A Label-Free Impedimetric Genosensor for the Nucleic Acid Amplification-Free Detection of Extracted RNA of Dengue Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Design and Production of DNA Samples
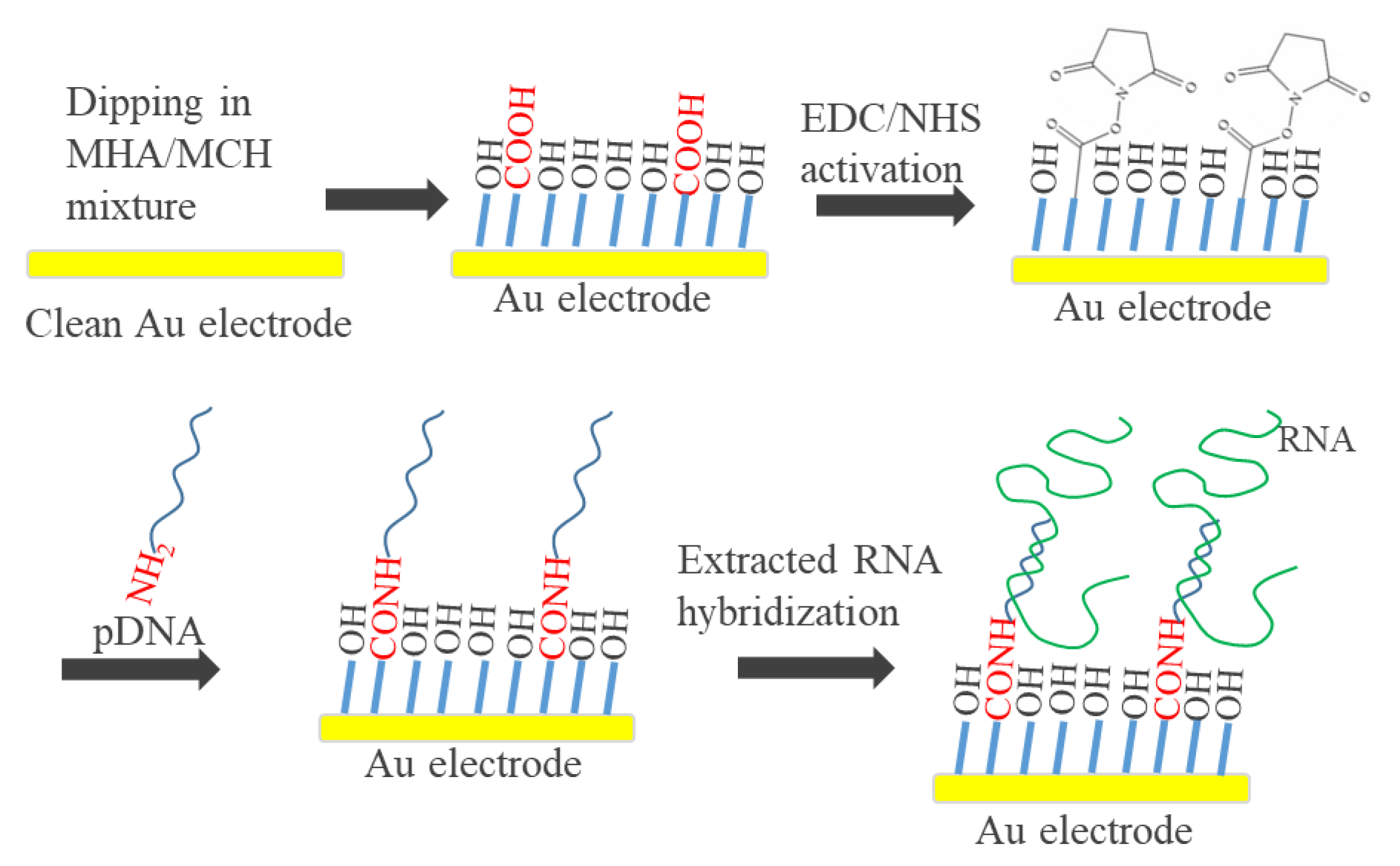
2.3. Preparation of Genosensor
2.4. Measurement of Self-Assembled Monolayer (SAM) Coverage and pDNA Density
2.5. Electrochemical Measurements
2.6. Extracted RNA Test
3. Results and Discussion
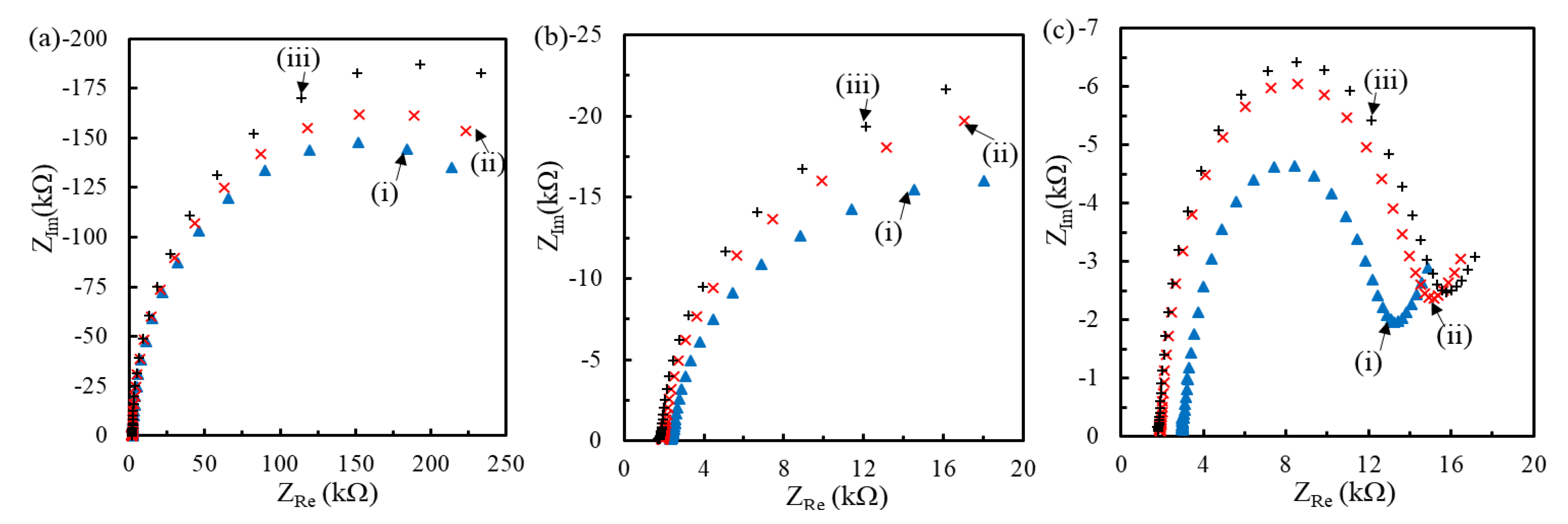
3.1. Effect of Binary SAM on pDNA Coverage
3.2. Effect of pDNA Density on Hybridization of Length-Varied tDNA
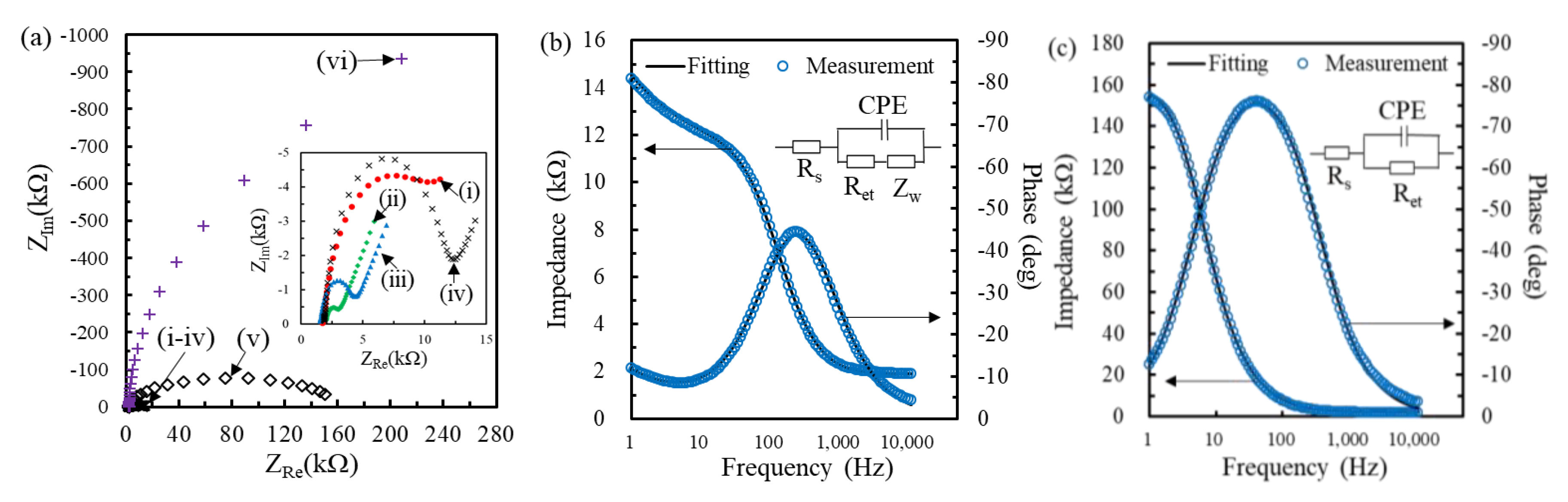
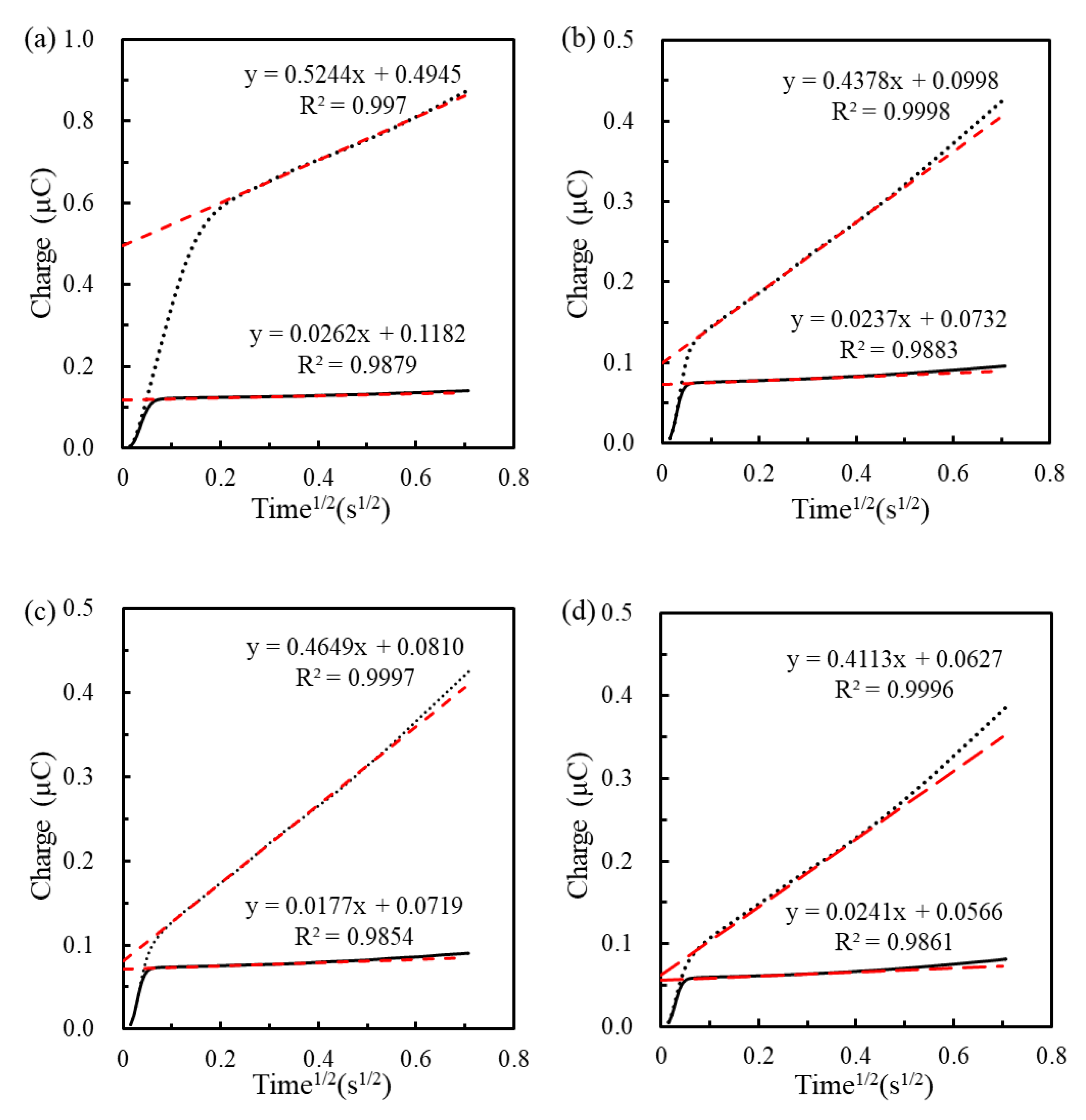
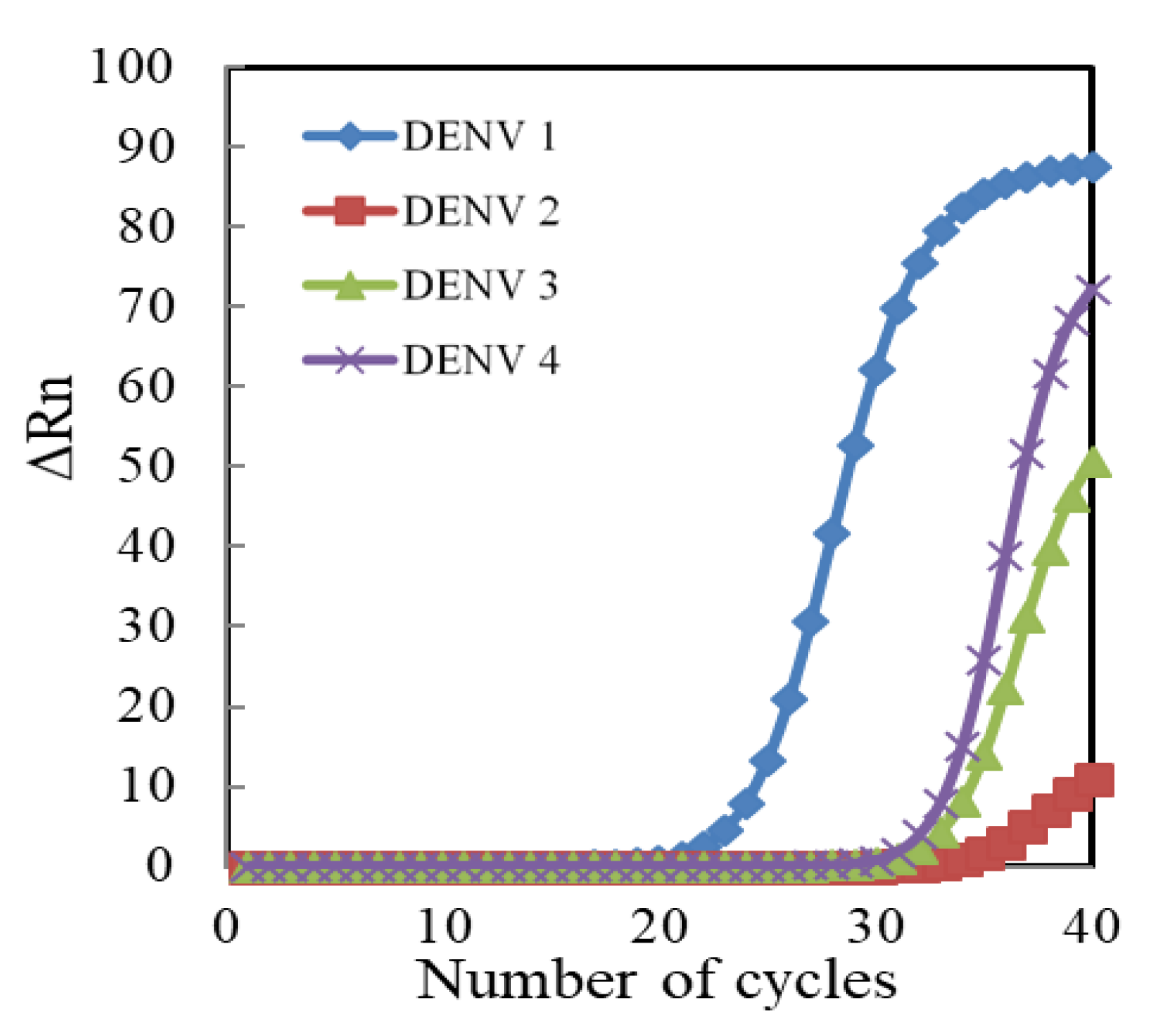
3.3. Sensing Properties of Genosensors for Extracted RNA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Fernandes, J.N.; Moise, I.K.; Maranto, G.L.; Beier, J.C. Revamping Mosquito-borne Disease Control to Tackle Future Threats. Trends Parasitol. 2018, 34, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chastel, C. Eventual role of asymptomatic cases of dengue for the introduction and spread of dengue viruses in non-endemic regions. Front Physiol. 2012, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, I.; Leder, K.; Black, J.; Torresi, J. Dengue fever and international travel. J. Travel Med. 2013, 20, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef]
- Malavige, G.N.; Fernando, S.; Fernando, D.J.; Seneviratne, S.L. Dengue viral infections. Postgrad. Med. J. 2004, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Lo, Y.C.; Perng, G.C. Novel concept on antiviral strategies to dengue. Curr. Opin. Virol. 2016, 18, 97–108. [Google Scholar] [CrossRef]
- Iswardy, E.; Tsai, T.C.; Cheng, I.F.; Ho, T.C.; Perng, G.C.; Chang, H.C. A bead-based immunofluorescence-assay on a microfluidic dielectrophoresis platform for rapid dengue virus detection. Biosens. Bioelectron. 2017, 95, 174–180. [Google Scholar] [CrossRef]
- Lim, J.M.; Kim, J.H.; Ryu, M.Y.; Cho, C.H.; Park, T.J.; Park, J.P. An electrochemical peptide sensor for detection of dengue fever biomarker NS1. Anal. Chim. Acta 2018, 1026, 109–116. [Google Scholar] [CrossRef]
- Nawaz, M.H.; Hayat, A.; Catanante, G.; Latif, U.; Marty, J.L. Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal. Chim. Acta 2018, 1026, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.R.; Crulhas, B.P.; Magro, M.; Vianello, F.; Pedrosa, V.A. A new immunoassay of hybrid nanomater conjugated to aptamers for the detection of dengue virus. Talanta 2019, 197, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.S.; Ho, J.S.; Tan, C.H.; Wong, J.P.; Ng, L.C.; Toh, C.S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta 2012, 725, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Vickers, I.; Harvey, K.; Nelson, K.; Brown, M.; Bullock-DuCasse, M.; Lindo, J. Evaluation of OneStep Dengue NS1 RapiDip InstaTest and OneStep Dengue Fever IgG/IgM RapiCard InstaTest during the course of a dengue type 1 epidemic. Diagn. Microbiol. Infect. Dis. 2017, 89, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Toh, C.S. Impedimetric DNA biosensor based on a nanoporous alumina membrane for the detection of the specific oligonucleotide sequence of dengue virus. Sensors 2013, 13, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-A.; Poudyal, S.; Marinero, E.E.; Kuhn, R.J.; Stanciu, L.A. Impedimetric Dengue Biosensor based on Functionalized Graphene Oxide Wrapped Silica Particles. Electrochim. Acta 2016, 194, 422–430. [Google Scholar] [CrossRef]
- Odeh, A.A.; Al-Douri, Y.; Voon, C.H.; Ayub, R.M.; Gopinath, S.C.B.; Odeh, R.A.; Ameri, M.; Bouhemadou, A. A needle-like Cu2CdSnS4 alloy nanostructure-based integrated electrochemical biosensor for detecting the DNA of Dengue serotype 2. Microchim. Acta 2017, 184, 2211–2218. [Google Scholar] [CrossRef]
- Oliveira, N.; Souza, E.; Ferreira, D.; Zanforlin, D.; Bezerra, W.; Borba, M.A.; Arruda, M.; Lopes, K.; Nascimento, G.; Martins, D.; et al. A sensitive and selective label-Free electrochemical DNA biosensor for the detection of specific dengue virus serotype 3 sequences. Sensors 2015, 15, 15562–15577. [Google Scholar] [CrossRef]
- Singhal, C.; Pundir, C.S.; Narang, J. A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef]
- Tripathy, S.; Vanjari, S.R.K.; Singh, V.; Swaminathan, S.; Singh, S.G. Electrospun manganese (III) oxide nanofiber based electrochemical DNA-nanobiosensor for zeptomolar detection of dengue consensus primer. Biosens. Bioelectron. 2017, 90, 378–387. [Google Scholar] [CrossRef]
- Sharp, T.M.; Perez-Padilla, J.; Waterman, S.H. Travel-Related Infectious Diseases. 2019. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/dengue (accessed on 26 October 2019).
- Boonnak, K.; Slike, B.M.; Burgess, T.H.; Mason, R.M.; Wu, S.J.; Sun, P.; Porter, K.; Rudiman, I.F.; Yuwono, D.; Puthavathana, P.; et al. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J. Virol. 2008, 82, 3939–3951. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.T.; Sekaran, S.D.; Khor, S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuator B-Chem. 2018, 255, 3316–3331. [Google Scholar] [CrossRef]
- Anusha, J.R.; Kim, B.C.; Yu, K.H.; Raj, C.J. Electrochemical biosensing of mosquito-borne viral disease, dengue: A review. Biosens. Bioelectron. 2019, 142, 111511. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gao, L.; Chen, S.; Huang, Z.; Wang, X. Electrochemical voltammetric behaviors of synthetic dengue virus RNAs at ITO sensing electrode. J. Electroanal. Chem. 2019, 851, 113463. [Google Scholar] [CrossRef]
- Lynch, C.A., III; Foguel, M.V.; Reed, A.J.; Balcarcel, A.M.; Calvo-Marzal, P.; Gerasimova, Y.V.; Chumbimuni-Torres, K.Y. Selective determination of isothermally amplified Zika virus RNA using a universal DNA-hairpin probe in less than 1 h. Anal. Chem. 2019, 91, 13458–13464. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.M.; Martin, C.P.; Armas, S.M.; Calvo-Marzal, P.; Kolpashchikov, D.M.; Chumbimuni-Torres, K.Y. A universal and label-free impedimetric biosensing platform for discrimination of single nucleotide substitutions in long nucleic acid strands. Biosens. Bioelectron. 2018, 100, 35–42. [Google Scholar] [CrossRef]
- Corrigan, D.K.; Schulze, H.; McDermott, R.A.; Schmüser, I.; Henihan, G.; Henry, J.B.; Bachmann, T.T.; Mount, A.R. Improving electrochemical biosensor performance by understanding the influence of target DNA length on assay sensitivity. J. Electroanal. Chem. 2014, 732, 25–29. [Google Scholar] [CrossRef]
- Riedel, M.; Kartchemnik, J.; Schoning, M.J.; Lisdat, F. Impedimetric DNA detection-steps forward to sensorial application. Anal. Chem. 2014, 86, 7867–7874. [Google Scholar] [CrossRef]
- Ding, S.-J.; Chang, B.-W.; Wu, C.-C.; Lai, M.-F.; Chang, H.-C. Impedance spectral studies of self-assembly of alkanethiols with different chain lengths using different immobilization strategies on Au electrodes. Anal. Chim. Acta 2005, 554, 43–51. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA Probes Immobilized on Gold Surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar] [CrossRef]
- Wu, C.-C.; Huang, W.-C.; Hu, C.-C. An ultrasensitive label-free electrochemical impedimetric DNA biosensing chip integrated with a DC-biased AC electroosmotic vortex. Sens. Actuator B-Chem. 2015, 209, 61–68. [Google Scholar] [CrossRef]
- Arihara, K.; Ariga, T.; Takashima, N.; Arihara, K.; Okajima, T.; Kitamura, F.; Tokuda, K.; Ohsaka, T. Multiple voltammetric waves for reductive desorption of cysteine and 4-mercaptobenzoic acid monolayers self-assembled on gold substrates. Phys. Chem. Chem. Phys. 2003, 5, 3758–3761. [Google Scholar] [CrossRef]
- Houng, H.-S.H.; Hritz, D.; Kanesa-thasan, N. Quantitative detection of dengue 2 virus using fluorogenic RT-PCR based on 3%-noncoding sequence. J. Virol. Methods 2000, 86, 1–11. [Google Scholar] [CrossRef]
- Chao, D.-Y.; Davis, B.S.; Chang, G.-J.J. Development of multiplex real-time reverse transcriptase PCR assays for Detecting Eight Medically Important Flaviviruses in Mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Darwish, N.T.; Alias, Y.B.; Khor, S.M. An introduction to dengue-disease diagnostics. TrAC Trend. Anal. Chem. 2015, 67, 45–55. [Google Scholar] [CrossRef]
- Zaytseva, N.V.; Montagna, R.A.; Baeumner, A.J. Microfluidic biosensor for the serotype-specific detection of dengue virus RNA. Anal. Biochem. 2005, 77, 7520–7527. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chuang, Y.-C.; Lu, Y.-C.; Lin, H.-C.; Yang, Y.-L.; Lin, C.-S. A method of layer-by-layer gold nanoparticle hybridization in a quartzcrystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef]
- Yin, Z.; Ramshani, Z.; Waggoner, J.J.; Pinsky, B.A.; Senapati, S.; Chang, H.-C. A non-optical multiplexed PCR diagnostic platform for serotype-specific detection of dengue virus. Sens. Actuator B-Chem. 2020, 310, 127854. [Google Scholar] [CrossRef]





| Name | Sequence from 5′to 3′ Terminal |
|---|---|
| pDNA | NH2-(CH2)6-AGT CCT TAC CAC CAG GGT AC |
| Complementary 20mtDNA | GTA CCC TGG TGG TAA GGA CT |
| 160mtDNA | GTA CCC TGG TGG TAA GGA CTA GAG GTT AGA GGA GAC CCC CCG CAC AAC AAT AAA CAG CAT ATT GAC GCT GGG AGA GAC CAG AGA TCC TGC TGT CTC TAC AGC ATC ATT CCA GGC ACA GAA CGC CAG AAA ATG GAA TGG TGC TGT TGA ATC AAC AGG TTC T |
| Ratio of MHA: MCH | 20mtDNA | 160mtDNA | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔRet (kΩ) | ΔRet Ratio (%) | ΔRet (kΩ) | ΔRet Ratio (%) | |||||
| 1 pM | 1 nM | 1 pM | 1 nM | 1 pM | 1 nM | 1 pM | 1 nM | |
| 0.10:1 | 18.3 ± 4.3 | 32.2 ± 7.1 | 10.1 ± 1.9 | 17.8 ± 5.5 | 63.7 ± 42.2 | 120.2 ± 47.4 | 16.2 ± 11.2 | 30.4 ± 12.5 |
| 0.04:1 | 1.8 ± 0.7 | 3.1 ± 0.9 | 17.0 ± 4.0 | 30.0 ± 4.3 | 11.0 ± 2.2 | 17.8 ± 2.8 | 32.9 ± 7.1 | 53.0 ± 9.3 |
| 0.02:1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 9.3 ± 1.8 | 13.8 ± 1.4 | 2.8 ± 0.30 | 3.2 ± 0.2 | 30.2 ± 3.6 | 35.1 ± 1.4 |
| Sensing Interface | Method/Target | Dynamic Range | LOD | Ref. |
|---|---|---|---|---|
| pDNA2-liposome/biotin-pDNA1-SMB | Fluorescence/synthetic DNA and RNA amplicon | 0.125 nM–50 pM | 0.125 nM | [39] |
| SH-pDNA-AuNP | QCM/PCR 130mtDNA amplicon | 2 × 100–2 × 106 PFU/mL | 2 PFU/mL | [40] |
| pDNA/IEM | I-V curve/RT-PCR DNA amplicon | -- | 0.17 aM | [41] |
| NH2-pDNA/APS/Pt/AAO | EIS or DPV/synthetic 30mtDNA | 1 pM–1 μM | 2.7 pM | [15] |
| pDNA/PGE | DPV/synthetic 22mtDNA | 10–100 nM | 3.1 nM | [18] |
| NH2-pDNA/chitosan/ZnO/Pt-Pd/FTO | CV/synthetic 35mtDNA | 1–100 μM | 43 μM | [19] |
| NH2-pDNA/MPA/Mn2O3 nanofiber/GCE | EIS or DPV/synthetic consensus dengue primer | 1 aM–1 μM | 0.12 aM | [20] |
| ITO | LSV/pDNA-RNA amplicon mixture | -- | 2 amol | [26] |
| pDNA/SiO2@APTES-GO/Pt | EIS/extracted RNA | -- | 1 fM | [16] |
| NH2-pDNA/MHA/MCH/Au | EIS/extracted RNA | 102–105 PFU/mL | 20 PFU/mL (~20 aM) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Yen, H.-Y.; Lai, L.-T.; Perng, G.-C.; Lee, C.-R.; Wu, S.-J. A Label-Free Impedimetric Genosensor for the Nucleic Acid Amplification-Free Detection of Extracted RNA of Dengue Virus. Sensors 2020, 20, 3728. https://doi.org/10.3390/s20133728
Wu C-C, Yen H-Y, Lai L-T, Perng G-C, Lee C-R, Wu S-J. A Label-Free Impedimetric Genosensor for the Nucleic Acid Amplification-Free Detection of Extracted RNA of Dengue Virus. Sensors. 2020; 20(13):3728. https://doi.org/10.3390/s20133728
Chicago/Turabian StyleWu, Ching-Chou, Hao-Yu Yen, Lu-Ting Lai, Guey-Chuen Perng, Cheng-Rei Lee, and Shuenn-Jue Wu. 2020. "A Label-Free Impedimetric Genosensor for the Nucleic Acid Amplification-Free Detection of Extracted RNA of Dengue Virus" Sensors 20, no. 13: 3728. https://doi.org/10.3390/s20133728
APA StyleWu, C.-C., Yen, H.-Y., Lai, L.-T., Perng, G.-C., Lee, C.-R., & Wu, S.-J. (2020). A Label-Free Impedimetric Genosensor for the Nucleic Acid Amplification-Free Detection of Extracted RNA of Dengue Virus. Sensors, 20(13), 3728. https://doi.org/10.3390/s20133728






