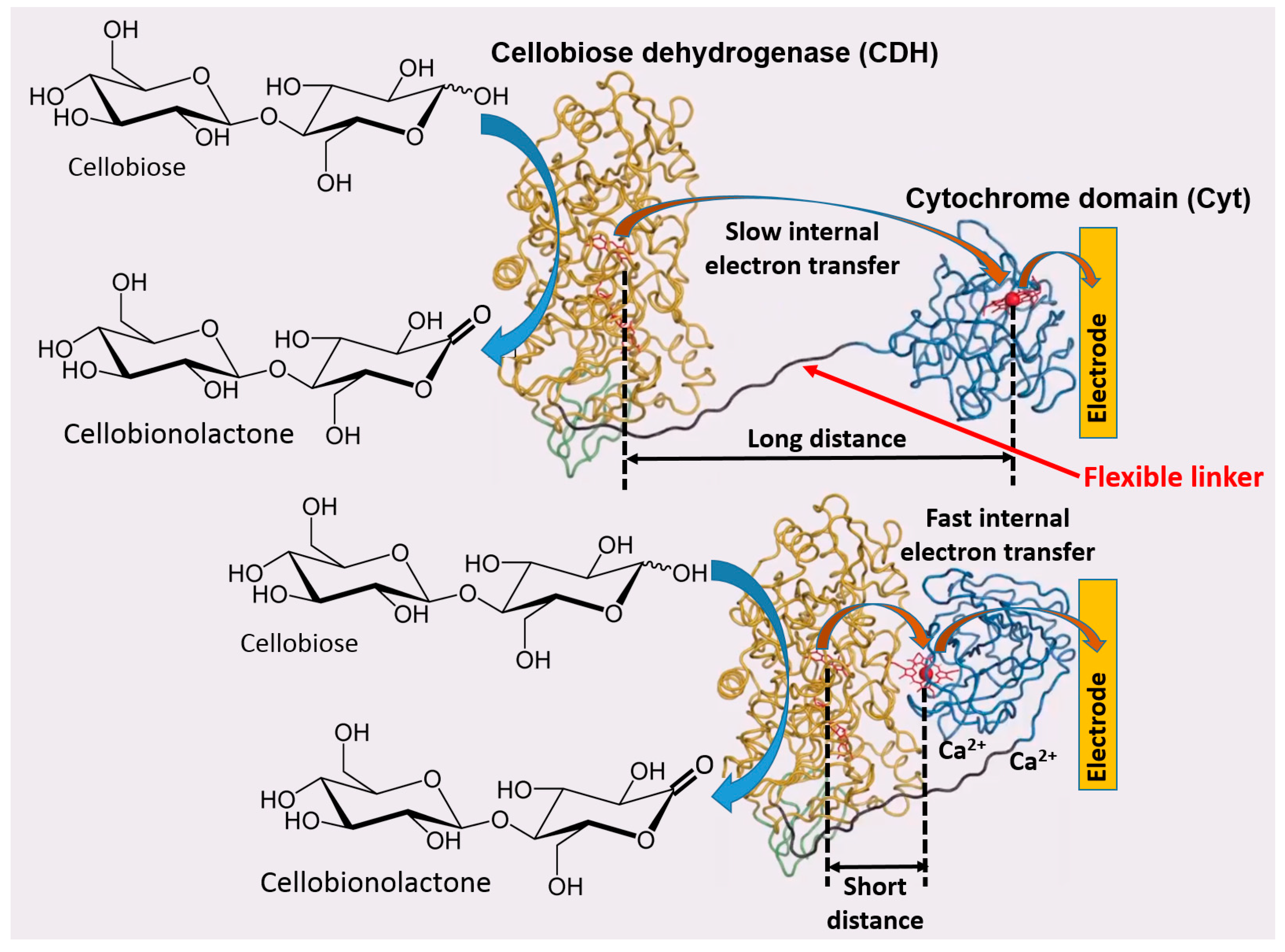

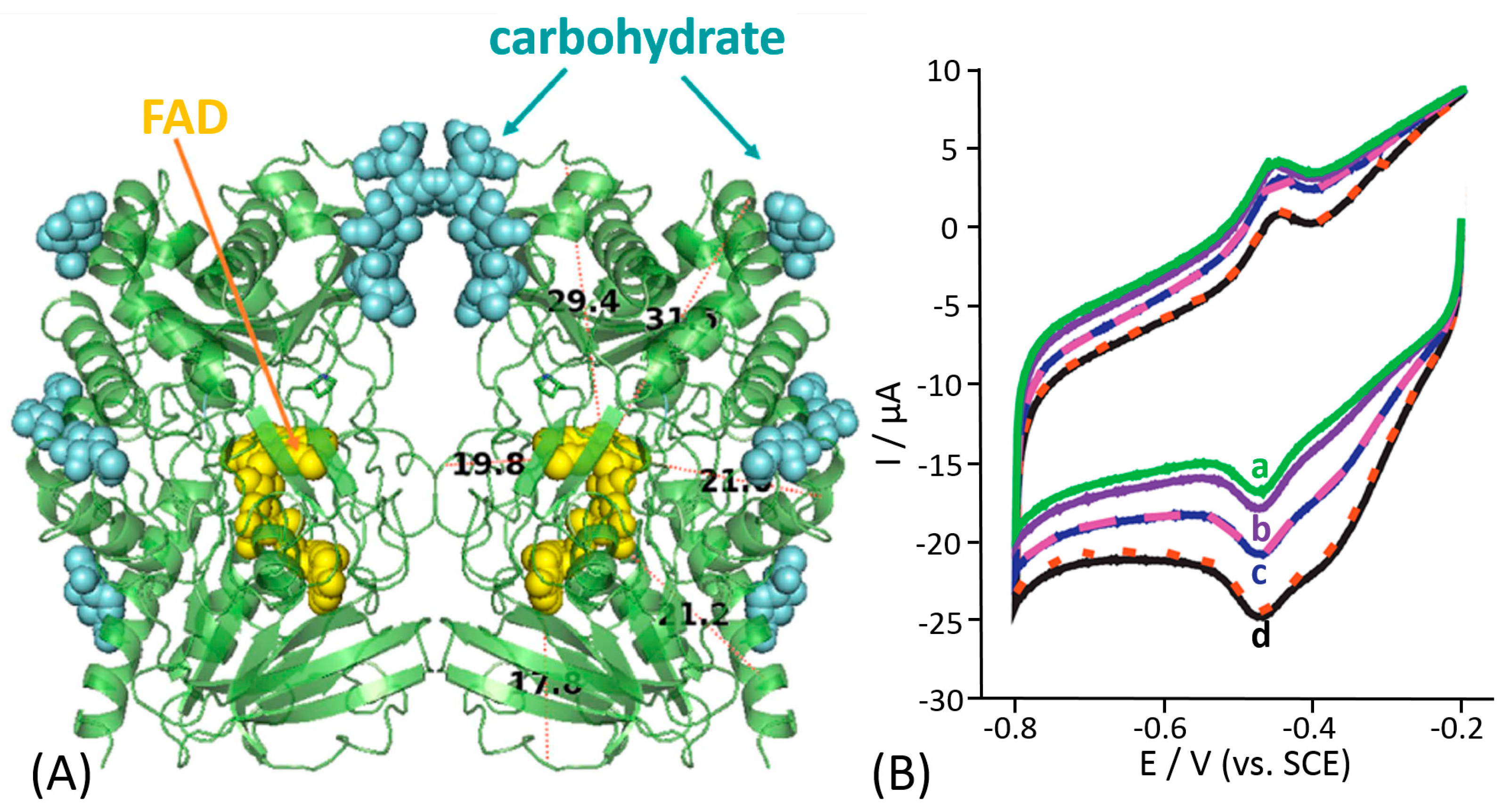
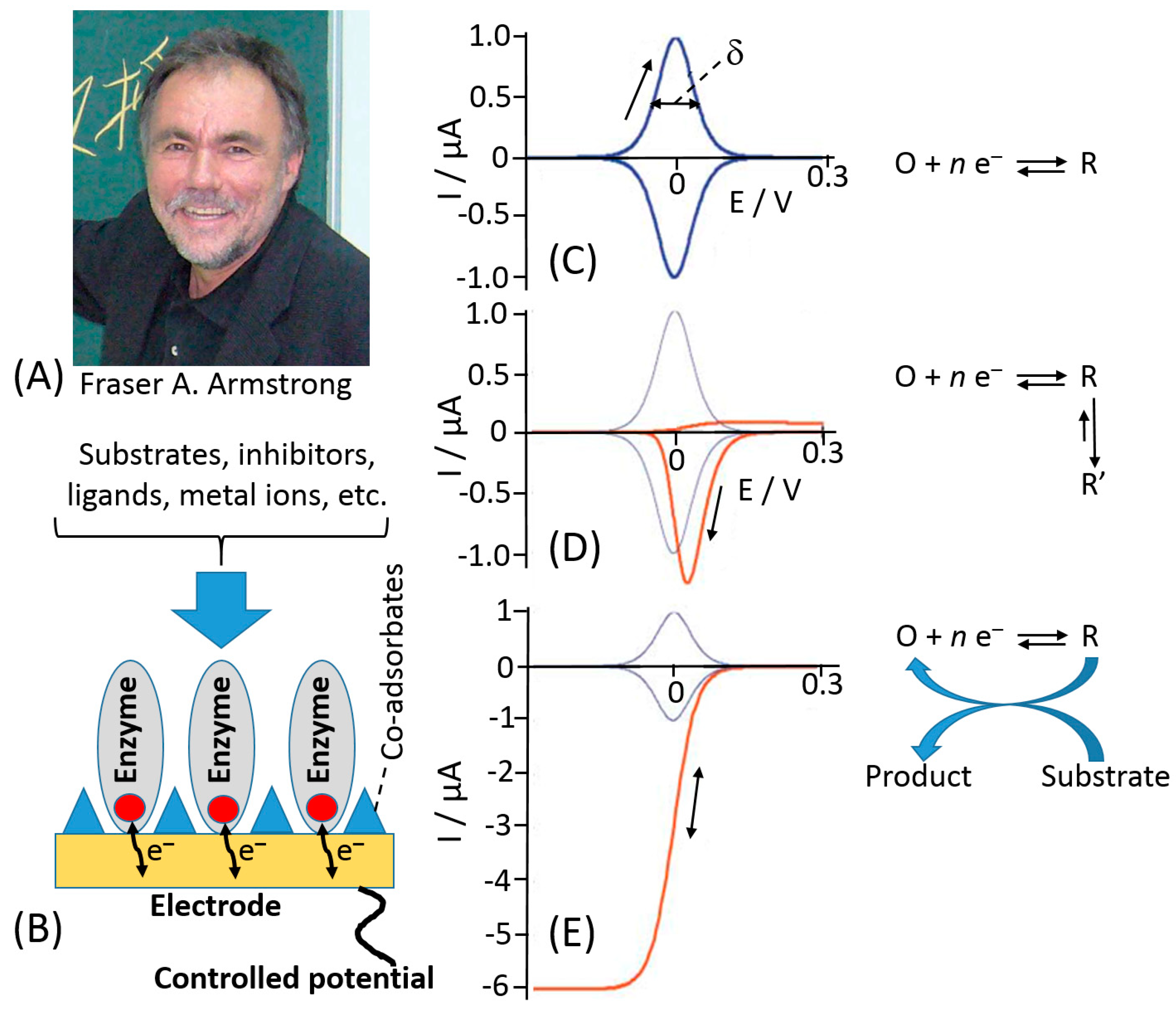

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. https://doi.org/10.3390/s20123517
Bollella P, Katz E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors. 2020; 20(12):3517. https://doi.org/10.3390/s20123517
Chicago/Turabian StyleBollella, Paolo, and Evgeny Katz. 2020. "Enzyme-Based Biosensors: Tackling Electron Transfer Issues" Sensors 20, no. 12: 3517. https://doi.org/10.3390/s20123517
APA StyleBollella, P., & Katz, E. (2020). Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors, 20(12), 3517. https://doi.org/10.3390/s20123517





