Machine Learning for Seed Quality Classification: An Advanced Approach Using Merger Data from FT-NIR Spectroscopy and X-ray Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
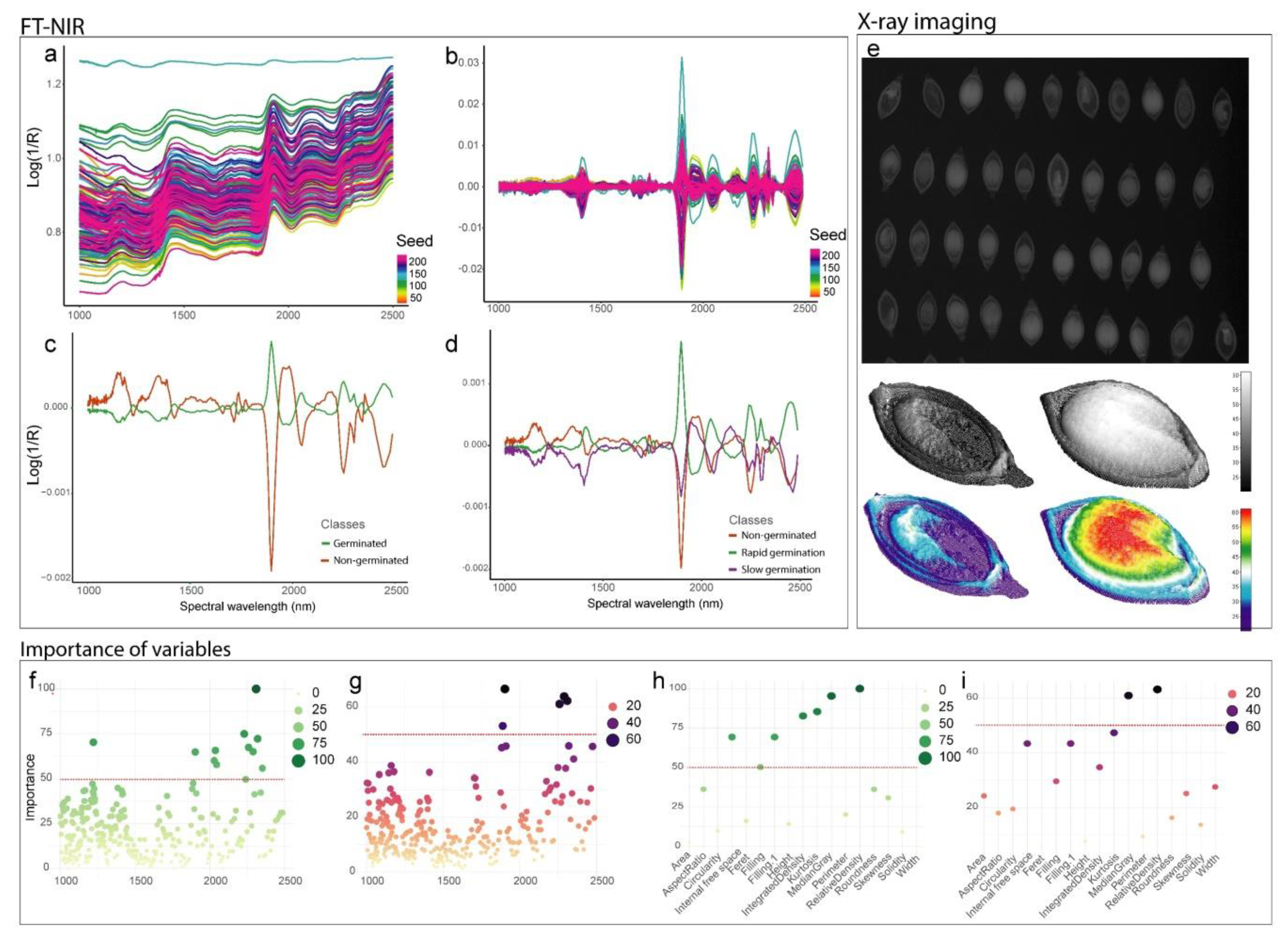
2.2. NIR Data Collection and Preprocessing
2.3. X-Ray Imaging
2.4. Physiological Analysis
2.5. Machine Learning for Seed Quality Classification
2.5.1. Germination and Vigor Classes
2.5.2. Machine Learning Methods
2.5.3. Model Validation
3. Results
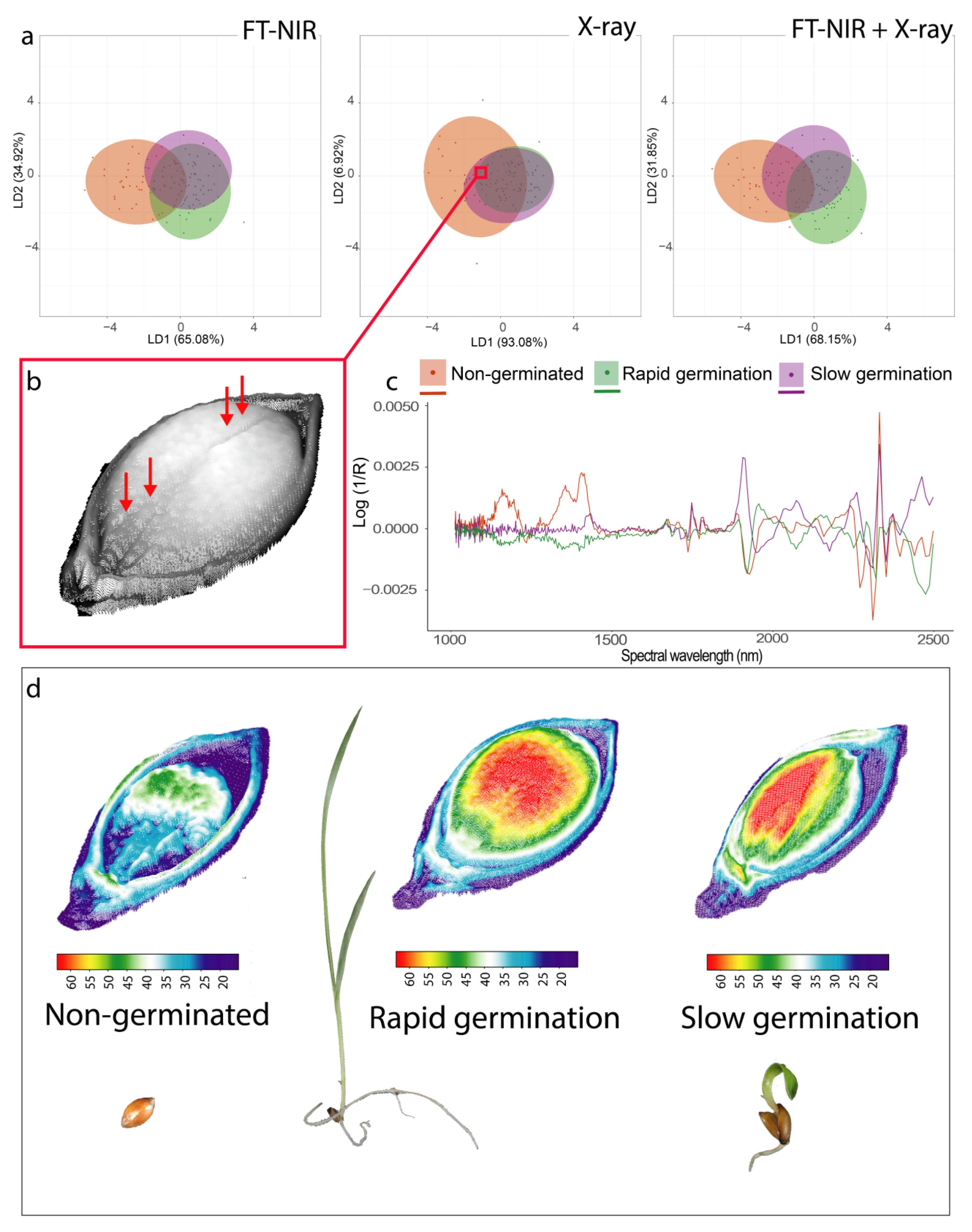
3.1. Spectral Overview and Internal Seed Morphology
3.2. Machine Learning Models
3.3. Germinated and Non-Germinated Seed Classification
3.4. Seed Vigor Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finch-Savage, W.E.E.; Bassel, G.W.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [Green Version]
- ElMasry, G.; Mandour, N.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Recent Applications of Multispectral Imaging in Seed Phenotyping and Quality Monitoring—An Overview. Sensors 2019, 19, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric. 2019, 1, 35–47. [Google Scholar] [CrossRef]
- Wakholi, C.; Kandpal, L.M.; Lee, H.; Bae, H.; Park, E.; Kim, M.S.; Mo, C.; Lee, W.H.H.; Cho, B.K.K. Rapid assessment of corn seed viability using short wave infrared line-scan hyperspectral imaging and chemometrics. Sens. Actuators B Chem. 2018, 255, 498–507. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Yasmin, J.; Collins, W.; Cho, B.K. X-ray CT image analysis for morphology of muskmelon seed in relation to germination. Biosyst. Eng. 2018, 175, 183–193. [Google Scholar] [CrossRef]
- De Medeiros, A.D.; Pinheiro, D.T.; Xavier, W.A.; da Silva, L.J.; dos Dias, D.C.F. Quality classification of Jatropha curcas seeds using radiographic images and machine learning. Ind. Crops Prod. 2020, 146, 112162. [Google Scholar] [CrossRef]
- De Medeiros, A.D.; Zavala-León, M.J.; da Silva, L.J.; Oliveira, A.M.S.; dos Dias, D.C.F. Relationship between internal morphology and physiological quality of pepper seeds during fruit maturation and storage. Agron. J. 2020. [Google Scholar] [CrossRef]
- Leão-Araújo, É.F.; Gomes-Junior, F.G.; da Silva, A.R.; Peixoto, N.; de Souza, E.R.B. Evaluation of the desiccation of campomanesia adamantium seed using radiographic analysis and the relation with physiological potential. Agron. J. 2019, 111, 592–600. [Google Scholar] [CrossRef]
- Kusumaningrum, D.; Lee, H.; Lohumi, S.; Mo, C.; Kim, M.S.; Cho, B.K. Non-destructive technique for determining the viability of soybean (Glycine max) seeds using FT-NIR spectroscopy. J. Sci. Food Agric. 2018, 98, 1734–1742. [Google Scholar] [CrossRef]
- Seo, Y.W.; Ahn, C.K.; Lee, H.; Park, E.; Mo, C.; Cho, B.K. Non-Destructive Sorting Techniques for Viable Pepper (Capsicum annuum L.) Seeds Using Fourier Transform Near-Infrared and Raman Spectroscopy. J. Biosyst. Eng. 2016, 41, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Andrade, G.C.; Medeiros Coelho, C.M.; Uarrota, V.G. Modelling the vigour of maize seeds submitted to artificial accelerated ageing based on ATR-FTIR data and chemometric tools (PCA, HCA and PLS-DA). Heliyon 2020, 6, e03477. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.; Lohumi, S.; Lee, W.H.H.; Cho, B.K. Comparative nondestructive measurement of corn seed viability using Fourier transform near-infrared (FT-NIR) and Raman spectroscopy. Sens. Actuators B Chem. 2016, 224, 500–506. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 72–126. [Google Scholar]
- Li, C.; Zhao, T.; Li, C.; Mei, L.; Yu, E.; Dong, Y.; Chen, J.; Zhu, S. Determination of gossypol content in cottonseeds by near infrared spectroscopy based on Monte Carlo uninformative variable elimination and nonlinear calibration methods. Food Chem. 2017, 221, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Mukasa, P.; Wakholi, C.; Mo, C.; Oh, M.; Joo, H.J.J.; Suh, H.K.; Cho, B.K.K. Determination of viability of Retinispora (Hinoki cypress) seeds using FT-NIR spectroscopy. Infrared Phys. Technol. 2019, 98, 62–68. [Google Scholar] [CrossRef]
- Jiang, G.L. Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding. Agronomy 2020, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Nugraha, B.; Verboven, P.; Janssen, S.; Wang, Z.; Nicolaï, B.M. Non-destructive porosity mapping of fruit and vegetables using X-ray CT. Postharvest Biol. Technol. 2019, 150, 80–88. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Benedet, L.; Faria, W.M.; Silva, S.H.G.; Mancini, M.; Guilherme, L.R.G.; Demattê, J.A.M.; Curi, N. Soil subgroup prediction via portable X-ray fluorescence and visible near-infrared spectroscopy. Geoderma 2020, 365, 114212. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez–Lopez, L. An Introduction to the Prospectr Package. Available online: https://cran.r-project.org/web/packages/prospectr/vignettes/prospectr-intro.pdf (accessed on 2 February 2020).
- R Core Team. R Development Core Team. R Lang. Environ. Stat. Comput. 2019, 55, 275–286. [Google Scholar]
- De Medeiros, A.D.; da Silva, L.J.; da Silva, J.M.; dos Dias, D.C.F.; Pereira, M.D. IJCropSeed: An open-access tool for high-throughput analysis of crop seed radiographs. Comput. Electron. Agric. 2020, 175, 105555. [Google Scholar] [CrossRef]
- Mapa, M. Rules for Seed Analysis; Secretaria de Defesa Agropecuária: Brasilia, Brazil, 2009; ISBN 978-85-99851-70-8. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28. [Google Scholar] [CrossRef] [Green Version]
- Guyon, I.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Dell’Aquila, A. Pepper seed germination assessed by combined X-radiography and computer-aided imaging analysis. Biol. Plant. 2007, 51, 777–781. [Google Scholar] [CrossRef]
- Gagliardi, B.; Marcos-Filho, J. Relationship between germination and bell pepper seed structure assessed by the X-ray test. Sci. Agric. 2011, 68, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Lahlali, R.; Liu, X.; Karunakaran, C. Infrared spectroscopy combined with imaging: A new developing analytical tool in health and plant science. Appl. Spectrosc. Rev. 2016, 51, 466–483. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Dumont, J.; Hirvonen, T.; Heikkinen, V.; Mistretta, M.; Granlund, L.; Himanen, K.; Fauch, L.; Porali, I.; Hiltunen, J.; Keski-Saari, S.; et al. Thermal and hyperspectral imaging for Norway spruce (Picea abies) seeds screening. Comput. Electron. Agric. 2015, 116, 118–124. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, S.; Wu, T. Individual wheat kernels vigor assessment based on NIR spectroscopy coupled with machine learning methodologies. Infrared Phys. Technol. 2020, 105, 103213. [Google Scholar] [CrossRef]
- He, X.; Feng, X.; Sun, D.; Liu, F.; Bao, Y.; He, Y. Rapid and nondestructive measurement of rice seed vitality of different years using near-infrared hyperspectral imaging. Molecules 2019, 24, 2227. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Mi, C.; Wu, N.; Liu, F.; He, Y. Rapid Classification of Wheat Grain Varieties Using Hyperspectral Imaging and Chemometrics. Appl. Sci. 2019, 9, 4119. [Google Scholar] [CrossRef] [Green Version]
- Baek, I.; Kusumaningrum, D.; Kandpal, L.M.; Lohumi, S.; Mo, C.; Kim, M.S.; Cho, B.K. Rapid measurement of soybean seed viability using Kernel-based multispectral image analysis. Sensors 2019, 19, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Algorithm | Hyperparameters | FT-NIR | X-Ray Imaging | FT-NIR + X-Ray Imaging |
|---|---|---|---|---|
| Values | ||||
| Classification of seed germination | ||||
| LDA | dimensions | 1 | 1 | 1 |
| PLS-DA | components | 6 | 1 | 3 |
| RF | trees | 36 | 15 | 290 |
| NB | Laplace correction, Kernel, adjust | 0, TRUE, 1 | 0, FALSE, 1 | 0, FALSE, 1 |
| SVM-r | Sigma, cost | 0.003315536, 4 | 0.05969127, 0.5 | 0.003371439, 2 |
| Classification of seed vigor | ||||
| LDA | dimensions | 2 | 2 | 2 |
| PLS-DA | components | 6 | 3 | 6 |
| RF | trees | 275 | 2 | 290 |
| NB | Laplace correction, Kernel, adjust | 0, TRUE, 1 | 0, TRUE, 1 | 0, TRUE, 1 |
| SVM-r | Sigma, cost | 0.002813337, 2 | 0.07259337, 0.25 | 0.002386695, 2 |
| Method | Feature | FT-NIR | X-Ray Imaging | FT-NIR + X-Ray Imaging | |||
|---|---|---|---|---|---|---|---|
| Cross-Validation | Testing | Cross-Validation | Testing | Cross-Validation | Testing | ||
| (n = 121) | (n = 79) | (n = 121) | (n = 79) | (n = 121) | (n = 79) | ||
| Hits (Total) | Hits (Total) | Hits (Total) | |||||
| LDA | Germinated | - | 47(56) | - | 54(56) | - | 47(56) |
| Non-germinated | - | 17(23) | - | 17(23) | - | 14(23) | |
| Accuracy | 0.68 ± 0.11 | 0.81 | 0.85 ± 0.07 | 0.90 | 0.74 ± 0.09 | 0.77 | |
| Sensitivity | 0.47 ± 0.16 | 0.74 | 0.63 ± 0.14 | 0.74 | 0.58 ± 0.09 | 0.61 | |
| Specificity | 0.78 ± 0.11 | 0.84 | 0.94 ± 0.04 | 0.96 | 0.81 ± 0.10 | 0.84 | |
| PLS-DA | Germinated | - | 54(56) | - | 55(56) | 82(86) | 50(56) |
| Non-germinated | - | 11(23) | - | 13(23) | 23(35) | 15(23) | |
| Accuracy | 0.83 ± 0.12 | 0.82 | 0.87 ± 0.04 | 0.86 | 0.80 ± 0.11 | 0.82 | |
| Sensitivity | 0.59 ± 0.26 | 0.48 | 0.57 ± 0.13 | 0.61 | 0.57 ± 0.19 | 0.65 | |
| Specificity | 0.93 ± 0.08 | 0.96 | 0.98 ± 0.02 | 0.96 | 0.90 ± 0.07 | 0.89 | |
| RF | Germinated | - | 54(56) | - | 54(56) | - | 53(56) |
| Non-germinated | - | 7(23) | - | 14(23) | - | 14(23) | |
| Accuracy | 0.73 ± 0.13 | 0.77 | 0.85 ± 0.09 | 0.86 | 0.84 ± 0.09 | 0.85 | |
| Sensitivity | 0.30 ± 0.23 | 0.30 | 0.57 ± 0.19 | 0.61 | 0.53 ± 0.14 | 0.61 | |
| Specificity | 0.93 ± 0.08 | 0.96 | 0.97 ± 0.03 | 0.96 | 0.97 ± 0.03 | 0.94 | |
| NB | Germinated | - | 44(56) | - | 49(56) | - | 46(56) |
| Non-germinated | - | 11(23) | - | 17(23) | - | 13(23) | |
| Accuracy | 0.65 ± 0.14 | 0.69 | 0.83 ± 0.06 | 0.84 | 0.73 ± 0.14 | 0.74 | |
| Sensitivity | 0.57 ± 0.17 | 0.48 | 0.60 ± 0.10 | 0.74 | 0.66 ± 0.10 | 0.57 | |
| Specificity | 0.69 ± 0.17 | 0.78 | 0.93 ± 0.06 | 0.87 | 0.75 ± 0.15 | 0.82 | |
| SVM-r | Germinated | - | 52(56) | - | 55(56) | 86(86) | 53(56) |
| Non-germinated | - | 11(23) | - | 14(23) | 24(35) | 11(23) | |
| Accuracy | 0.78 ± 0.11 | 0.79 | 0.84 ± 0.06 | 0.86 | 0.79 ± 0.11 | 0.81 | |
| Sensitivity | 0.38 ± 0.27 | 0.48 | 0.58 ± 0.09 | 0.61 | 0.51 ± 0.23 | 0.48 | |
| Specificity | 0.93 ± 0.04 | 0.93 | 0.95 ± 0.04 | 0.96 | 0.92 ± 0.06 | 0.97 | |
| Method | Feature | FT-NIR | X-Ray Imaging | FT-NIR + X-Ray Imaging | |||
|---|---|---|---|---|---|---|---|
| Cross-Validation | Testing | Cross-Validation | Testing | Cross-Validation | Testing | ||
| (n = 121) | (n = 79) | (n = 121) | (n = 79) | (n = 121) | (n = 79) | ||
| Hits (Total) | Hits (Total) | Hits (Total) | |||||
| LDA | Non-germinated | - | 13(25) | - | 16(25) | - | 14(25) |
| Rapid germination | - | 29(38) | - | 37(38) | - | 28(38) | |
| Slow germination | - | 6(16) | - | 0(16) | - | 3(16) | |
| Accuracy | 0.52 ± 0.06 | 0.61 | 0.61 ± 0.11 | 0.67 | 0.50 ± 0.08 | 0.57 | |
| Sensitivity | 0.51 ± 0.20 | 0.55 | 0.51 ± 0.34 | 0.54 | 0.48 ± 0.21 | 0.49 | |
| Specificity | 0.75 ± 0.11 | 0.79 | 0.79 ± 0.18 | 0.79 | 0.74 ± 0.12 | 0.76 | |
| PLS-DA | Non-germinated | - | 15(25) | - | 16(25) | - | 12(25) |
| Rapid germination | - | 33(38) | - | 38(38) | - | 31(38) | |
| Slow germination | - | 0(16) | - | 0(16) | - | 3(16) | |
| Accuracy | 0.57 ± 0.09 | 0.61 | 0.62 ± 0.09 | 0.68 | 0.58 ± 0.05 | 0.58 | |
| Sensitivity | 0.50 ± 0.32 | 0.49 | 0.49 ± 0.40 | 0.55 | 0.50 ± 0.27 | 0.49 | |
| Specificity | 0.77 ± 0.18 | 0.77 | 0.77 ± 0.25 | 0.8 | 0.78 ± 0.17 | 0.76 | |
| RF | Non-germinated | - | 15(25) | - | 15(25) | - | 13(25) |
| Rapid germination | - | 25(38) | - | 74(38) | - | 35(38) | |
| Slow germination | - | 2(16) | - | 0(16) | - | 1(16) | |
| Accuracy | 0.54 ± 0.12 | 0.53 | 0.59 ± 0.05 | 0.66 | 0.59 ± 0.10 | 0.62 | |
| Sensitivity | 0.46 ± 0.29 | 0.46 | 0.49 ± 0. 40 | 0.52 | 0.51 ± 0.34 | 0.50 | |
| Specificity | 0.74 ± 0.23 | 0.73 | 0.76 ± 0. 26 | 0.78 | 0.77 ± 0.23 | 0.77 | |
| NB | Non-germinated | - | 12(25) | - | 15(25) | - | 13(25) |
| Rapid germination | - | 15(38) | - | 30(38) | - | 17(38) | |
| Slow germination | - | 7(16) | - | 1(16) | - | 8(16) | |
| Accuracy | 0.46 ± 0.12 | 0.43 | 0.56 ± 0.06 | 0.58 | 0.45 ± 0.12 | 0.48 | |
| Sensitivity | 0.49 ± 0.16 | 0.44 | 0.48± 0. 32 | 0.48 | 0.49 ± 0.18 | 0.49 | |
| Specificity | 0.74 ± 0.13 | 0.72 | 0.77 ± 0. 16 | 0.76 | 0.74 ± 0.11 | 0.75 | |
| SVM-r | Non-germinated | - | 12(25) | - | 16(25) | - | 13(25) |
| Rapid germination | - | 28(38) | - | 36(38) | - | 30(38) | |
| Slow germination | - | 2(16) | - | 0(16) | - | 2(16) | |
| Accuracy | 0.56 ± 0.12 | 0.50 | 0.64 ± 0.05 | 0.66 | 0.59 ± 0.07 | 0.57 | |
| Sensitivity | 0.50 ± 0.26 | 0.45 | 0.53 ± 0.41 | 0.53 | 0.52 ± 0.28 | 0.48 | |
| Specificity | 0.76 ± 0.17 | 0.73 | 0.79 ± 0.25 | 0.78 | 0.77 ± 0.19 | 0.75 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.D.d.; Silva, L.J.d.; Ribeiro, J.P.O.; Ferreira, K.C.; Rosas, J.T.F.; Santos, A.A.; Silva, C.B.d. Machine Learning for Seed Quality Classification: An Advanced Approach Using Merger Data from FT-NIR Spectroscopy and X-ray Imaging. Sensors 2020, 20, 4319. https://doi.org/10.3390/s20154319
Medeiros ADd, Silva LJd, Ribeiro JPO, Ferreira KC, Rosas JTF, Santos AA, Silva CBd. Machine Learning for Seed Quality Classification: An Advanced Approach Using Merger Data from FT-NIR Spectroscopy and X-ray Imaging. Sensors. 2020; 20(15):4319. https://doi.org/10.3390/s20154319
Chicago/Turabian StyleMedeiros, André Dantas de, Laércio Junio da Silva, João Paulo Oliveira Ribeiro, Kamylla Calzolari Ferreira, Jorge Tadeu Fim Rosas, Abraão Almeida Santos, and Clíssia Barboza da Silva. 2020. "Machine Learning for Seed Quality Classification: An Advanced Approach Using Merger Data from FT-NIR Spectroscopy and X-ray Imaging" Sensors 20, no. 15: 4319. https://doi.org/10.3390/s20154319






