Sensing Exposure Time to Oxygen by Applying a Percolation-Induced Principle
Abstract
1. Introduction
1.1. Oxygen Sensors in the Food Industry
1.2. Bio-Based Sensors
1.3. Composite-Based Oxygen Sensors
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Characterization of the Oil and the Composite
2.3.1. Gas Chromatography with Flame-Ionization Detection
2.3.2. X-ray Photoelectron Spectroscopy
2.3.3. Rheology
2.3.4. Scanning Electron Microscopy
2.3.5. Electrical Conductivity
2.3.6. Thermogravimetric Analysis
2.3.7. X-ray Scattering
3. Results and Discussion
3.1. Characterization
3.1.1. Matrix Oil Composition and Viscosity
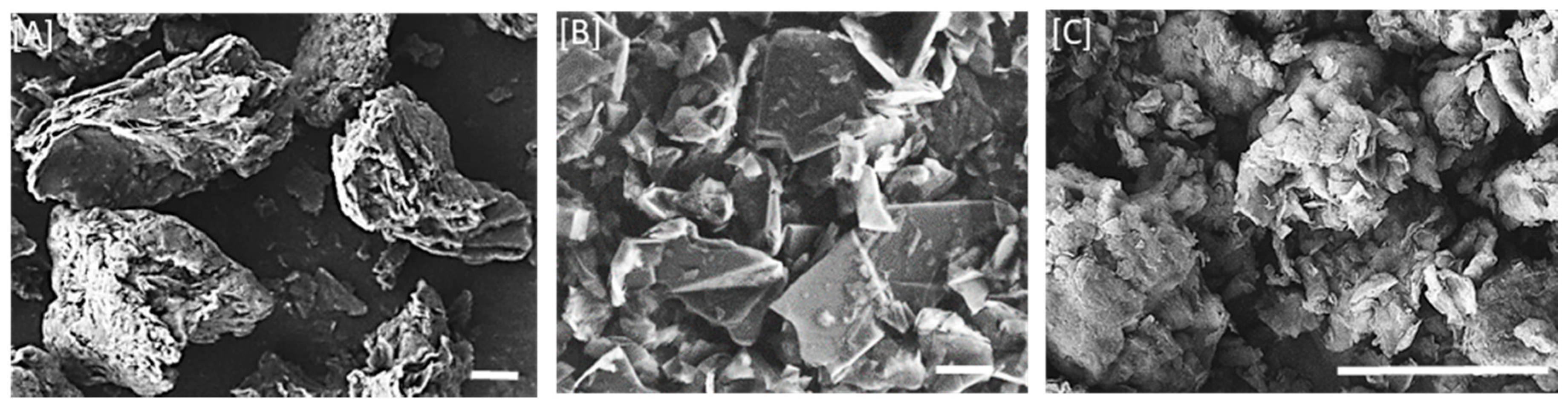
3.1.2. SEM Imaging of the Fillers
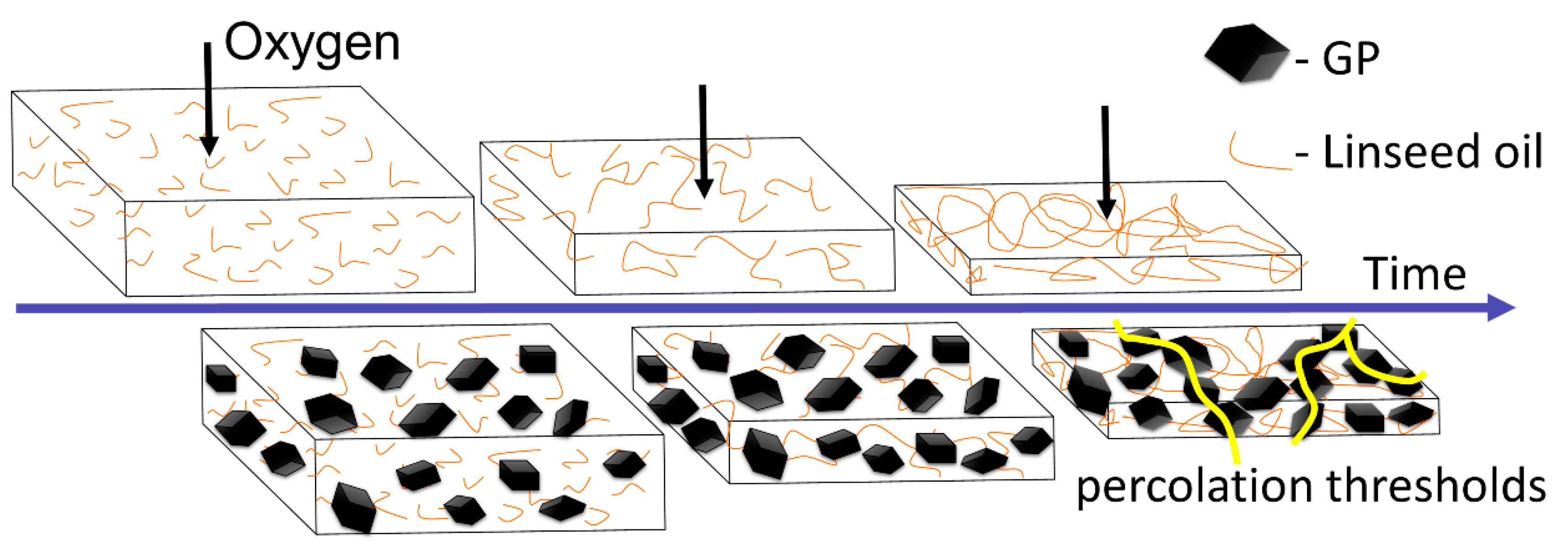
3.2. The 'Shrink-Percolate' Approach
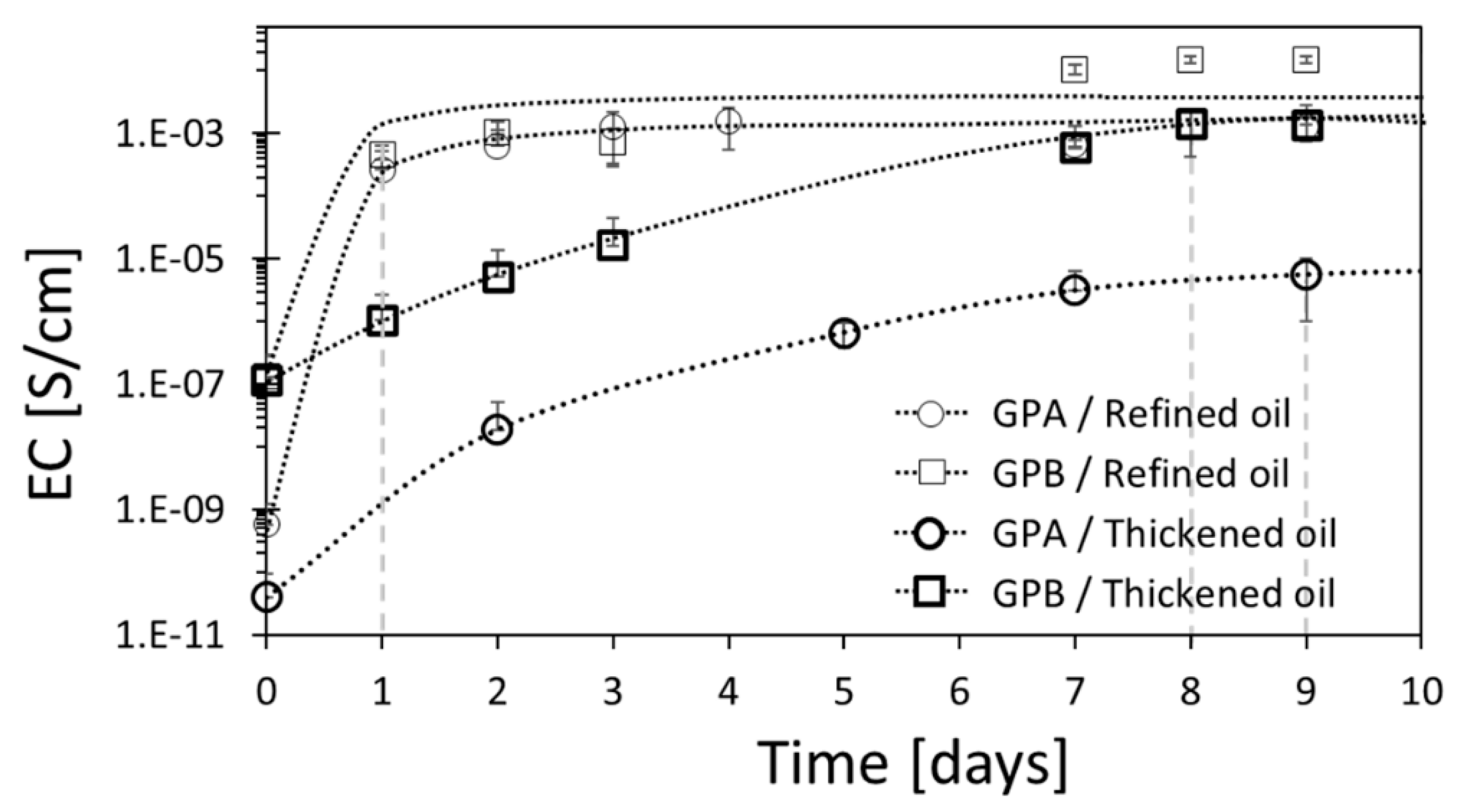
3.3. Effect of the Degree of Saturation of the Oil on the Electrical Conductivity
3.4. Effect of the Aspect Ratio of the Graphite Powder on the Electrical Conductivity
3.5. Clay Addition to Form a Hybrid Composite System
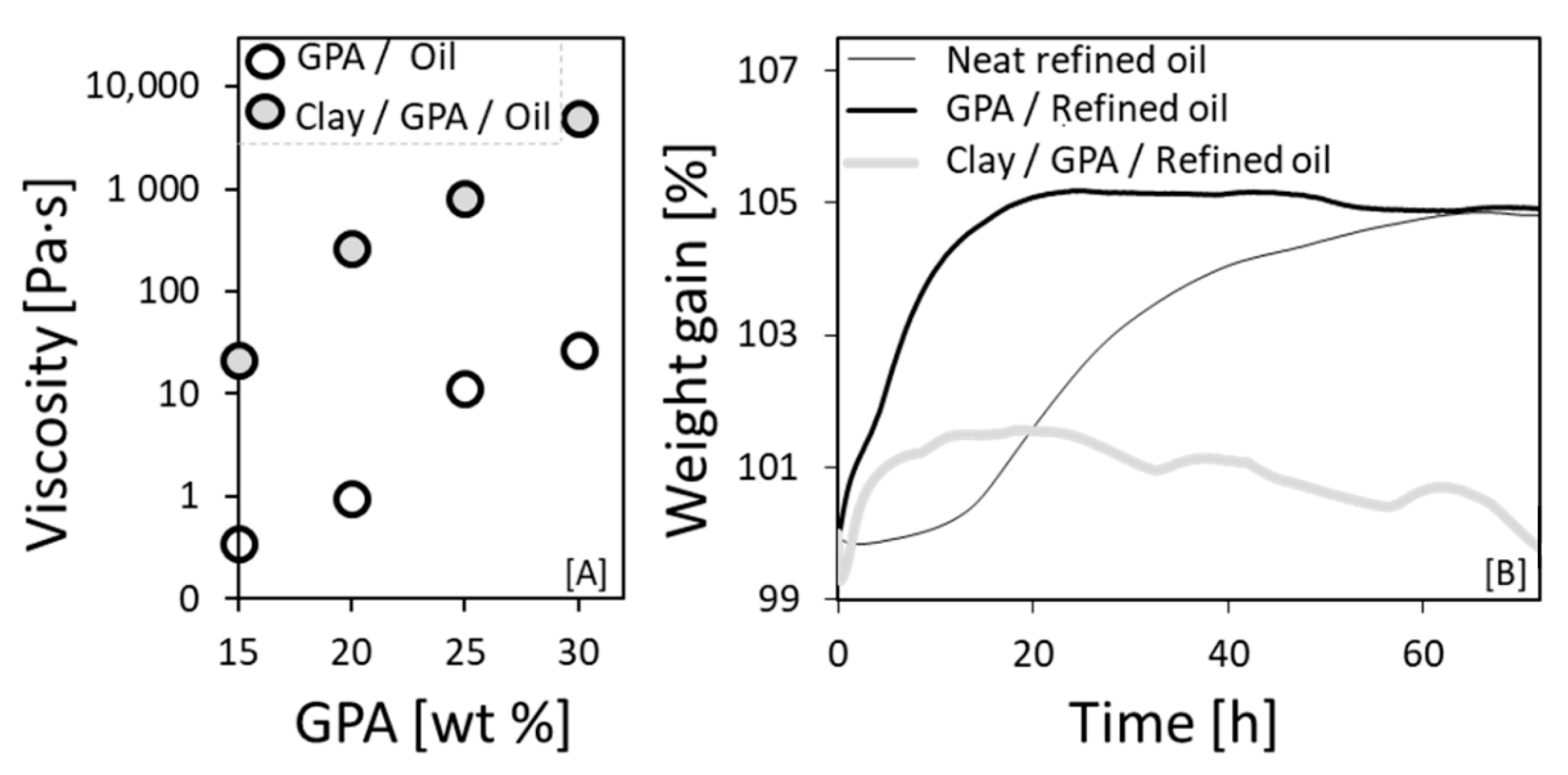
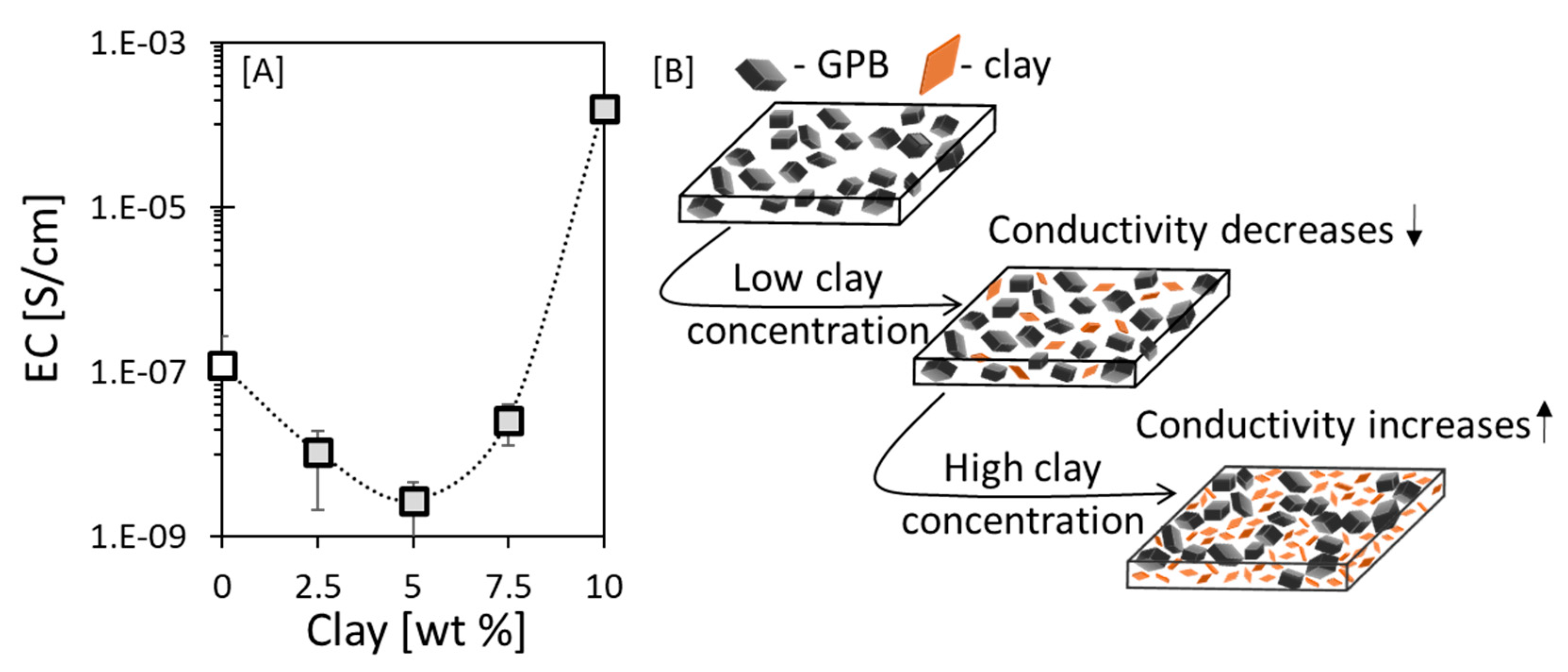
3.5.1. Clay-Induced Excluded Volume Effect
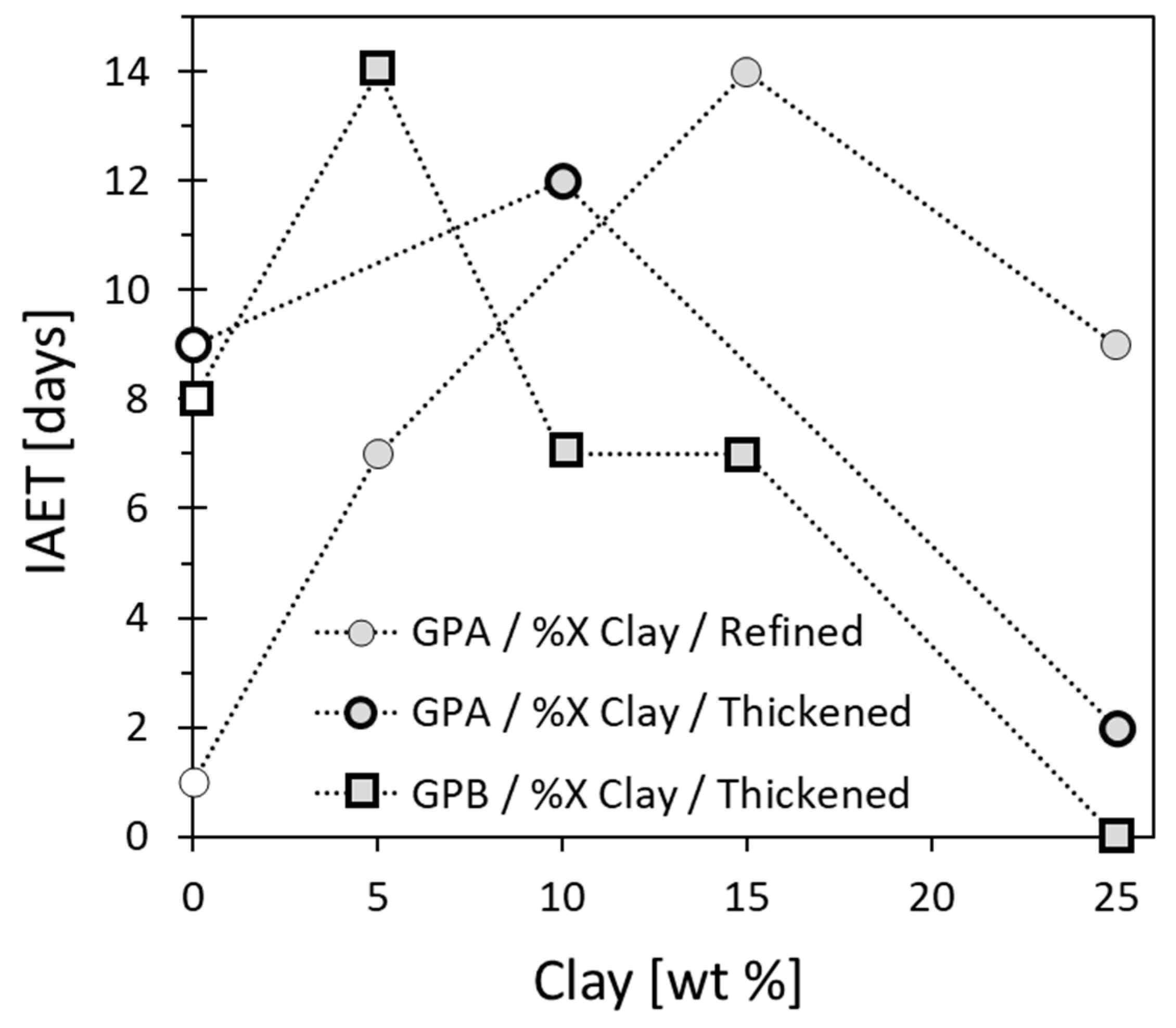
3.5.2. Manipulation of the IAET
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Electrical Conductivity |
| MAP | Modified Atmosphere Packaging |
| GPA | Graphite Powder Alfa Aesar |
| GPB | Graphite Powder BTC |
| IAET | Indication of Air-Exposure Time |
References
- Cinti, S.; Volpe, G.; Piermarini, S.; Delibato, E.; Palleschi, G. Electrochemical biosensors for rapid detection of foodborne Salmonella: A critical overview. Sensors (Switzerland) 2017, 17, 1910. [Google Scholar] [CrossRef]
- Páez-Avilés, C.; Juanola-Feliu, E.; Punter-Villagrasa, J.; del Moral Zamora, B.; Homs-Corbera, A.; Colomer-Farrarons, J.; Miribel-Català, P.L.; Samitier, J. Combined dielectrophoresis and impedance systems for bacteria analysis in microfluidic on-chip platforms. Sensors (Switzerland) 2016, 16, 1514. [Google Scholar] [CrossRef] [PubMed]
- Salter, S.J. The food-borne identity. Nat. Rev. Microbiol. 2014, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, D.; Xu, P.; Zhang, T.; Ou, Q.; Bai, C.; Yao, Z. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. Environ. Res. 2016, 150, 528–540. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Nitzan, N.; Vaxman, A.; Segal, E. Active packaging containing encapsulated carvacrol for control of postharvest decay. Postharvest Biol. Technol. 2016, 118, 175–182. [Google Scholar] [CrossRef]
- Akkerman, R.; Farahani, P.; Grunow, M. Quality, safety and sustainability in food distribution: A review of quantitative operations management approaches and challenges. OR Spectr. 2010, 32, 863–904. [Google Scholar] [CrossRef]
- Kader, A.A.; Watkins, C.B. Modified atmosphere packaging-Toward 2000 and beyond. Horttechnology 2000, 10, 483–486. [Google Scholar] [CrossRef]
- Mills, A. Oxygen indicators and intelligent inks for packaging food, Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste–Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar] [CrossRef]
- Vogliano, C.; Brown, K. The State of America’s Wasted Food and Opportunities to Make a Difference. J. Acad. Nutr. Diet. 2016, 116, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; WHO: Geneva, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kirwan, M.; Brown, H.; Williams, J. Packaged Product Quality and Shelf Life. In Food and Beverage Packaging Technology, 2nd ed.; Coles, R., Kirwan, M., Eds.; Wiley-Blackwell: London, UK, 2011; pp. 59–83. [Google Scholar] [CrossRef]
- Farber, J.M. Microbiological Aspects of Modified-Atmosphere Packaging Technology-A Review. J. Food Prot. 2016, 54, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Hazafy, D. A solvent-based intelligence ink for oxygen. Analyst 2008, 133, 213–218. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Bennik, M.H.J.; Smid, E.J.; Rombouts, F.M.; Gorris, L.G.M. Growth of psychrotrophic foodborne pathogens in a solid surface model system under the influence of carbon dioxide and oxygen. Food Microbiol. 1995, 12, 509–519. [Google Scholar] [CrossRef]
- Marek, P.; Velasco-Veléz, J.J.; Haas, T.; Doll, T.; Sadowski, G. Time-monitoring sensor based on oxygen diffusion in an indicator/polymer matrix. Sens. Actuators B Chem. 2013, 178, 254–262. [Google Scholar] [CrossRef]
- Lee, S.K.; Sheridan, M.; Mills, A. Novel UV-activated colorímetric oxygen indicator. Chem. Mater. 2005, 17, 2744–2751. [Google Scholar] [CrossRef]
- Roberts, L.; Lines, R.; Reddy, S.; Hay, J. Investigation of Polyviologens as Oxygen Indicators in Food Packaging Luke Roberts. Sens. Actuators B Chem. 2011, 152, 63–67. [Google Scholar] [CrossRef]
- Araque, P.E.; de Vargas Sansalvador, I.M.P.; Ruiz, N.L.; Erenas, M.M.; Rodríguez, M.A.C.; Olmos, A.M. Non-Invasive Oxygen Determination in Intelligent Packaging Using a Smartphone. IEEE Sens. J. 2018, 18, 4351–4357. [Google Scholar] [CrossRef]
- Weigel, C.; Schneider, M.; Schmitt, J.; Hoffmann, M.; Kahl, S.; Jurisch, R. Self-sufficient sensor for oxygen detection in packaging via radio-frequency identification. J. Sens. Sens. Syst. 2015, 4, 179–186. [Google Scholar] [CrossRef]
- Chu, C.S.; Lin, K.Z.; Tang, Y.H. A new optical sensor for sensing oxygen based on phase shift detection. Sens. Actuators B Chem. 2016, 223, 606–612. [Google Scholar] [CrossRef]
- Mills, A. Controlling the sensitivity of optical oxygen sensors. Sens. Actuators B Chem. 1998, 51, 60–68. [Google Scholar] [CrossRef]
- Ogurtsov, V.I.; Papkovsky, D.B. Modelling of phase-fluorometric oxygen sensors: Consideration of temperature effects and operational requirements. Sens. Actuators B Chem. 2006, 113, 917–929. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors (Switzerland) 2017, 17, 2715. [Google Scholar] [CrossRef]
- Matindoust, S.; Baghaei-Nejad, M.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Food quality and safety monitoring using gas sensor array in intelligent packaging. Sens. Rev. 2016, 36, 169–183. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Maier, D.E.; Channaiah, L.H.; Martinez-Kawas, A.; Lawrence, J.; Chaves, E.; Coradi, P.; Fromme, G. Monitoring carbon dioxide concentration for early detection of spoilage in stored grain. Jul.-Kühn-Arch. 2010, 425, 505–509. [Google Scholar] [CrossRef]
- Altaf, S.; Ahmad, S.; Zaindin, M.; Soomro, M.W. Xbee-based WSN architecture for monitoring of banana ripening process using knowledge-level artificial intelligent technique. Sensors (Switzerland) 2020, 20, 4033. [Google Scholar] [CrossRef]
- OxySence Inc. OxySence. Available online: http://www.Oxysense.Com/Index2.Htm (accessed on 8 August 2020).
- Saini, D. Real-time Oxygen Monitoring for Modified Atmosphere Processing Using the OxySentry System, OxySense White Pap. 2014. Available online: http://www.oxysense.com/OxySense-white-papers/real-time-oxygen-monitoring-OxySentry-System.pdf (accessed on 8 August 2020).
- Mitsubishi Gas Chemical Company Inc. Ageless Eye Oxygen Indicator. Available online: http://www.mgc.co.jp/eng/products/abc/ageless/eye.html (accessed on 20 March 2020).
- Kim, J.; Jeerapan, I.; Ciui, B.; Hartel, M.C.; Martin, A.; Wang, J. Edible Electrochemistry: Food Materials Based Electrochemical Sensors. Adv. Healthc. Mater. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors–A Review. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Corradini, M.G.; Wang, Y.L.; Le, A.; Waxman, S.M.; Zelent, B.; Chib, R.; Gryczynski, I.; Ludescher, R.D. Identifying and selecting edible luminescent probes as sensors of food quality. AIMS Biophys. 2016, 3, 319–339. [Google Scholar] [CrossRef]
- Wang, T.; Ramnarayanan, A.; Cheng, H. Real time analysis of bioanalytes in healthcare, food, zoology and botany. Sensors (Switzerland) 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Dudnyk, I.; Janeček, E.R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, A.; Xie, K.; Gao, A. Smart color-changing paper packaging sensors with pH sensitive chromophores based on azo-anthraquinone reactive dyes. Sens. Actuators B Chem. 2019, 286, 362–369. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, D. Vegetable oil-based polymer composites: Synthesis, properties and their applications. Handb. Compos. Renew. Mater. 2017, 8, 441–470. [Google Scholar] [CrossRef]
- Samarth, N.B.; Mahanwar, P.A. Modified Vegetable Oil Based Additives as a Future Polymeric Material—Review. Open J. Org. Polym. Mater. 2015, 5, 1–22. [Google Scholar] [CrossRef]
- Zovi, O.; Lecamp, L.; Loutelier-bourhis, C.; Lange, M.; Bunel, C. A solventless synthesis process of new UV-curable materials based on linseed oil. Green Chem. 2011, 13, 1014–1022. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Hess, P.S.; O'Hare, G.A. Oxidation of linseed oil. Ind. Eng. Chem. 1947, 42, 1424–1431. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Oxidation reactions and spontaneous ignition of linseed oil. Proc. Combust. Inst. 2011, 33, 2625–2632. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Low temperature oxidation of linseed oil: A review. Fire Sci. Rev. 2012, 1, 3. [Google Scholar] [CrossRef]
- Kasprzycka-Guttman, T.; Odzeniak, D. Thermoanalytical investigation of edible oils. Thermochim. Acta 1992, 204, 303–310. [Google Scholar] [CrossRef]
- Hendriks, C.F.; Heertjes, P.M.; van Beek, H.C. Autoxidation of Methyl Linoleate and Methyl Linolenate and Reactions of the Hydroperoxides Formed in n-Heptane Solution. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 212–216. [Google Scholar] [CrossRef]
- Partanen, R.; Raula, J.; Seppänen, R.; Buchert, J.; Kauppinen, E.; Forssell, P. Effect of relative humidity on oxidation of flaxseed oil in spray dried whey protein emulsions. J. Agric. Food Chem. 2008, 56, 5717–5722. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Brier, J.C. Influence of Antioxidants on the Rate of Oxidation of Linseed Oil: I—Hydroquinone. Ind. Eng. Chem. 1931, 23, 40–49. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Yamamoto, K.; Nozawa, M.; Miyashita, K.; Murakami, T. Lipid Profiles and Oxidative Stability of Silkworm Pupal Oil. J. Oleo Sci. 2002, 51, 681–690. [Google Scholar] [CrossRef]
- Holman, R.T.; Elmer, O.C. The Rates of Oxidation of Unsaturated Fatty Acids and Esters. J. Am. Oil Chem. Soc. 1947, 24, 127–129. [Google Scholar] [CrossRef]
- Natural Pigments Inc. Available online: https://www.naturalpigments.com/sun-thickened-linseed-oil.html (accessed on 8 August 2020).
- Yeomans, D.J. How to Make Sun Thickened Linseed Oil. Available online: https://danieljamesyeomans.com/how-to-make-sun-thickened-linseed-oil/ (accessed on 17 February 2020).
- Utrecht Art Supplies. “Bodied” Oils for Painting; Dick Blick Holdings Inc. Available online: https://assets.ctfassets.net/f1fikihmjtrp/VRUWCXVQUlaPjS0iZh7yf/5f450bbdf819c25c1c4c2fc2d812a599/PP_bodied_oils.pdf (accessed on 10 August 2020).
- Costa, L.; Almeida, T.; Carvalho, L.; Canedo, E. Stabilization during processing of PHB/organoclay. In Proceedings of the 2nd Brazilian Conference on Composite Materials, São Paulo, Brazil, 15–18 September 2014. [Google Scholar]
- Thelakkadan, A.S.; Coletti, G.; Guastavino, F.; Fina, A. Effect of the Nature of Clay on the Thermo-Mechanodynamical and Electrical Properties of Epoxy/Clay Nanocomposites A.S. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Ahmed, A.; Hafiz, A. Synthesis and Characterization of EVA-Cloisite Clay Nanocomposites. Master’s Thesis, The American University in Cairo, New Cairo, Egypt, 2013. [Google Scholar]
- Utracki, L.A. Clay-containing polymeric nanocomposites and their properties. IEEE Electr. Insul. Mag. 2010, 26, 6–15. [Google Scholar] [CrossRef]
- Zagho, M.M.; Khader, M.M. The Impact of Clay Loading on the Relative Intercalation of Poly (Vinyl Alcohol)-Clay Composites. J. Mater. Sci. Chem. Eng. 2016, 4, 20–31. [Google Scholar] [CrossRef]
- Xiang, F.; Tzeng, P.; Sawyer, J.S.; Regev, O.; Grunlan, J.C. Improving the Gas Barrier Property of Clay−Polymer Multilayer Thin Films Using Shorter Deposition Times. ACS Appl. Mater. Interfaces 2014, 6, 6040–6048; [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.F. Principles of Composite Material Mechanics, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Chawla, K.K. Composite Materials: Science and Engineering; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Kaw, A.K. Mechanics of Composite Material, 2nd ed.; CRC Press LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Schadler, L.S. Polymer-Based and Polymer-Filled Nanocomposites. In Nanocomposite Science and Technology; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 77–153. [Google Scholar] [CrossRef]
- Varenik, M.; Nadiv, R.; Levy, I.; Vasilyev, G.; Regev, O. Breaking through the Solid/Liquid Processability Barrier: Thermal Conductivity and Rheology in Hybrid Graphene-Graphite Polymer Composites. ACS Appl. Mater. Interfaces. 2017, 9, 7556–7564. [Google Scholar] [CrossRef]
- Levy, I.; Wormser, E.M.; Varenik, M.; Buzaglo, M.; Nadiv, R.; Regev, O. Graphene–graphite hybrid epoxy composites with controllable workability for thermal management. Beilstein J. Nanotechnol. 2019, 10, 95–104. [Google Scholar] [CrossRef]
- Arshak, K.; Adley, C.; Moore, E.; Cunniffe, C.; Campion, M.; Harris, J. Characterisation of polymer nanocomposite sensors for quantification of bacterial cultures. Sens. Actuators B Chem. 2007, 126, 226–231. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Müller, M.T.; Boldt, R.; Pötschke, P. The in fluence of matrix viscosity on MWCNT dispersion and electrical properties in different thermoplastic nanocomposites. Polymer (Guildf) 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Lundberg, B.; Sundqvist, B. Resistivity of a composite conducting polymer as a function of temperature, pressure, and environment: Applications as a pressure and gas concentration transducer. J. Appl. Phys. 1986, 60, 1074–1079. [Google Scholar] [CrossRef]
- Selampinar, F.; Toppare, L.; Akbulut, U.; Yalçin, T.; Süzer, Ş. A conducting composite of polypyrrole II. As a gas sensor. Synth. Met. 1995, 68, 109–116. [Google Scholar] [CrossRef]
- Van Hieu, N.; Thuy, L.T.B.; Chien, N.D. Highly sensitive thin film NH3 gas sensor operating at room temperature based on SnO2/MWCNTs composite. Sens. Actuators B Chem. 2008, 129, 888–895. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Dong, X.M.; Fu, R.W.; Zhang, M.Q.; Zhang, B.; Rong, M.Z. Electrical resistance response of carbon black filled amorphous polymer composite sensors to organic vapors at low vapor concentrations. Carbon 2004, 42, 2551–2559. [Google Scholar] [CrossRef]
- Singh, R.P. Scientific Principles of Shelf Life Evaluation Shelf Life Evaluation of Foods; Singh, R.P. Blackie Academic & Professional: London, UK, 1994. [Google Scholar]
- Cui, L.; Ma, X.; Paul, D.R. Morphology and properties of nanocomposites formed from ethylene-vinyl acetate copolymers and organoclays. Polymer 2007, 48, 6325–6339. [Google Scholar] [CrossRef]
- BYK Cloisite® 20A Nanoclay, Songhan Plast. Technol. Co., Ltd. pp. 1–2. Available online: http://www.lookpolymers.com/polymer_BYK-Cloisite-20A-Nanoclay.php (accessed on 8 August 2020).
- Cao, T.; Fasulo, P.D.; Rodgers, W.R. Investigation of the shear stress effect on montmorillonite platelet aspect ratio by atomic force microscopy. Appl. Clay Sci. 2010, 49, 21–28. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Keramat, M.; Zare Darniyani, L. A Kinetic Approach to the Oxidation of Linseed Oil as Influenced by Fruit Peel and Seeds of Pomegranate. Eur. J. Lipid Sci. Technol. 2020, 122, 1–7. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Leenson, I.A. The Arrhenius Law and Storage of Food in a Freezer. J. Chem. Educ. 1999, 76, 504–505. [Google Scholar] [CrossRef]
- Hammond, S.T.; Brown, J.H.; Burger, J.R.; Tatiana, P. Food Spoilage, Storage, and Transport : Implications for a Sustainable Future. BioScience 2015, 65, 758–768. [Google Scholar] [CrossRef]
- Ha, H.; Kim, S.C.; Ha, K. Effect of molecular weight of polymer matrix on the dispersion of MWNTs in HDPE/MWNT and PC/MWNT composites. Macromol. Res. 2010, 18, 512–518. [Google Scholar] [CrossRef]
- Bai, J.B.; Allaoui, A. Effect of the length and the aggregate size of MWNTs on the improvement efficiency of the mechanical and electrical properties of nanocomposites-Experimental investigation. Compos. Part A Appl. Sci. Manuf. 2003, 34, 689–694. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Narkis, M.; Siegmann, A. Nanocomposite systems based on unsaturated polyester and organo-clay. Polym. Eng. Sci. 2005, 45, 174–186. [Google Scholar] [CrossRef]
- Ser, È.; Jank, M.; Ritter, A.; Cking, È.; Ko, H.; Lu, F.; Powder, I. Graphite and Activated Carbon as Catalysts for The Oxidation of 4-Chlorophenol with Hydrogen Peroxide in Aqueous Solution. Water Res. 1998, 32, 2607–2614. [Google Scholar]
- Domínguez, C.M.; Ocón, P.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Applied Catalysis B : Environmental Graphite and carbon black materials as catalysts for wet peroxide oxidation. Appl. Catal. B: Environ. 2014, 144, 599–606. [Google Scholar] [CrossRef]
- Ammar, L.B.; Fakhfakh, S.; Jbara, O.; Hadjadj, A.; Rondot, S. Polypropylene-nanoclay composites under electron irradiation in SEM: Structure, charge trapping and electron emission properties. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1878–1887. [Google Scholar] [CrossRef]


| Viscosity [Pa S] | Mean Particle Size [µm] | Aspect Ratio | |
|---|---|---|---|
| Refined linseed oil | 0.039 ± 0.002 | ||
| Thickened linseed oil | 2.4 ± 0.1 | ||
| GPA [69,70] | 27 ± 4 | 1.5 ± 0.4 | |
| GPB | 10.0 ± 0.2 | 39 ± 6 | |
| Clay—Cloisite 20A [80,81] | 6.0 * | 192—2800 ** |
| Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 cis-9 | Linoleic C18:2 cis-9,12 | Linolenic C18:3 cis-9,12,15 | |
|---|---|---|---|---|---|
| Area % | 6.1 | 5.0 | 20.5 | 15.6 | 45.1 |
| Peak | Binding Energy [eV] | Atomic % |
|---|---|---|
| O1s | 529.44 | 16.28 |
| C1s | 281.84 | 83.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afik, N.; Yadgar, O.; Volison-Klimentiev, A.; Peretz-Damari, S.; Ohayon-Lavi, A.; Alatawna, A.; Yosefi, G.; Bitton, R.; Fuchs, N.; Regev, O. Sensing Exposure Time to Oxygen by Applying a Percolation-Induced Principle. Sensors 2020, 20, 4465. https://doi.org/10.3390/s20164465
Afik N, Yadgar O, Volison-Klimentiev A, Peretz-Damari S, Ohayon-Lavi A, Alatawna A, Yosefi G, Bitton R, Fuchs N, Regev O. Sensing Exposure Time to Oxygen by Applying a Percolation-Induced Principle. Sensors. 2020; 20(16):4465. https://doi.org/10.3390/s20164465
Chicago/Turabian StyleAfik, Noa, Omri Yadgar, Anastasiya Volison-Klimentiev, Sivan Peretz-Damari, Avia Ohayon-Lavi, Amr Alatawna, Gal Yosefi, Ronit Bitton, Naomi Fuchs, and Oren Regev. 2020. "Sensing Exposure Time to Oxygen by Applying a Percolation-Induced Principle" Sensors 20, no. 16: 4465. https://doi.org/10.3390/s20164465
APA StyleAfik, N., Yadgar, O., Volison-Klimentiev, A., Peretz-Damari, S., Ohayon-Lavi, A., Alatawna, A., Yosefi, G., Bitton, R., Fuchs, N., & Regev, O. (2020). Sensing Exposure Time to Oxygen by Applying a Percolation-Induced Principle. Sensors, 20(16), 4465. https://doi.org/10.3390/s20164465






