Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensor Materials and Design
2.2. The Microfabrication Process
2.3. Temperature Measurement Circuit and Setup
2.4. In Situ Cantilever Curvature Measurement System
2.5. Reagents
3. Results
3.1. Microfabricated Temperature Sensors
3.2. Temperature Sensing Analysis
3.3. The Funtional Stability of Sensors
3.3.1. Thermal Stability
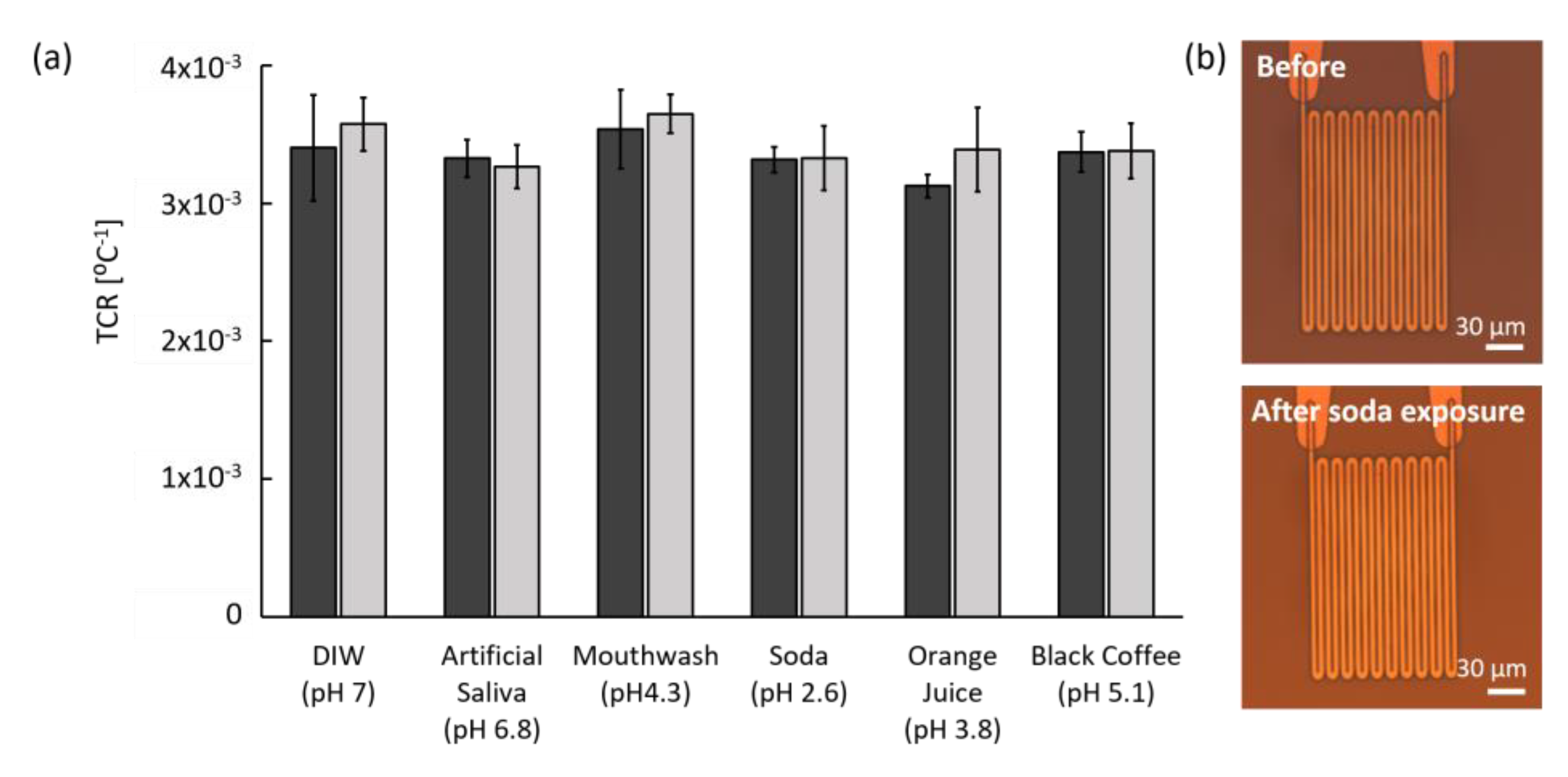
3.3.2. Chemical Stability
3.3.3. Mechanical Stability
3.4. The Physical Stability of Polyimide Film
3.4.1. Thermal Stress Measurement
3.4.2. Humidity Stress Measurement
4. Discussion
- Efficient localized monitoring. Compared to other saliva sensors, our sensor is small but efficient for onsite sensing with multi-channels around dental implants. The sensor can retrieve localized information from proximity soft tissue, where the inflammation is likely to occur.
- Flexible sensor design. By changing photomasks, additional channels can be easily integrated on the sensor without any outline change. Furthermore, other electrochemical or biological sensors could be integrated with the temperature sensor for various parameter sensing such as pH, bacteria, or specific biomarkers for advanced diagnosis.
- No calibration requirement. Unlike most lab-made sensors, our temperature sensor does not require calibration before measurement because it monitors the baseline change of temperature for a long period of time and is based on a reproducible resistance–temperature relationship. This strategy is beneficial because intraoral temperature is patient and location specific, and transient temperature changes due to events like drinking hot or cold beverages are not significant for disease diagnosis. Therefore, our sensor is effective for long-term monitoring of relative temperature changes.
- Safe for long-term implantation. Our sensor is built with previously tested biocompatible materials and shows a constant TCR value throughout testing without polymer film damage. In addition, the sensor pattern shows no deformation after exposure to both DI water and artificial saliva for two months at 37 °C. Furthermore, preliminary data using Streptococcus mutans (strain UA159) shows that biofilm formation on the sensor induced negligible temperature difference.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramanauskaite, A.; Juodzbalys, G. Diagnostic principles of peri-implantitis: A systematic review and guidelines for peri-implantitis diagnosis proposal. J. Oral Maxillofac. Res. 2016, 7. [Google Scholar] [CrossRef]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T.; HeitzMayfield, L.J.; Pjetursson, B.E.; Salvi, G.E.; Sanz, M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int. J. Oral Maxillofac. Implant. 2004, 19, 150–154. [Google Scholar]
- Froum, S.J.; Rosen, P.S. A proposed classification for peri-implantitis. Int. J. Periodontics Restor. Dent. 2012, 32, 533. [Google Scholar]
- Padial-Molina, M.; Suarez, F.; Rios, H.F.; Galindo-Moreno, P.; Wang, H.-L. Guidelines for the diagnosis and treatment of peri-implant diseases. Int. J. Periodontics Restor. Dent. 2014, 34, 102–111. [Google Scholar] [CrossRef]
- Lee, D.-S.; Jeon, B.G.; Ihm, C.; Park, J.-K.; Jung, M.Y. A simple and smart telemedicine device for developing regions: A pocket-sized colorimetric reader. Lab A Chip 2011, 11, 120–126. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Kuan, C.-M.; Chen, C.-F. In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Graf, H.; Mühlemann, H. Telemetry of plaque pH from interdental area. Helv. Odontol. Acta 1966, 10, 94. [Google Scholar]
- Quadir, N.A.; Albasha, L.; Taghadosi, M.; Qaddoumi, N.; Hatahet, B. Low-power implanted sensor for orthodontic bond failure diagnosis and detection. IEEE Sens. J. 2018, 18, 3003–3009. [Google Scholar] [CrossRef]
- Graf, H.; Mühlemann, H. Oral telemetry of fluoride ion activity. Arch. Oral Biol. 1969, 14, 259–263. [Google Scholar] [CrossRef]
- Li, X.; Luo, C.; Fu, Q.; Zhou, C.; Ruelas, M.; Wang, Y.; He, J.; Wang, Y.; Zhang, Y.S.; Zhou, J. A transparent, wearable fluorescent mouthguard for high-sensitive visualization and accurate localization of hidden dental lesion sites. Adv. Mater. 2020, 32, 2000060. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Koh, A.; Gutbrod, S.R.; Meyers, J.D.; Lu, C.; Webb, R.C.; Shin, G.; Li, Y.; Kang, S.K.; Huang, Y.; Efimov, I.R. Ultrathin injectable sensors of temperature, thermal conductivity, and heat capacity for cardiac ablation monitoring. Adv. Healthc. Mater. 2016, 5, 373–381. [Google Scholar] [CrossRef]
- Chaimanonart, N.; Young, D.J. A wireless batteryless in vivo EKG and body temperature sensing microsystem with adaptive RF powering for genetically engineered mice monitoring. In Proceedings of the 15th International Conference on Solid-State Sensors, Actuators and Microsystems, Dever, CO, USA, 21–25 June 2009; pp. 1473–1476. [Google Scholar]
- Kang, S.-K.; Murphy, R.K.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938. [Google Scholar] [CrossRef]
- Lou, D.; Pang, Q.; Pei, X.; Dong, S.; Li, S.; Tan, W.Q.; Ma, L. Flexible wound healing system for pro-regeneration, temperature monitoring and infection early warning. Biosens. Bioelectron 2020, 162, 112275. [Google Scholar] [CrossRef]
- Kung, R.T.; Ochs, B.; Goodson, J. Temperature as a periodontal diagnostic. J. Clin. Periodontol. 1990, 17, 557–563. [Google Scholar] [CrossRef]
- Choi, J.E.; Lyons, K.M.; McLean, M.C.; Waddell, N.J. Interarch comparison of intraoral pH and temperature: A pilot study. BDJ Open 2016, 2, 16008. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Garbarini, L.; Kaplan, D.L.; Omenetto, F.G. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater. 2018, 30, 1703257. [Google Scholar] [CrossRef] [PubMed]
- Moser, Y.; Gijs, M.A. Miniaturized flexible temperature sensor. J. Microelectromechanical Syst. 2007, 16, 1349–1354. [Google Scholar] [CrossRef]
- Sun, Y.; Lacour, S.; Brooks, R.; Rushton, N.; Fawcett, J.; Cameron, R. Assessment of the biocompatibility of photosensitive polyimide for implantable medical device use. J. Biomed. Mater. Res. Part. A 2009, 90, 648–655. [Google Scholar] [CrossRef]
- Tyson, J.; Tran, M.; Slaughter, G. Biocompatibility of a quad-shank neural probe. Solid-State Electron. 2017, 136, 113–119. [Google Scholar] [CrossRef]
- Kireev, D.; Zadorozhnyi, I.; Qiu, T.; Sarik, D.; Brings, F.; Wu, T.; Seyock, S.; Maybeck, V.; Lottner, M.; Blaschke, B.M.; et al. Graphene field-effect transistors for in vitro and ex vivo recordings. IEEE Trans. Nanotechnol. 2016, 16, 140–147. [Google Scholar] [CrossRef]
- Markov, A.; Maybeck, V.; Wolf, N.; Mayer, D.; Offenhäusser, A.; Wördenweber, R. Engineering of neuron growth and enhancing cell-chip communication via mixed SAMs. J. Acs Appl. Mater. Interfaces 2018, 10, 18507–18514. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Shin, J.W.; Eastman, S.A.; Rowe, B.W.; Kim, S.; Kusoglu, A.; Yager, K.G.; Stafford, G.R. In situ method for measuring the mechanical properties of Nafion thin films during hydration cycles. Acs Appl. Mater. Interfaces 2015, 7, 17874–17883. [Google Scholar] [CrossRef]
- Frieberg, B.R.; Page, K.A.; Graybill, J.R.; Walker, M.L.; Stafford, C.M.; Stafford, G.R.; Soles, C.L. Mechanical response of thermally annealed nafion thin films. Acs Appl. Mater. Interfaces 2016, 8, 33240–33249. [Google Scholar] [CrossRef] [PubMed]
- Floro, J.; Chason, E.; Lee, S.; Twesten, R.; Hwang, R.; Freund, L. Real-time stress evolution during Si1-x Gex heteroepitaxy: Dislocations, islanding, and segregation. J. Electron. Mater. 1997, 26, 969–979. [Google Scholar] [CrossRef]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Available online: https://www.jstor.org/stable/92886?seq=1#metadata_info_tab_contents (accessed on 14 July 2020).
- Levallois, B.; Fovet, Y.; Lapeyre, L.; Gal, J.Y. In vitro fluoride release from restorative materials in water versus artificial saliva medium (SAGF). Dent Mater 1998, 14, 441–447. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2017, 143, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Lyons, K.M.; Kieser, J.A.; Waddell, N.J. Diurnal variation of intraoral pH and temperature. BDJ Open 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed]
- Fierheller, M.; Sibbald, R.G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Ski. Wound Care 2010, 23, 369–379. [Google Scholar] [CrossRef]
- Fedi Jr, P.F.; Killoy, W.J. Temperature differences at periodontal sites in health and disease. J. Periodontol. 1992, 63, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Chanmugam, A.; Langemo, D.; Thomason, K.; Haan, J.; Altenburger, E.A.; Tippett, A.; Henderson, L.; Zortman, T.A. Relative temperature maximum in wound infection and inflammation as compared with a control subject using long-wave infrared thermography. Adv. Ski. Wound Care 2017, 30, 406–414. [Google Scholar] [CrossRef]
- Li, Y.-J.; Lu, C.-C. A novel scheme and evaluations on a long-term and continuous biosensor platform integrated with a dental implant fixture and its prosthetic abutment. Sensors 2015, 15, 24961–24976. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chen, Y.-C.; Chen, W.-J.; Huang, P.; Chu, H.-h. Sensor-embedded teeth for oral activity recognition. In Proceedings of the 2013 International Symposium on Wearable Computers, Zurich, Switzerland, 8–12 September 2013; pp. 41–44. [Google Scholar]
- Song, J.K.; Cho, T.H.; Pan, H.; Song, Y.M.; Kim, I.S.; Lee, T.H.; Hwang, S.J.; Kim, S.J. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics 2009, 30, 374–384. [Google Scholar] [CrossRef]
- Amar, A.B.; Kouki, A.B.; Cao, H. Power approaches for implantable medical devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Albasha, L.; Qaddoumi, N.; Hatahet, B.; Quadir, N.; Taghadosi, M. Design Challenges in Wireless Sensors for Dental Applications. In The IoT Physical Layer; Springer: Cham, Switzerland, 2019; pp. 105–126. [Google Scholar]
- Badr, B.M.; Somogyi-Csizmazia, R.; Leslie, P.; Delaney, K.R.; Dechev, N. Design of a wireless measurement system for use in wireless power transfer applications for implants. Wirel. Power Transf. 2017, 4, 21–32. [Google Scholar] [CrossRef]
- Ahn, D.; Ghovanloo, M. Optimal design of wireless power transmission links for millimeter-sized biomedical implants. Ieee Trans. Biomed. Circuits Syst. 2016, 10, 125–137. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.J.; Stafford, G.R.; Beauchamp, C.; Kim, S.A. Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease. Sensors 2020, 20, 3953. https://doi.org/10.3390/s20143953
Kim JJ, Stafford GR, Beauchamp C, Kim SA. Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease. Sensors. 2020; 20(14):3953. https://doi.org/10.3390/s20143953
Chicago/Turabian StyleKim, Jeffrey J., Gery R. Stafford, Carlos Beauchamp, and Shin Ae Kim. 2020. "Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease" Sensors 20, no. 14: 3953. https://doi.org/10.3390/s20143953
APA StyleKim, J. J., Stafford, G. R., Beauchamp, C., & Kim, S. A. (2020). Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease. Sensors, 20(14), 3953. https://doi.org/10.3390/s20143953




