Wearable Monitoring and Interpretable Machine Learning Can Objectively Track Progression in Patients during Cardiac Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Multidisciplinary CR Program
2.3. Study Design
2.4. Feature Extraction
2.5. Linear Regression Model
2.6. Machine Learning Model Derivation
2.7. Feature Analysis and Tracking
3. Results
3.1. Functional Capacity
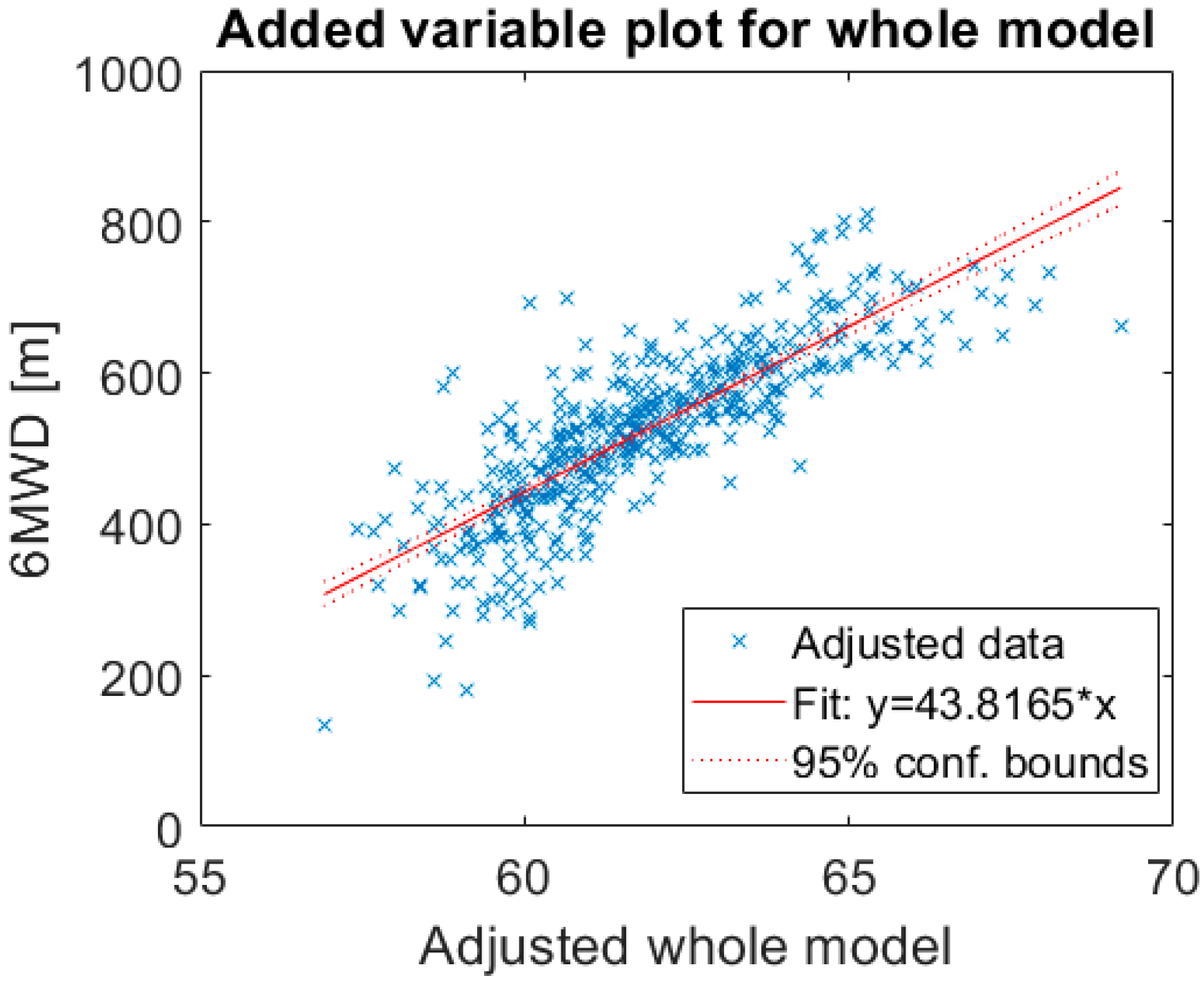
3.2. Linear Regression Model
3.3. Machine Learning Model Derivation
3.4. Feature Analysis and Tracking
3.5. Rehabilitation Tracking
4. Discussion
4.1. Main Findings
4.2. Linear and Nonlinear Regression Models
4.3. 3D Visualization of Functional Capacity on Population and Personal Level
4.4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregory, A.R.; Degu, A.; Kalkidan, H.A.; Solomon, M.A.; Cristiana, A.; Nooshin, A.; Hedayat, A.; Foad, A.-A.; Jemal, A.; Ahmed, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- Frederix, I.; Dendale, P.; Schmid, J.P. Who needs secondary prevention? Eur. J. Prev. Cardiol. 2017, 24 (Suppl. 3), 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Volkmar, F.; José, R.G.-J.; Veli-Pekka, H.; Ewa, A.J.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Thomas, R.J.; Balady, G.; Banka, G.; Beckie, T.M.; Chiu, J.; Gokak, S. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 2018, 71, 1814–1837. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Gregg, C.F.; Stephen, A.G.; Tamara, H.; James, L.J.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [Green Version]
- Dalal, H.M.; Doherty, P.; Taylor, R.S. Cardiac rehabilitation. BMJ (Clin. Res. Ed.) 2015, 351, h5000. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Corra, U.; Adamopoulos, S.; Benzer, W.; Bjarnason-Wehrens, B.; Cupples, M. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: A policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2014, 21, 664–681. [Google Scholar]
- Thomas, R.J.; Beatty, A.L.; Beckie, T.M.; Brewer, L.C.; Brown, T.M.; Forman, D.E.; Franklin, B.A.; Keteyian, S.J.; Kitzman, D.W.; Regensteiner, J.G.; et al. Home-Based Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2019, 39, 208–225. [Google Scholar] [CrossRef]
- Vegesna, A.; Tran, M.; Angelaccio, M.; Arcona, S. Remote Patient Monitoring via Non-Invasive Digital Technologies: A Systematic Review. Telemed. eHealth 2016, 23, 3–17. [Google Scholar] [CrossRef]
- Baril, J.-F.; Bromberg, S.; Moayedi, Y.; Taati, B.; Manlhiot, C.; Ross, H.; A Cafazzo, J. Use of Free-Living Step Count Monitoring for Heart Failure Functional Classification: Validation Study. JMIR Cardio 2019, 3, e12122. [Google Scholar] [CrossRef]
- Moayedi, Y.; Abdulmajeed, R.; Posada, J.D.; Foroutan, F.; Alba, A.C.; A Cafazzo, J.; Ross, H. Assessing the Use of Wrist-Worn Devices in Patients With Heart Failure: Feasibility Study. JMIR Cardio 2017, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Thijs, I.; Fresiello, L.; Oosterlinck, W.; Sinnaeve, P.; Rega, F.; Goris, J.; Vogt, F.; Pietilä, J. Assessment of Physical Activity by Wearable Technology During Rehabilitation After Cardiac Surgery: Explorative Prospective Monocentric Observational Cohort Study. JMIR mHealth uHealth 2019, 7, e9865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.-S.; Jhang, R.-J.; Lin, B.-S. Wearable Cardiopulmonary Function Evaluation System for Six-Minute Walking Test. Sensors 2019, 19, 4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehn, M.; Prescher, S.; Koehler, K.; Von Haehling, S.; Winkler, S.; Deckwart, O.; Honold, M.; Sechtem, U.; Baumann, G.; Halle, M.; et al. Tele-accelerometry as a novel technique for assessing functional status in patients with heart failure: Feasibility, reliability and patient safety. Int. J. Cardiol. 2013, 168, 4723–4728. [Google Scholar] [CrossRef] [PubMed]
- Jehn, M.; Schmidt-Trucksäess, A.; Schuster, T.; Hanssen, H.; Weiß, M.; Halle, M.; Koehler, F. Accelerometer-Based Quantification of 6-Minute Walk Test Performance in Patients with Chronic Heart Failure: Applicability in Telemedicine. J. Card. Fail. 2009, 15, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.; Mikalsen, M.H.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; A Hopstock, L.; Grimsgaard, S.; Sanders, J.; Wark, P.; Winfree, K.; et al. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e110. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.K.; Deen, J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- O’Driscoll, R.; Turicchi, J.; Beaulieu, K.; Scott, S.; Matu, J.; Deighton, K.; Finlayson, G.; Stubbs, J. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br. J. Sports Med. 2018. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Martin, S.S.; Steinhubl, S.R.; Muse, E.D. Connected Health Technology for Cardiovascular Disease Prevention and Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 29. [Google Scholar] [CrossRef]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, T.B.A.P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart (Br. Card. Soc.) 2018, 104, 1156–1164. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Adams, V.; Bahls, M.; Bowen, T.S.; Cornelissen, V.; Dörr, M.; Hansen, D.; Mc Kemps, H.; Leeson, P.; Van Craenenbroeck, E.M.; et al. Towards a personalised approach in exercise-based cardiovascular rehabilitation: How can translational research help? A ’call to action’ from the Section on Secondary Prevention and Cardiac Rehabilitation of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2019, 2019. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, T.; Groenendaal, W.; Ruyssinck, J.; Dreesen, P.; Klerkx, S.; Smeets, C.; De Francisco, R.; Deschrijver, D.; Dhaene, T. Systematic Comparison of Respiratory Signals for the Automated Detection of Sleep Apnea. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; Volume 2018, pp. 449–452. [Google Scholar]
- Varon, C.; Testelmans, D.; Buyse, B.; Suykens, J.A.K.; Van Huffel, S. Robust artefact detection in long-term ECG recordings based on autocorrelation function similarity and percentile analysis. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 3151–3154. [Google Scholar]
- Moeyersons, J.; Amoni, M.; Van Huffel, S.; Willems, R.; Varon, C. R-DECO: An open-source Matlab based graphical user interface for the detection and correction of R-peaks. bioRxiv 2019, 5, e226. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Di, C.; Xiao, L.; Evenson, K.R.; Lacroix, A.Z.; Crainiceanu, C.M.; Buchner, D.M. An Activity Index for Raw Accelerometry Data and Its Comparison with Other Activity Metrics. PLoS ONE 2016, 11, e0160644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7–14. [Google Scholar]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Nyström, C.D.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Kecman, V.; Huang, T.-M.; Vogt, M. Iterative Single Data Algorithm for Training Kernel Machines from Huge Data Sets: Theory and Performance. In Integration of Fuzzy Logic and Chaos Theory; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; Volume 177, pp. 255–274. [Google Scholar]
- Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Altini, M.; Casale, P.; Penders, J.; Amft, O. Cardiorespiratory fitness estimation in free-living using wearable sensors. Artif. Intell. Med. 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Juen, J.; Cheng, Q.; Schatz, B. A Natural Walking Monitor for Pulmonary Patients Using Mobile Phones. IEEE J. Biomed. Health Inform. 2015, 19, 1399–1405. [Google Scholar] [CrossRef]
- Lynch, J.; Fang, Q.; Salvi, D.; Poffley, E.; Orchard, E.; Tarassenko, L. The Mobile-Based 6-Minute Walk Test: Usability Study and Algorithm Development and Validation. JMIR mHealth uHealth 2020, 8, e13756. [Google Scholar] [CrossRef] [Green Version]
- Rasekaba, T.; Lee, A.L.; Naughton, M.T.; Williams, T.J.; Holland, A.E. The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern. Med. J. 2009, 39, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.-P.; Zurek, M.; Saner, H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur. J. Prev. Cardiol. 2012, 20, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, A.; Van Der Lingen, A.-L.C.; Handoko, M.L.; Van Rossum, A.C.; Allaart, C. Chronotropic Incompetence in Chronic Heart Failure. Circ. Heart Fail. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Lund, L.H.; Rao, P.; Ghosh, R.; Warier, P.; Vaccaro, B.; Dahlström, U.; O’Connor, C.M.; Felker, G.M.; Desai, N.R. Machine Learning Methods Improve Prognostication, Identify Clinically Distinct Phenotypes, and Detect Heterogeneity in Response to Therapy in a Large Cohort of Heart Failure Patients. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P. Faculty Opinions recommendation of Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. Faculty Opin. Post Publ. Peer Rev. Biomed. Lit. 2019, 73, 1317–1335. [Google Scholar] [CrossRef]
- Kao, D.P.; Lewsey, J.D.; Anand, I.; Massie, B.M.; Zile, M.R.; Carson, P.E.; McKelvie, R.S.; Komajda, M.; McMurray, J.J.; Lindenfeld, J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur. J. Heart Fail. 2015, 17, 925–935. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, M.; Bauman, A.; Neubeck, L.; Gallagher, R. Validation of Fitbit-Flex as a measure of free-living physical activity in a community-based phase III cardiac rehabilitation population. Eur. J. Prev. Cardiol. 2016, 23, 1476–1485. [Google Scholar] [CrossRef]
- Etiwy, M.; Akhrass, Z.; Gillinov, L.; Alashi, A.; Wang, R.; Blackburn, G.; Gillinov, S.; Phelan, D.; Gillinov, A.M.; Houghtaling, P.L.; et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc. Diagn. Ther. 2019, 9, 262–271. [Google Scholar] [CrossRef]




| Variable | Total Population (n = 89) |
|---|---|
| Anthropometric Features | |
| Male | 65 (73%) |
| Age, yrs | 63 ± 1 |
| Height, m | 1.72 [1.70–1.74] |
| Weight, kg | 79.2 ± 1.4 |
| BMI, kg/m2 | 26.7 ± 0.4 |
| LV ejection fraction, % | 46 [43–49] |
| Comorbidities | |
| Atrial fibrillation | 22 (25%) |
| Hypertension | 38 (43%) |
| Dyslipidemia | 39 (44%) |
| Diabetes | 12 (14%) |
| NYHA Class | |
| Class I | 26 (29%) |
| Class II | 44 (49%) |
| Class III | 19 (21%) |
| 6MWD, m | |
| Baseline | 484 ± 96 |
| 1st follow-up | 533 ± 100 |
| 2nd follow-up | 564 ± 100 |
| 3rd follow-up | 570 ± 103 |
| End of study | 585 ± 104 |
| Baseline VO2 max, mL/kg/min | 17.0 ± 5.1 |
| Kernel Type | MAE ± STD | Features |
|---|---|---|
| RBF | 42.8 m ± 36.8 m | IMU effort, chronotropic response |
| Linear | 55.2 m ± 51.3 | |
| Polynomial order 2 | 45.3 m ± 43.3 m | |
| Polynomial order 3 | 58.3 m ± 55 m | |
| Polynomial order 4 | 259.4 m ± 68 m | |
| RBF | 40.1 m ± 39.1 m | IMU effort, height |
| Linear | 67.6 m ± 62.5 m | |
| Polynomial order 2 | 257.3 m ± 70.3 m | |
| Polynomial order 3 | 98.6 m ± 77.5 m | |
| Polynomial order 4 | 284.1 m ± 92.6 m | |
| RBF | 47.2 m ± 47.6 m | IMU effort |
| Linear | 67.7 m ± 63.2 m | |
| Polynomial order 2 | 90.7 m ± 60.7 m | |
| Polynomial order 3 | 267.6 m ± 57.6 m | |
| Polynomial order 4 | 285.6 m ± 67.6 m | |
| RBF | 37.7 m ± 36.2 m | IMU effort, chronotropic response, height |
| Linear | 55.0 m ± 51.8 m | |
| Polynomial order 2 | 42.6 m ± 40.4 m | |
| Polynomial order 3 | 44.6 m ± 50.7 m | |
| Polynomial order 4 | 263.5 m ± 176 m | |
| RBF | 60.0 m ± 58.7 m | Chronotropic response |
| Linear | 131.0 m ± 110.4 m | |
| Polynomial order 2 | 75.7 m ± 71.4 m | |
| Polynomial order 3 | 83.2 m ± 71.7 m | |
| Polynomial order 4 | 89.9 m ± 81.1 m | |
| RBF | 67.5 m ± 67.1 m | Chronotropic response, height |
| Linear | 100.1 m ± 85.3m | |
| Polynomial order 2 | 65.5 m ± 59.3 m | |
| Polynomial order 3 | 193.9 m ± 123.1 m | |
| Polynomial order 4 | 250.5 m ± 408.4 m |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cannière, H.; Corradi, F.; Smeets, C.J.P.; Schoutteten, M.; Varon, C.; Van Hoof, C.; Van Huffel, S.; Groenendaal, W.; Vandervoort, P. Wearable Monitoring and Interpretable Machine Learning Can Objectively Track Progression in Patients during Cardiac Rehabilitation. Sensors 2020, 20, 3601. https://doi.org/10.3390/s20123601
De Cannière H, Corradi F, Smeets CJP, Schoutteten M, Varon C, Van Hoof C, Van Huffel S, Groenendaal W, Vandervoort P. Wearable Monitoring and Interpretable Machine Learning Can Objectively Track Progression in Patients during Cardiac Rehabilitation. Sensors. 2020; 20(12):3601. https://doi.org/10.3390/s20123601
Chicago/Turabian StyleDe Cannière, Hélène, Federico Corradi, Christophe J. P. Smeets, Melanie Schoutteten, Carolina Varon, Chris Van Hoof, Sabine Van Huffel, Willemijn Groenendaal, and Pieter Vandervoort. 2020. "Wearable Monitoring and Interpretable Machine Learning Can Objectively Track Progression in Patients during Cardiac Rehabilitation" Sensors 20, no. 12: 3601. https://doi.org/10.3390/s20123601
APA StyleDe Cannière, H., Corradi, F., Smeets, C. J. P., Schoutteten, M., Varon, C., Van Hoof, C., Van Huffel, S., Groenendaal, W., & Vandervoort, P. (2020). Wearable Monitoring and Interpretable Machine Learning Can Objectively Track Progression in Patients during Cardiac Rehabilitation. Sensors, 20(12), 3601. https://doi.org/10.3390/s20123601





