A 3D Scanning System for Inverse Analysis of Moist Biological Samples: Design and Validation Using Tendon Fascicle Bundles
Abstract
:1. Introduction
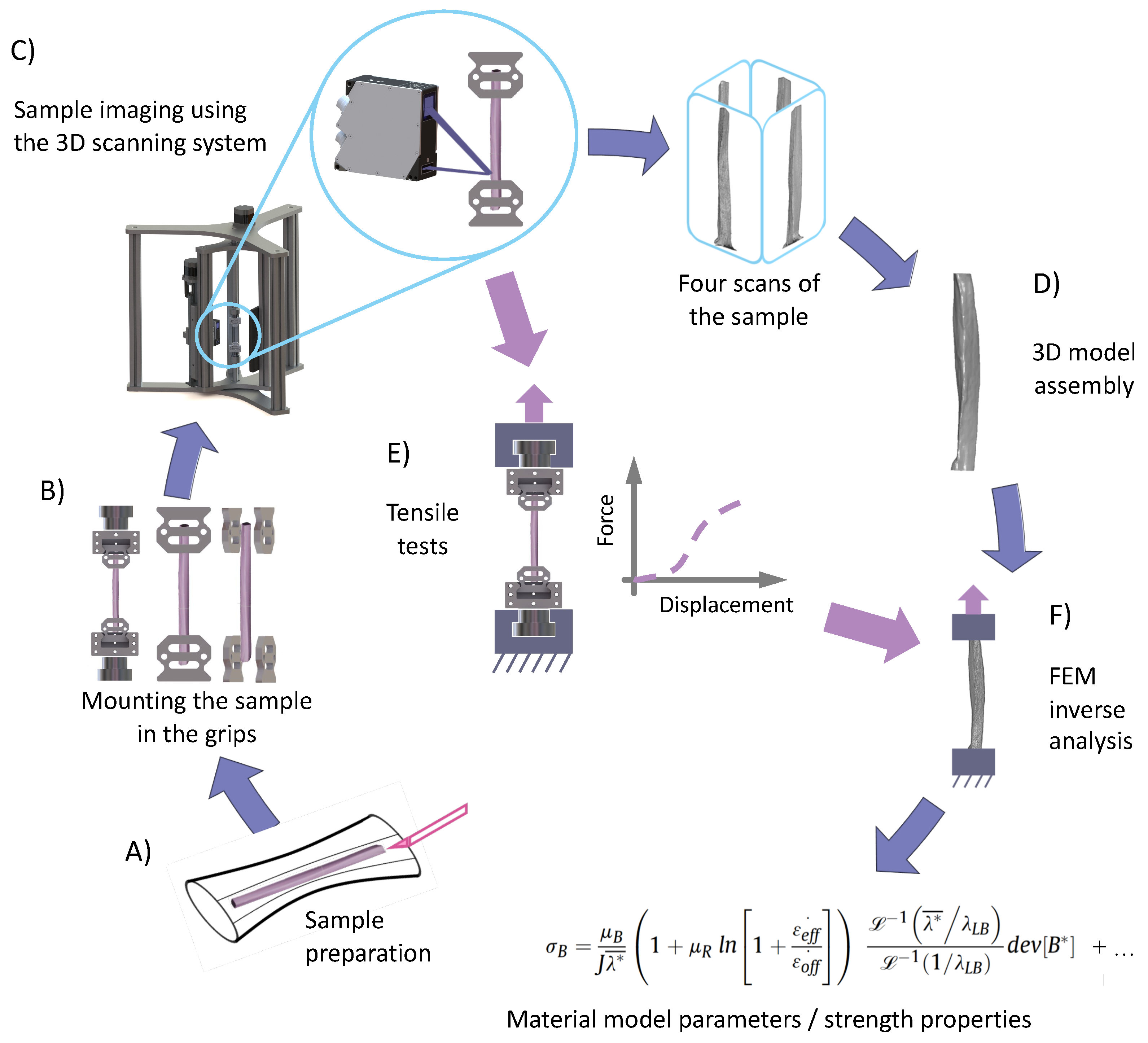
2. Development of a 3D Scanning System for the Imaging of Tendon Fascicle Bundles
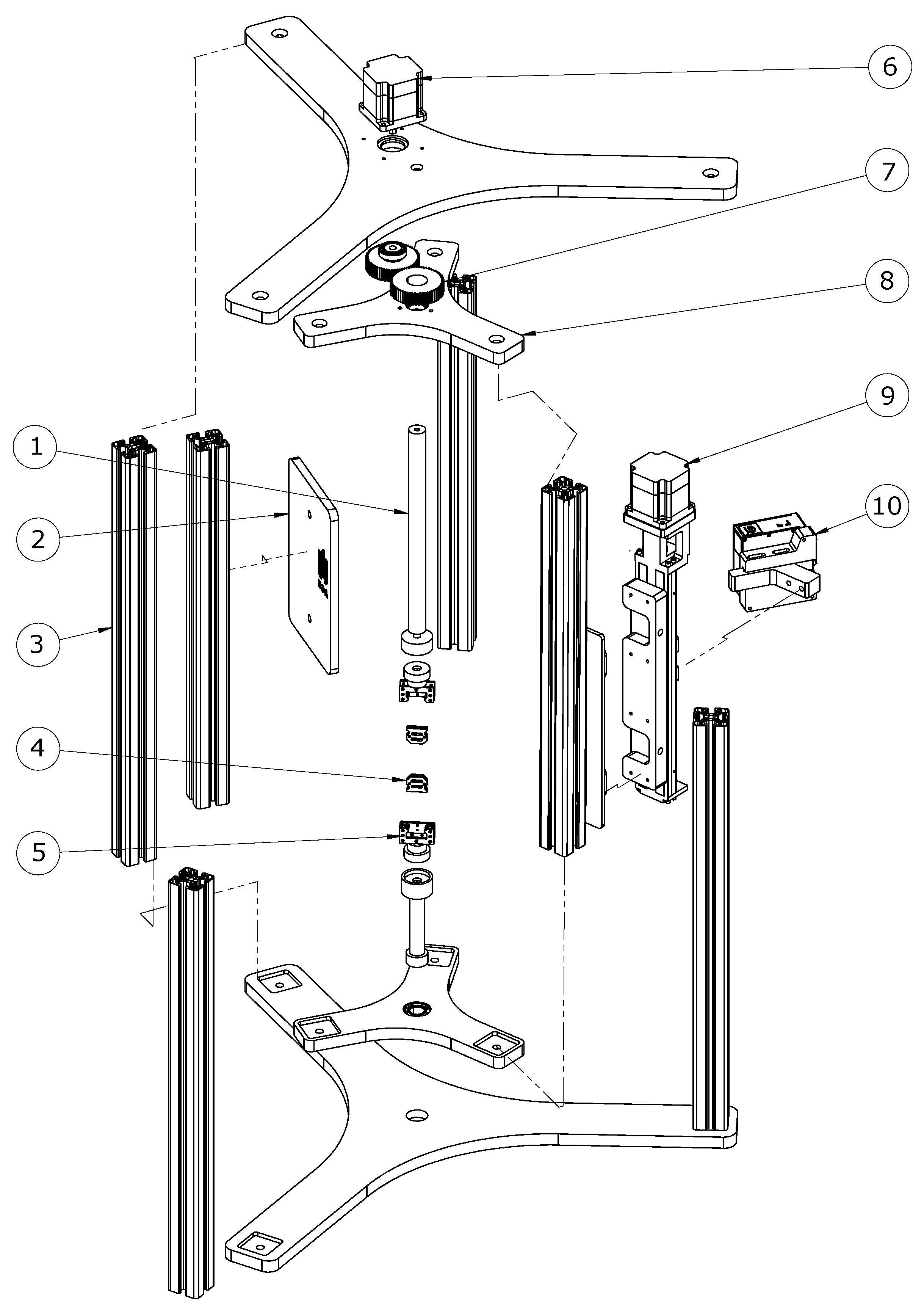
2.1. Three-Dimensional Scanning System: Design
2.2. Validation Procedure of Our 3D Scanning System on Synthetic Regular Samples and a Wooden Twig
2.3. Validation Procedure of Our 3D Scanning System on Soft Tissues: Tendon Fascicle Bundles
3. Results
3.1. Regular Cylindrical/Hexagonal Prismatic Shapes and Irregular Biological Samples: Comparison between the True Value, Vision-Based Method, and 3D Scanning System
3.2. Tissue Samples: Comparison between the Vision-Based Method and 3D Scanning System Using Talc-Coated and Uncoated Samples
4. Conclusions
- it used a blue laser profile sensor, which enabled the measurement of samples with a diameter of up to 15 mm with ±2 μm accuracy and a relative error of less than 1.2%
- it enabled the creation of 3D computational models of biological samples, including their concavities, within a matter of minutes
- samples were mounted on replaceable inserts, which were fitted to the 3D scanning system, as well as the tensile machine; this ensured no change in the boundary conditions between 3D scanning and mechanical tests, making inverse analysis feasible
- the process of sample scanning was fully automated, which ensured the high repeatability and accuracy of measurements
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Handsfield, G.G.; Slane, L.C.; Screen, H.R. Nomenclature of the tendon hierarchy: An overview of inconsistent terminology and a proposed size-based naming scheme with terminology for multi-muscle tendons. J. Biomech. 2016, 49, 3122–3124. [Google Scholar] [CrossRef] [PubMed]
- Muench, J.R.; Thelen, D.G.; Henak, C.R. Interfibrillar shear behavior is altered in aging tendon fascicles. Biomech. Model. Mechanobiol. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodenberg, R.E.; Bowman, E.; Ravindran, R. Overuse injuries. Prim. Care Clin. Off. Pract. 2013, 40, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, M.; Yin, G.; Zhang, J.; Zhang, Z.; Lai, P.; Yan, B.; Chen, Y.; Jin, D.; Wang, L. Characterization of a Novel Calcific Achilles Tendinopathy Model in Mice: Contralateral Tendinopathy Induced by Unilateral Tenotomy. Calcif. Tissue Int. 2018, 103, 698–707. [Google Scholar] [CrossRef]
- Kaleagasoglu, F.; Olcay, E.; Olgac, V. Statin-induced calcific Achilles tendinopathy in rats: Comparison of biomechanical and histopathological effects of simvastatin, atorvastatin and rosuvastatin. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1884–1891. [Google Scholar] [CrossRef]
- Arya, S.; Kulig, K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J. Appl. Physiol. 2010, 108, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Child, S.; Bryant, A.L.; Clark, R.A.; Crossley, K.M. Mechanical properties of the achilles tendon aponeurosis are altered in athletes with achilles tendinopathy. Am. J. Sports Med. 2010, 38, 1885–1893. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Kjaer, M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur. J. Appl. Physiol. 2003, 90, 549–553. [Google Scholar] [CrossRef]
- Fallah, A.; Ahmadian, M.T.; Mohammadi Aghdam, M. Rate-dependent behavior of connective tissue through a micromechanics-based hyper viscoelastic model. Int. J. Eng. Sci. 2017, 121, 91–107. [Google Scholar] [CrossRef]
- Chatzistergos, P.E.; Tsitsilonis, S.I.; Mitousoudis, A.S.; Perrea, D.N.; Zoubos, A.B.; Kourkoulis, S.K. The fracture stress of rat achilles tendons. Scand. J. Lab. Anim. Sci. 2010, 37, 149–156. [Google Scholar]
- Zhang, X.; Aoyama, T.; Takaishi, R.; Higuchi, S.; Yamada, S.; Kuroki, H.; Takakuwa, T. Spatial change of cruciate ligaments in rat embryo knee joint by three-dimensional reconstruction. PLoS ONE 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodship, A.E.; Birch, H.L. Cross sectional area measurement of tendon and ligament in vitro: A simple, rapid, non-destructive technique. J. Biomech. 2005, 38, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Q.; Woo, S.L.Y. A new method for determining cross-sectional shape and area of soft tissues. J. Biomech. Eng. 1988, 110, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Danto, M.I.; Ohland, K.J.; Lee, T.Q.; Newton, P.O. The use of a laser micrometer system to determine the cross-sectional shape and area of ligaments: A comparative study with two existing methods. J. Biomech. Eng. 1990, 112, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Wolfram, U.; Schmidt, H.; Wilke, H.J. A method to obtain surface strains of soft tissues using a laser scanning device. J. Biomech. 2008, 41, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Chandrashekar, N.; Cowden, C.; Slauterbeck, J. An alternative method of anthropometry of anterior cruciate ligament through 3-D digital image reconstruction. J. Biomech. 2005, 38, 551–555. [Google Scholar] [CrossRef]
- Hayes, A.; Easton, K.; Devanaboyina, P.T.; Wu, J.P.; Kirk, T.B.; Lloyd, D. Structured white light scanning of rabbit Achilles tendon. J. Biomech. 2016, 49, 3753–3758. [Google Scholar] [CrossRef]
- Ahn, H.W.; Chang, Y.J.; Kim, K.A.; Joo, S.H.; Park, Y.G.; Park, K.H. Measurement of three-dimensional perioral soft tissue changes in dentoalveolar protrusion patients after orthodontic treatment using a structured light scanner. Angle Orthod. 2014, 84, 795–802. [Google Scholar] [CrossRef]
- Salisbury, S.T.; Buckley, C.P.; Zavatsky, A.B. Image-based non-contact method to measure cross-sectional areas and shapes of tendons and ligaments. Meas. Sci. Technol. 2008, 19. [Google Scholar] [CrossRef]
- Moon, D.K.; Abramowitch, S.D.; Woo, S.L.Y. The development and validation of a charge-coupled device laser reflectance system to measure the complex cross-sectional shape and area of soft tissues. J. Biomech. 2006, 39, 3071–3075. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ghassemi, P.; Wang, J.; Wang, Q.; Chen, Y.; Pfefer, J. Performance evaluation of CCD-and mobile-phone-based near-infrared fluorescence imaging systems with molded and 3D-printed phantoms. Des. Qual. Biomed. Technol. IX 2016, 9700, 970006. [Google Scholar] [CrossRef]
- Huang, C.; Irwin, D.; Lin, Y.; Shang, Y.; He, L.; Kong, W.; Luo, J.; Yu, G. Speckle contrast diffuse correlation tomography of complex turbid medium flow. Med. Phys. 2015, 42, 4000–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohut, P.; Holak, K.; Obuchowicz, R.; Ekiert, M.; Mlyniec, A.; Ambrozinski, L.; Tomaszewski, K.A.; Uhl, T. Modeling and identification of the mechanical properties of achilles tendon with application in health monitoring. J. Nondestr. Eval. Diagn. Progn. Eng. Syst. 2019, 2. [Google Scholar] [CrossRef]
- Li, C.; Dong, B.; Pan, B. A flexible and easy-to-implement single-camera microscopic 3D digital image correlation technique. Meas. Sci. Technol. 2019, 30. [Google Scholar] [CrossRef]
- Lionello, G.; Sirieix, C.; Baleani, M. An effective procedure to create a speckle pattern on biological soft tissue for digital image correlation measurements. J. Mech. Behav. Biomed. Mater. 2014, 39, 1–8. [Google Scholar] [CrossRef]
- Mallett, K.F.; Arruda, E.M. Digital image correlation-aided mechanical characterization of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Acta Biomater. 2017, 56, 44–57. [Google Scholar] [CrossRef]
- Feng, X.; Xue, F. Characterization of 3D printed bolts based on digital image correlation and infrared thermography. Mater. Des. 2020, 191, 108641. [Google Scholar] [CrossRef]
- Zheng, Q.; Mashiwa, N.; Furushima, T. Evaluation of large plastic deformation for metals by a non-contacting technique using digital image correlation with laser speckles. Mater. Des. 2020, 191, 108626. [Google Scholar] [CrossRef]
- Obuchowicz, R.; Ekiert, M.; Kohut, P.; Holak, K.; Ambrozinski, L.; Tomaszewski, K.A.; Uhl, T.; Mlyniec, A. Interfascicular matrix-mediated transverse deformation and sliding of discontinuous tendon subcomponents control the viscoelasticity and failure of tendons. J. Mech. Behav. Biomed. Mater. 2019, 97, 238–246. [Google Scholar] [CrossRef]
- Mao, H.; Rumpler, R.; Göransson, P. An inverse method for characterisation of the static elastic Hooke’s tensors of solid frame of anisotropic open-cell materials. Int. J. Eng. Sci. 2020, 147, 103198. [Google Scholar] [CrossRef]
- Mlyniec, A.; Tomaszewski, K.A.; Spiesz, E.M.; Uhl, T. Molecular-based nonlinear viscoelastic chemomechanical model incorporating thermal denaturation kinetics of collagen fibrous biomaterials. Polym. Degrad. Stab. 2015, 119, 87–95. [Google Scholar] [CrossRef]
- Shariff, M. On the spectral constitutive modelling of transversely isotropic soft tissue: Physical invariants. Int. J. Eng. Sci. 2017, 120, 199–219. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Tao, J.; Jia, B.; Li, D.; Zhai, Y. A viscoelastic constitutive relation for the rate-dependent mechanical behavior of rubber-like elastomers based on thermodynamic theory. Mater. Des. 2019, 178, 107876. [Google Scholar] [CrossRef]
- Bouaziz, R.; Ahosea, K.D.; Lejeunes, S.; Eyheramendy, D.; Sosson, F. Characterization and modeling of filled rubber submitted to thermal aging. Int. J. Solids Struct. 2019, 169, 122–140. [Google Scholar] [CrossRef]
- Adams, R.; Soe, S.P.; Santiago, R.; Robinson, M.; Hanna, B.; McShane, G.; Alves, M.; Burek, R.; Theobald, P. A novel pathway for efficient characterisation of additively manufactured thermoplastic elastomers. Mater. Des. 2019, 180, 107917. [Google Scholar] [CrossRef]
- Curless, B.; Levoy, M. Better optical triangulation through spacetime analysis. In Proceedings of the IEEE International Conference on Computer Vision, Cambridge, MA, USA, 20–23 June 1995; pp. 987–994. [Google Scholar] [CrossRef]
- Son, Y.; Yoon, S.; Oh, S.Y.; Han, S. A Lightweight and Cost-Effective 3D Omnidirectional Depth Sensor Based on Laser Triangulation. IEEE Access 2019, 7, 58740–58750. [Google Scholar] [CrossRef]
- Sansoni, G.; Trebeschi, M.; Docchio, F. State-of-the-art and applications of 3D imaging sensors in industry, cultural heritage, medicine, and criminal investigation. Sensors 2009, 9, 568–601. [Google Scholar] [CrossRef]
- Siekański, P.; Magda, K.; Malowany, K.; Rutkiewicz, J.; Styk, A.; Krzesłowski, J.; Kowaluk, T.; Zagórski, A. On-line laser triangulation scanner for wood logs surface geometry measurement. Sensors 2019, 19, 1074. [Google Scholar] [CrossRef] [Green Version]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source mesh processing tool. In Proceedings of the 6th Eurographics Italian Chapter Conference, Salerno, Italy, 22–25 January 2008; pp. 129–136. [Google Scholar]
- Imran, R.; Rogers, T.L. Resolving Reflection and Resolution in 3D Imaging of Fresh Bone. J. Forensic Sci. 2020, 65, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Haut, R.C. The influence of specimen length on the tensile failure properties of tendon collagen. J. Biomech. 1986, 19, 951–955. [Google Scholar] [CrossRef]
- Sanjeevi, R.; Somanathan, N.; Ramaswamy, D. A viscoelastic model for collagen fibres. J. Biomech. 1982, 15, 181–183. [Google Scholar] [CrossRef]
- Legerlotz, K.; Riley, G.P.; Screen, H.R.C. Specimen dimensions influence the measurement of material properties in tendon fascicles. J. Biomech. 2010, 43, 2274–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, W.; Shim, V.B.; Obst, S.; Lloyd, D.G.; Newsham-West, R.; Barrett, R.S. Achilles tendon stress is more sensitive to subject-specific geometry than subject-specific material properties: A finite element analysis. J. Biomech. 2017, 56, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kumar, A.; Berkson, E.; Verma, N.; Bach, B.R.; Hallab, N. A biomechanical analysis of bone-patellar tendon-bone grafts after repeat freeze-thaw cycles in a cyclic loading model. J. Knee Surg. 2009, 22, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Louis-Ugbo, J.; Leeson, B.; Hutton, W.C. Tensile Properties of Fresh Human Calcaneal (Achilles) Tendons. Clin. Anat. 2004, 17, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Udeze, C.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. Specialization of tendon mechanical properties results from interfascicular differences. J. R. Soc. Interface 2012, 9, 3108–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: The CAD models, as well as the software and validation data will be made available upon request. |

| Sample | True Value | 3D Model | Vision-Based | |||
|---|---|---|---|---|---|---|
| Number | CSA | CSA | Error | CSA | Error | |
| (Section) | (mm2) | (mm2) | (%) | (mm2) | (%) | |
| 1 (circular) | 9.46 ± 0.04 | 9.45 ± 0.02 | 0.06 | 10.02 ± 0.01 | 5.92 | p < 0.001 |
| 2 (circular) | 8.98 ± 0.04 | 9.02 ± 0.02 | 0.47 | 9.70 ± 0.09 | 8.00 | p < 0.001 |
| 3 (circular) | 2.32 ± 0.05 | 2.31 ± 0.02 | 0.13 | 2.47 ± 0.10 | 6.66 | p < 0.001 |
| 4 (hexagonal) | 8.06 ± 0.05 | 8.06 ± 0.02 | 0.07 | 8.44 ± 0.01 | 4.68 | p < 0.001 |
| 5 (hexagonal) | 6.61 ± 0.01 | 6.61 ± 0.06 | 0.01 | 6.90 ± 0.01 | 4.29 | p < 0.001 |
| 6 (hexagonal) | 3.34 ± 0.03 | 3.33 ± 0.02 | 0.30 | 3.37 ± 0.01 | 4.13 | p < 0.05 |
| Section | True Value | Reconstructed 3D Model | Vision-Based Method | |||
|---|---|---|---|---|---|---|
| Number | CSA | CSA | Error | CSA | Error | |
| (mm2) | (mm2) | (%) | (mm2) | (%) | ||
| 1 | 29.21 | 28.89 ± 0.16 | 1.12 | 35.56 ± 2.00 | 17.85 | p < 0.001 |
| 2 | 34.51 | 34.82 ± 0.42 | 0.90 | 39.10 ± 1.66 | 11.74 | p < 0.001 |
| 3 | 29.40 | 29.38 ± 0.51 | 0.06 | 44.01 ± 2.26 | 33.20 | p < 0.001 |
| 4 | 33.99 | 33.89 ± 0.51 | 0.15 | 45.89 ± 0.67 | 25.86 | p < 0.001 |
| 5 | 46.66 | 47.20 ± 0.18 | 1.13 | 67.99 ± 1.10 | 31.36 | p < 0.001 |
| 6 | 30.27 | 29.94 ± 0.34 | 1.10 | 45.20 ± 0.82 | 33.03 | p < 0.001 |
| Tendon | 3D Model | 3D Model | Vision-Based | ||
|---|---|---|---|---|---|
| Fascicle | Uncoated | Talc-Coated | Error | CSA | Error |
| Bundle | CSA (mm2) | CSA (mm2) | (%) | (mm2) | (%) |
| 1 | 22.97 ± 0.32 | 26.96 ± 0.69 | 17.37 | 24.85 ± 0.30 | 8.18 |
| 2 | 30.27 ± 0.36 | 34.82 ± 0.84 | 15.03 | 32.70 ± 0.32 | 8.00 |
| 3 | 26.05 ± 0.40 | 30.84 ± 0.93 | 18.39 | 28.42 ± 0.41 | 9.10 |
| 4 | 17.40 ± 0.28 | 20.95 ± 0.64 | 20.40 | 19.94 ± 0.28 | 14.60 |
| 5 | 20.57 ± 0.78 | 20.15 ± 0.73 | −2.04 | 23.65 ± 0.25 | 14.97 |
| 6 | 24.00 ± 0.76 | 27.02 ± 0.30 | 12.58 | 29.84 ± 0.78 | 24.33 |
| 7 | 17.98 ± 0.21 | 21.78 ± 0.53 | 21.13 | 19.86 ± 0.34 | 10.46 |
| 8 | 24.57 ± 0.29 | 28.27 ± 0.51 | 15.06 | 32.10 ± 0.45 | 30.65 |
| 9 | 14.88 ± 0.54 | 16.26 ± 0.37 | 9.27 | 18.88 ± 0.52 | 26.88 |
| 10 | 16.23 ± 0.61 | 19.70 ± 0.41 | 21.38 | 18.10 ± 0.41 | 11.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowska, S.; Ekiert, M.; Wojcik, K.; Kalemba, M.; Mlyniec, A. A 3D Scanning System for Inverse Analysis of Moist Biological Samples: Design and Validation Using Tendon Fascicle Bundles. Sensors 2020, 20, 3847. https://doi.org/10.3390/s20143847
Dabrowska S, Ekiert M, Wojcik K, Kalemba M, Mlyniec A. A 3D Scanning System for Inverse Analysis of Moist Biological Samples: Design and Validation Using Tendon Fascicle Bundles. Sensors. 2020; 20(14):3847. https://doi.org/10.3390/s20143847
Chicago/Turabian StyleDabrowska, Sylwia, Martyna Ekiert, Kaja Wojcik, Marek Kalemba, and Andrzej Mlyniec. 2020. "A 3D Scanning System for Inverse Analysis of Moist Biological Samples: Design and Validation Using Tendon Fascicle Bundles" Sensors 20, no. 14: 3847. https://doi.org/10.3390/s20143847
APA StyleDabrowska, S., Ekiert, M., Wojcik, K., Kalemba, M., & Mlyniec, A. (2020). A 3D Scanning System for Inverse Analysis of Moist Biological Samples: Design and Validation Using Tendon Fascicle Bundles. Sensors, 20(14), 3847. https://doi.org/10.3390/s20143847





