Development of an Inverted Epifluorescence Microscope for Long-Term Monitoring of Bacteria in Multiplexed Microfluidic Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging System Design and Components
2.1.1. General Setup
2.1.2. Excitation Path
2.1.3. Emission Path
2.1.4. Software
2.2. Microdevice Fabrication
2.3. Time-Lapse Imaging of Fluorescent Beads
2.4. Cell Culture for Single-Cell Analysis Applications
3. Results
3.1. Real Setup and Microscope Characterization
3.2. Time-Lapse Imaging of The Microfluidic Devices
3.3. Applications for Single-Cell Analysis
4. Discussion
Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Syal, K.; Mo, M.; Yu, H.; Iriya, R.; Jing, W.; Guodong, S.; Wang, S.; Grys, T.E.; Haydel, S.E.; Tao, N. Current and emerging techniques for antibiotic susceptibility tests. Theranostics 2017, 7, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Baltekin, Ö.; Boucharin, A.; Tano, E.; Andersson, D.I.; Elf, J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc. Natl. Acad. Sci. USA 2017, 114, 9170–9175. [Google Scholar] [CrossRef] [PubMed]
- Karale, C.K.; Nikumbh, K.K.; Wagh, D.S.; Thorat, S.S. Microfluidics in Drug Discovery: An Overview. Inventi Rapid Pharm. Process Dev. 2013, 2013, 15. [Google Scholar]
- Salim, A.; Lim, S. Review of Recent Metamaterial Microfluidic Sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Njoloma, J.P.; Oota, M.; Saeki, Y.; Akao, S. Detection of gfp expression from gfp-labelled bacteria spot inoculated onto sugarcane tissues. Afr. J. Biotechnol. 2005, 4, 7. [Google Scholar]
- Qiu, Y.; Zhang, J.; Li, B.; Wen, X.; Liang, P.; Huang, X. A novel microfluidic system enables visualization and analysis of antibiotic resistance gene transfer to activated sludge bacteria in biofilm. Sci. Total Environ. 2018, 642, 582–590. [Google Scholar] [CrossRef]
- Sabhachandani, P.; Sarkar, S.; Zucchi, P.C.; Whitfield, B.A.; Kirby, J.E.; Hirsch, E.B.; Konry, T. Integrated microfluidic platform for rapid antimicrobial susceptibility testing and bacterial growth analysis using bead-based biosensor via fluorescence imaging. Microchim. Acta 2017, 184, 4619–4628. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and Emerging Methods of Antibiotic Susceptibility Testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- Mohan, R.; Sanpitakseree, C.; Desai, A.V.; Sevgen, S.E.; Schroeder, C.M.; Kenis, P.J.A. A microfluidic approach to study the effect of bacterial interactions on antimicrobial susceptibility in polymicrobial cultures. RSC Adv. 2015, 5, 35211–35223. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Ceri, H.; Oliveira, R. Evaluation of antimicrobial activity of certain combinations of antibiotics against in vitro Staphylococcus epidermidis biofilms. Indian J. Med. Res. 2012, 135, 542. [Google Scholar] [PubMed]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Bacterial Viability in Starved and Revitalized Biofilms: Comparison of Viability Staining and Direct Culture. J. Endod. 2010, 36, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Deore, A.B.; Dhumane, J.R.; Wagh, R.; Sonawane, R. The Stages of Drug Discovery and Development Process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar] [CrossRef]
- Sarrion-Perdigones, A.; Chang, L.; Gonzalez, Y.; Gallego-Flores, T.; Young, D.W.; Venken, K.J.T. Examining multiple cellular pathways at once using multiplex hextuple luciferase assaying. Nat. Commun. 2019, 10, 5710. [Google Scholar] [CrossRef] [PubMed]
- Manina, G.; Griego, A.; Singh, L.K.; McKinney, J.D.; Dhar, N. Preexisting variation in DNA damage response predicts the fate of single mycobacteria under stress. EMBO J. 2019, 38, e101876. [Google Scholar] [CrossRef]
- He, J.; Mu, X.; Guo, Z.; Hao, H.; Zhang, C.; Zhao, Z.; Wang, Q. A novel microbead-based microfluidic device for rapid bacterial identification and antibiotic susceptibility testing. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2223–2230. [Google Scholar] [CrossRef]
- Mohan, R.; Mukherjee, A.; Sevgen, S.E.; Sanpitakseree, C.; Lee, J.; Schroeder, C.M.; Kenis, P.J. A multiplexed microfluidic platform for rapid antibiotic susceptibility testing. Biosens. Bioelectron. 2013, 49, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wistrand-Yuen, P.; Malmberg, C.; Fatsis-Kavalopoulos, N.; Lübke, M.; Tängdén, T.; Kreuger, J. A Multiplex Fluidic Chip for Rapid Phenotypic Antibiotic Susceptibility Testing. mBio 2020, 11. [Google Scholar] [CrossRef]
- Bullen, A. Microscopic imaging techniques for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 54–67. [Google Scholar] [CrossRef]
- Bian, Z.; Jiang, S.; Song, P.; Zhang, H.; Hoveida, P.; Hoshino, K.; Zheng, G. Ptychographic modulation engine: A low-cost DIY microscope add-on for coherent super-resolution imaging. J. Phys. Appl. Phys. 2019, 53, 014005. [Google Scholar] [CrossRef]
- Webb, D.J.; Brown, C.M. Epi-fluorescence microscopy. Methods Mol. Biol. Clifton NJ 2013, 931, 29–59. [Google Scholar] [CrossRef]
- Combs, C.A.; Shroff, H. Fluorescence Microscopy: A Concise Guide to Current Imaging Methods. Curr. Protoc. Neurosci. 2017, 79, 2.1.1–2.1.25. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Moran, B.M.; Woehl, J.C. Development of a confocal scanning microscope for fluorescence imaging and spectroscopy at variable temperatures. Rev. Sci. Instrum. 2019, 90, 043702. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhaes, P.J.; Ram, S. J Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Piruska, A.; Nikcevic, I.; Lee, S.H.; Ahn, C.; Heineman, W.R.; Limbach, P.A.; Seliskar, C.J. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab. Chip 2005, 5, 1348–1354. [Google Scholar] [CrossRef]
- Escámez, M.J.; Carretero, M.; García, M.; Martínez-Santamaría, L.; Mirones, I.; Duarte, B.; Holguín, A.; García, E.; García, V.; Meana, A.; et al. Assessment of Optimal Virus-Mediated Growth Factor Gene Delivery for Human Cutaneous Wound Healing Enhancement. J. Investig. Dermatol. 2008, 128, 1565–1575. [Google Scholar] [CrossRef][Green Version]
- Hell, S.W.; Schrader, M.; van der Voort, H.T.M. Far-field fluorescence microscopy with three-dimensional resolution in the 100-nm range. J. Microsc. 1997, 187, 1–7. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Sha, J.; Zhang, Z.; Tu, Q.; Chen, P.; Wang, J. High-throughput microfluidic system for long-term bacterial colony monitoring and antibiotic testing in zero-flow environments. Biosens. Bioelectron. 2011, 26, 1993–1999. [Google Scholar] [CrossRef]
- Souza, A.; Ribeiro, J.; Araújo, F. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Kim, B.; Kang, D.; Choi, S. Handheld Microflow Cytometer Based on a Motorized Smart Pipette, a Microfluidic Cell Concentrator, and a Miniaturized Fluorescence Microscope. Sensors 2019, 19, 2761. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction, Global Edition; Pearson Education Limited: London, UK, 2015. [Google Scholar]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. MMBR 1998, 62, 1094–1156. [Google Scholar] [CrossRef] [PubMed]
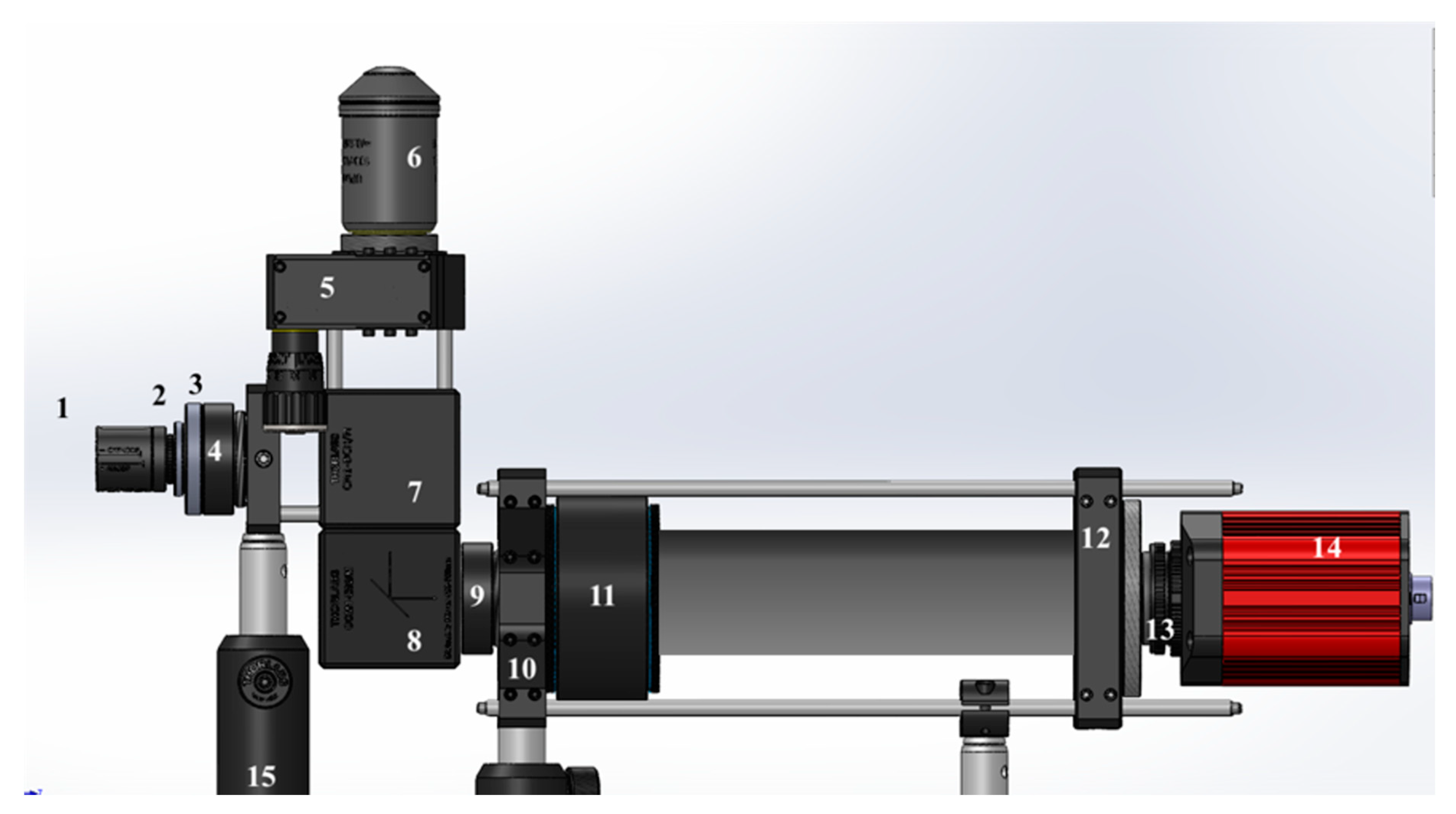





| Number | Component | Model |
|---|---|---|
| 1 | Blue LED | LUMILEDS blue light-emitting diode. |
| 2 | Glass Ground Diffuser | Thorlabs Unmounted N-BK7 Ground Glass Diffuser DG10-220 |
| 3 | Condensing Lens: achromatic doublets lens | Thorlabs AC254-030-A |
| 4 | Excitation Filter | Thorlabs MF469-35 |
| 5 | Tunable lens | Optotune EL-16-40-TC |
| 6 | Magnification Objective | Either Olympus UPlanFLN 100× or Motic CCIS Plan achromatic phase objective UC Ph2 20× |
| 7 | Dichroic Mirror | Thorlabs MD498 |
| 8 | Dielectric Turning Mirror | Thorlabs CCM1-E02/M 30 |
| 9 | Emission Filter | Thorlabs MF525-35 |
| 10 | Cage Adapter | Thorlabs LCP02/M |
| 11 | Tube Lens | Thorlabs TTL180-A |
| 12 | Cage Adapter | Thorlabs LCP01/M |
| 13 | C-mount Adapter | Thorlabs SM2A31 |
| 14 | CCD Sensor | Allied Vision Manta G-145B NIR CCD Camera |
| 15 | Post holder |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Simón, A.; Marino, M.H.; Gómez-Cruz, C.; Cañadas, M.; Marco, M.; Ripoll, J.; Vaquero, J.J.; Muñoz-Barrutia, A. Development of an Inverted Epifluorescence Microscope for Long-Term Monitoring of Bacteria in Multiplexed Microfluidic Devices. Sensors 2020, 20, 4140. https://doi.org/10.3390/s20154140
Torres-Simón A, Marino MH, Gómez-Cruz C, Cañadas M, Marco M, Ripoll J, Vaquero JJ, Muñoz-Barrutia A. Development of an Inverted Epifluorescence Microscope for Long-Term Monitoring of Bacteria in Multiplexed Microfluidic Devices. Sensors. 2020; 20(15):4140. https://doi.org/10.3390/s20154140
Chicago/Turabian StyleTorres-Simón, Amaro, María Henar Marino, Clara Gómez-Cruz, Marina Cañadas, Miguel Marco, Jorge Ripoll, Juan José Vaquero, and Arrate Muñoz-Barrutia. 2020. "Development of an Inverted Epifluorescence Microscope for Long-Term Monitoring of Bacteria in Multiplexed Microfluidic Devices" Sensors 20, no. 15: 4140. https://doi.org/10.3390/s20154140
APA StyleTorres-Simón, A., Marino, M. H., Gómez-Cruz, C., Cañadas, M., Marco, M., Ripoll, J., Vaquero, J. J., & Muñoz-Barrutia, A. (2020). Development of an Inverted Epifluorescence Microscope for Long-Term Monitoring of Bacteria in Multiplexed Microfluidic Devices. Sensors, 20(15), 4140. https://doi.org/10.3390/s20154140





