Multi-Beat Averaging Reveals U Waves Are Ubiquitous and Standing Tall at Elevated Heart Rates Following Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. ECG Recordings
2.2. Beat Averaging Algorithm to Recover U Waves
2.3. Measurement of U and T Wave Amplitudes
2.4. Statistical Analysis
3. Results
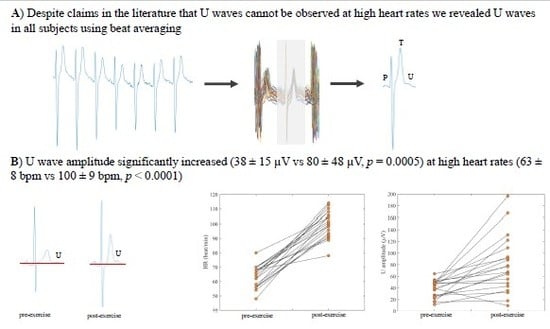
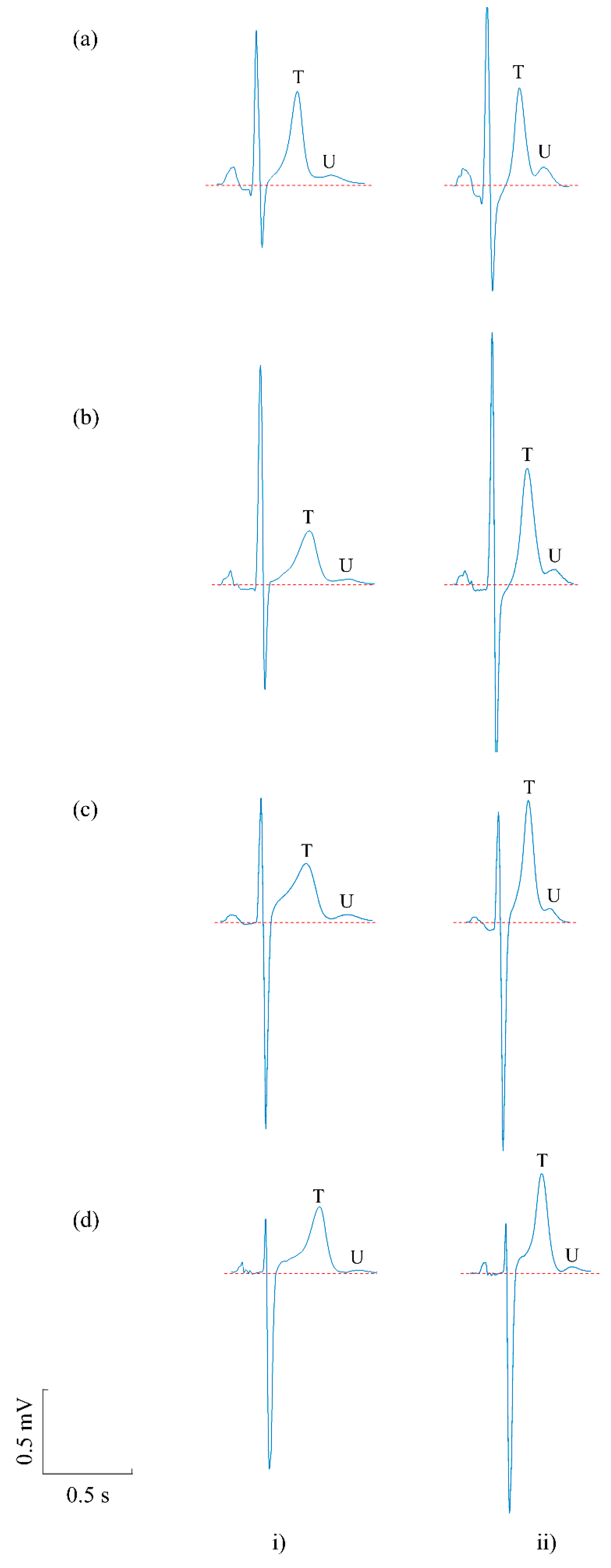
3.1. Prevalence of U Waves in Pre- and Post-Exercise Recordings
3.2. The Effect of Exercise on U Waves
4. Discussion
4.1. Prevalence of U Waves in Post-Exercise Recordings
4.2. The Effect of Exercise on U Waves
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Surawicz, B. U wave: Facts, hypotheses, misconceptions, and misnomers. J. Cardiovasc. Electrophysiol. 1998, 9, 1117–1128. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U saves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias C. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez Riera, A.R.; Ferreira, C.; Filho, C.F.; Ferreira, M.; Meneghini, A.; Uchida, A.H.; Schapachnik, E.; Dubner, S.; Zhang, L. The enigmatic sixth wave of the electrocardiogram: The U wave. Cardiol. J. 2008, 15, 408–421. [Google Scholar]
- Di Stolfo, G.; Mastroianno, S.; Amico, C.; Salvatori, M.; Pacilli, M.; Fanelli, M.; D’arienzo, C.; Massaro, R.; Potenza, D.; Russo, A.; et al. Electrical remodeling of repolarization abnormality after surgical treatment in pheochromocytoma: A retrospective analysis. EP Eur. 2016, 18, i166. [Google Scholar] [CrossRef]
- Al-Karadi, M.S.; Wilkinson, A.J.; Caldwell, J.; Langley, P. Validation of an algorithm to reveal the U wave in atrial fibrillation. Sci. Rep. 2018, 8, 11946. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, B.; Kemp, R.L.; Bellet, S. Polarity and Amplitude of the U Wave of the Electrocardiogram in Relation to that of the T Wave. Circulation 1957, 15, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Figueredo, V.M.; Greenspan, A.M.; Pressman, G.S. Giant U waves: An important clinical clue. Res. Rep. Clin. Cardiol. 2011, 2, 51. [Google Scholar] [CrossRef] [Green Version]
- Schimpf, R.; Antzelevitch, C.; Haghi, D.; Giustetto, C.; Pizzuti, A.; Gaita, F.; Veltmann, C.; Wolpert, C.; Borggrefe, M. Electromechanical coupling in patients with the short QT syndrome: Further insights into the mechanoelectrical hypothesis of the U wave. Hear. Rhythm. 2008, 5, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, P.; Di Bernardo, D.; Murray, A. Quantification of T wave shape changes following exercise. PACE Pacing Clin. Electrophysiol. 2002, 25, 1230–1234. [Google Scholar] [CrossRef]
- Klabunde, R. Cardiovascular Physiology Concepts, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; ISBN 9781451113846. [Google Scholar]
- Gomes, J.A. Signal Averaged Electrocardiography: Concepts, Methods and Applications; Springer: Dordrecht, The Netherlands, 1993; ISBN1 0792323904. ISBN2 9780792323907. [Google Scholar]
- Jané, R.; Rix, H.; Caminal, P.; Laguna, P. Alignment methods for averaging of high resolution cardiac signals: A comparative study of performance. IEEE Trans. Biomed. Eng. 1991, 38, 571–579. [Google Scholar] [CrossRef]
- Rompelman, O.; Ros, H.H. Coherent averaging technique: A tutorial review Part 1: Noise reduction and the equivalent filter. J. Biomed. Eng. 1986, 8, 24–29. [Google Scholar] [CrossRef]
- Kohl, P.; Schimpf, R.; Borggrefe, M. Is the U wave in the Electrocardiogram a Mechano-Electrical Phenomenon? In Cardiac Mechano-Electric Coupling and Arrhythmias; OUP: Oxford, UK, 2011; pp. 274–280. ISBN1 0199570167. ISBN2 9780199570164. [Google Scholar]
- Ishida, Y.; Meisner, J.S.; Tsujioka, K.; Gallo, J.I.; Yoran, C.; Frater, R.W.; Yellin, E.L. Left ventricular filling dynamics: Influence of left ventricular relaxation and left atrial pressure. Circulation 1986, 74, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bers, D.M. Cardiac Excitation–Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P.; Bollensdorff, C.; Garny, A. Effects of mechanosensitive ion channels on ventricular electrophysiology: Experimental and theoretical models. Exp. Physiol. 2006, 91, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Murray, A. Origin on the electrocardiogram of U-waves and abnormal U-wave inversion. Cardiovasc. Res. 2002, 53, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Arteyeva, N.V.; Azarov, J.E. Effect of action potential duration on Tpeak-Tend interval, T-wave area and T-wave amplitude as indices of dispersion of repolarization: Theoretical and simulation study in the rabbit heart. J. Electrocardiol. 2017, 50, 919–924. [Google Scholar] [CrossRef]
- Bernardo, D.; Langley, P.; Murray, A. Effect of changes in heart rate and in action potential duration on the electrocardiogram T wave shape. Physiol. Meas. 2002, 23, 355–364. [Google Scholar] [CrossRef]
- Antzelevitch, C. Cellular basis for the repolarization waves of the ECG. Ann. N. Y. Acad. Sci. 2006, 1080, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Gerson, M.C.; Phillips, J.F.; Morris, S.N.; McHenry, P.L. Exercise-induced U-wave inversion as a marker of stenosis of the left anterior descending coronary artery. Circulation 1979, 60, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveendran, S.; Hadfield, R.; Petkar, S.; Malik, N. Significance of Exercise Induced U Wave Inversion as a Marker for Coronary Artery Disease; BMJ Case Reports; BMJ Publishing Group: London, UK, 2012; Volume 2012. [Google Scholar]
- Kishida, H.; Cole, J.S.; Surawicz, B. Negative U wave: A highly specific but poorly understood sign of heart disease. Am. J. Cardiol. 1982, 49, 2030–2036. [Google Scholar] [CrossRef]
- Tai Fu, L.; Kato, N.; Takahashi, N. Ischaemia-induced negative U waves in electrocardiograms (an experimental study in canine hearts). Cardiovasc. Res. 1982, 16, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sovari, A.A.; Farokhi, F.; Kocheril, A.G. Inverted U wave, a specific electrocardiographic sign of cardiac ischemia. Am. J. Emerg. Med. 2007, 25, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, Y.; Yufu, T.; Yumoto, S. Inverted U-wave and myocardial ischemia. QJM 2018, 111, 493. [Google Scholar] [CrossRef] [PubMed]
- Yasue, H.; Nakagawa, H.; Itoh, T.; Harada, E.; Mizuno, Y. Coronary artery spasm—Clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 2008, 51, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Surawicz, B.; Knilans, T. QT Interval, U Wave Abnormalities, and Cardiac Alternans. In Chou’s Electrocardiography in Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; pp. 569–585. ISBN 9781416037743. [Google Scholar]
- Kirchhof, P.; Franz, M.R.; Bardai, A.; Wilde, A.M. Giant T–U Waves Precede Torsades de Pointes in Long QT Syndrome. J. Am. Coll. Cardiol. 2009, 54, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Fioretti, P.; Brower, R.W.; Meester, G.T.; Serruys, P.W. Interaction of left ventricular relaxation and filling during early diastole in human subjects. Am. J. Cardiol. 1980, 46, 197–203. [Google Scholar] [CrossRef] [Green Version]




| Subject | Lead | Pre-Exercise | Post-Exercise | ∆ Amplitudes (µV) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (bpm) | U Amp (µV) | T Amp (µV) | HR (bpm) | U Amp (µV) | T Amp (µV) | ∆U | ∆T | ||
| 1 | V4 | 70 | 48 | 654 | 98 | 48 | 601 | 0 | −53 |
| 2 | V2 | 56 | 48 | 413 | 98 | 93 | 867 | 45 | 454 |
| 3 | V2 | 54 | 11 | 387 | 94 | 35 | 588 | 24 | 201 |
| 4 | V4 | 64 | 39 | 789 | 108 | 78 | 726 | 39 | −63 |
| 5 | V4 | 48 | 47 | 720 | 106 | 168 | 890 | 121 | 170 |
| 6 | V4 | 62 | 54 | 550 | 100 | 109 | 579 | 55 | 29 |
| 7 | V4 | 56 | 36 | 321 | 91 | 88 | 688 | 52 | 367 |
| 8 | V4 | 66 | 28 | 1226 | 110 | 43 | 996 | 15 | −230 |
| 9 | V4 | 70 | 27 | 278 | 104 | 32 | 482 | 5 | 204 |
| 10 | V4 | 56 | 47 | 927 | 113 | 197 | 1085 | 150 | 158 |
| 11 | V2 | 68 | 39 | 722 | 78 | 9 | 562 | −30 | −160 |
| 12 | V2 | 56 | 64 | 734 | 92 | 91 | 774 | 27 | 40 |
| 13 | V2 | 68 | 53 | 503 | 101 | 67 | 621 | 14 | 118 |
| 14 | V4 | 66 | 17 | 413 | 108 | 122 | 592 | 105 | 179 |
| 15 | V4 | 62 | 50 | 739 | 114 | 77 | 744 | 27 | 5 |
| 16 | V4 | 70 | 48 | 704 | 102 | 55 | 857 | 7 | 153 |
| 17 | V4 | 58 | 22 | 284 | 96 | 67 | 503 | 45 | 219 |
| 18 | V4 | 80 | 43 | 551 | 104 | 131 | 603 | 88 | 52 |
| 19 | V4 | 68 | 25 | 243 | 90 | 64 | 709 | 39 | 466 |
| 20 | V4 | 68 | 16 | 221 | 89 | 18 | 418 | 2 | 197 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Karadi, M.S.; Langley, P. Multi-Beat Averaging Reveals U Waves Are Ubiquitous and Standing Tall at Elevated Heart Rates Following Exercise. Sensors 2020, 20, 4029. https://doi.org/10.3390/s20144029
Al-Karadi MS, Langley P. Multi-Beat Averaging Reveals U Waves Are Ubiquitous and Standing Tall at Elevated Heart Rates Following Exercise. Sensors. 2020; 20(14):4029. https://doi.org/10.3390/s20144029
Chicago/Turabian StyleAl-Karadi, Marwa S., and Philip Langley. 2020. "Multi-Beat Averaging Reveals U Waves Are Ubiquitous and Standing Tall at Elevated Heart Rates Following Exercise" Sensors 20, no. 14: 4029. https://doi.org/10.3390/s20144029
APA StyleAl-Karadi, M. S., & Langley, P. (2020). Multi-Beat Averaging Reveals U Waves Are Ubiquitous and Standing Tall at Elevated Heart Rates Following Exercise. Sensors, 20(14), 4029. https://doi.org/10.3390/s20144029