Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review
Abstract
:1. Introduction: Definition of Nanozymes, Classification, Advantages vs. Natural Enzymes, and Potential Practical Applications
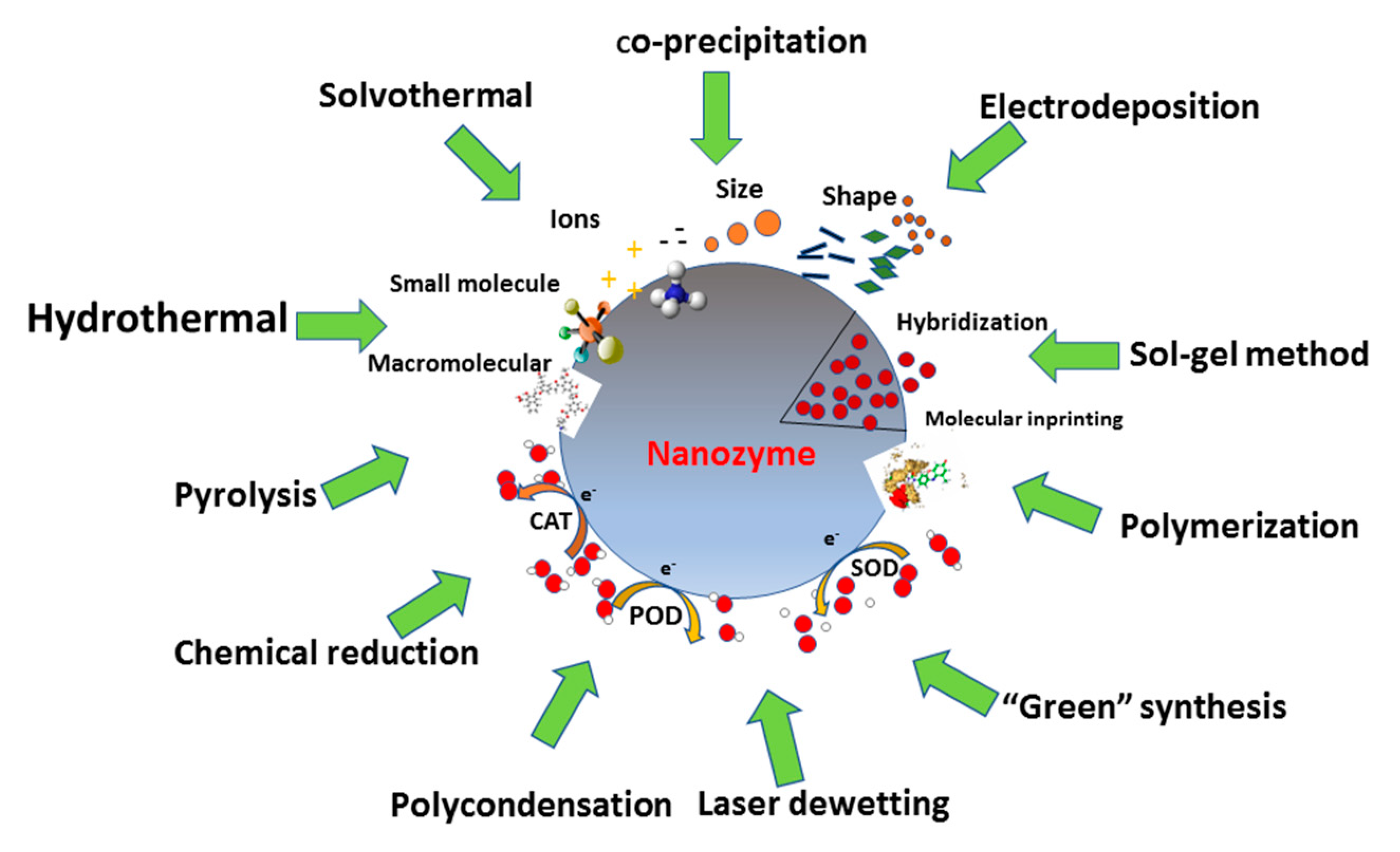
2. Methods for the Synthesis of Catalytic Nanomaterials
2.1. Hydrothermal and Solvothermal Methods
2.2. Chemical Reduction
2.3. Sol-gel Method
2.4. Co-Precipitation
2.5. Polymerization and Polycondensation
2.6. Electrochemical Deposition
3. Catalytic Performance of Nanozymes

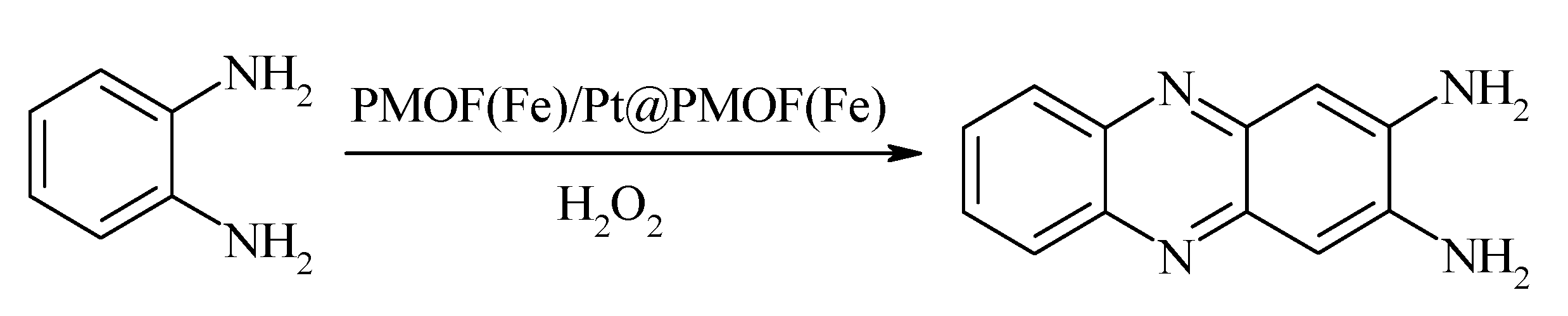
4. Nanozymes as Peroxidase Mimetics
5. Nanooxidases
6. Laccase-Mimicking Nanozymes
7. Conclusions and Potential Applications
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ABTS | 2,2’-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) |
| AKCN | Alkalized graphitic carbon nitride |
| AMP | Adenosine monophosphate |
| AOx | Alcohol oxidase |
| AP | Acetamidophenol |
| AuNPs | Gold nanoparticles |
| Au/Co@HNCF | Gold/cobalt nanoporous carbon framework |
| AuNBP/MWCNTs Fer/rGO | Gold nanobipyramids supported by multi-walled carbon nanotubes ferumoxytol and reduced graphene oxide |
| BET | Brunauer-Emmett-Teller |
| BG | Bucky gel consisting of carbon nanotubes and ionic liquid |
| biot-GOx | Biotinylated glucose oxidase |
| BNNS@CuS | Boron nitride nanosheet and copper sulfide nanohybrids |
| BSA | Bovine serum albumin |
| C60[C(COOH)2]2 | C60-carboxyfullerene |
| CeNPs | Nanoceria particles |
| CF@CuAl-LDH | Carbon fiber-supported ultrathin cual layered double hydroxides (LDH) nanosheets |
| CF-H-Au | Carbon microfibers-hemin-gold nanoparticles |
| CMC@Pd/Al-LDH | Pd/Al layered double hydroxide/carboxymethyl cellulose nanocomposite; |
| CNE | Carbon nanoelectrodes fabricated within a quartz nanopipette and electrochemically etched |
| CNFs | Carbon nanofibers |
| CNTs | Multi-walled carbon nanotubes |
| COx | Cholesterol oxidase |
| CP | Carbon paste |
| CPS | Carbon paste sensors |
| CQDs | Carbon quantum dots |
| cTnI | Cardiac troponin I |
| CTMB | Cetyltrimethylammonium bromide |
| Cu-Cys | Copper(II) complex of cysteine |
| Cu-MOG | Cu-based metal-organic gel |
| CuNWs/rGO | One-dimensional copper nanowires and two-dimensional reduced graphene oxide nanosheets |
| DA | Dopamine |
| 2,4-DCP | 2,4-Dichlorophenol |
| 3D GNCs | 3D graphene-supported quantum dots |
| 3DG | Three-dimensional graphene |
| DBD | Diamond Boron-doped |
| EMSN-AuNPs | Expanded mesoporous silica-encapsulated gold nanoparticles |
| ENC | Electronanocatalyst |
| ERGO | Electrochemically reduced graphene oxide |
| FePP | Iron porphyrins |
| GBR | Metal-free brominated graphene |
| GCE | Glassy carbon electrode |
| GCE/MWCNTs-Av/RuNPs/biot-GOx | Glassy carbon electrode/avidin-functionalized multi-walled carbon/nanotubes/Ru nanoparticles/biotinylated glucose oxidase |
| GDCh | Glucose derived sheet-like carbons |
| GE | Graphite electrode |
| GNP/Cu-Cys | Gold nanoparticles with the copper(II) complex of cysteine |
| GNPs | Gold nanoparticles |
| GNs | Graphene nanosheets |
| GO | Graphene oxide |
| GOx | Glucose oxidase |
| GOx/PtNP/PAni/Pt | Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel |
| H2TCPP | meso-Tetrakis(4-carboxyphenyl)-porphyrin |
| HCC | Carbon cubic nanomaterial |
| HCF | Hexacyanoferrate |
| h-CuS NCs | Hollow copper sulfide nanocubes |
| H-GNs | Hemin-graphene hybrid nanosheets |
| His@AuNCs | Histidine-capped gold nanoclusters |
| HNCs | Hollow nanocubes |
| H-rGO-Au | Hemin-graphene-gold nanoparticles |
| HRP | Horseradish peroxidase |
| H/WS2-NSs | Hemin-functionalized/Tungsten disulfide nanosheets |
| ITO | Indium tin oxide |
| L-Cys | L-Cysteine |
| LOD | Limit of detection |
| LOx | Lactate oxidase |
| LR | Linear range |
| MCM-41 | Mobil composition of matter No. 41 |
| MCNs | Mesoporous carbon nanospheres |
| MCP | Microchannel plate |
| MIP | Molecularly imprinted polymer |
| MMT | Montmorillonite |
| MC | Magnetic carbon |
| Mn-MPSA-HCC | Mn nanomaterials: hollow carbon cubic |
| Mn-MPSA-HCS | Mn nanomaterials: hollow carbon sphere |
| MOFs | Metal-Organic Frameworks |
| MWCNTs | Multi-walled carbon nanotubes |
| MWCNTs-Av | Avidin-functionalized multi-walled carbon nanotubes |
| NCs | Nanoclusters |
| NCF | Nitrogen doped cotton carbon fiber composite |
| N-CNFht | Nitrogen-doped carbon nanofibers |
| NFs | Nanoflakes |
| NPs | Nanoparticles |
| NTH | Nanotetrahedron |
| NW/CF | Nanowires/copper foam |
| NZ | Nanozyme |
| OPD | o-Phenylenediamine |
| PAni | Polyaniline |
| PANI/MXene | Polyaniline and Ti3C2Tx |
| PB | Prussian Blue |
| PEG-HCCs | Poly(ethylene glycolated) hydrophilic carbon clusters |
| PFOP | Heptadecafluoro-n-octyl bromide |
| PMMA | Poly(methylmethacrylate) |
| PNAANI | Poly(N-acetylaniline) |
| PO | Natural horseradish peroxidase |
| RDE | Rotating disk electrode |
| RGO | Reduced graphene oxide |
| rGO | Reduced graphene oxide |
| SOD | Superoxide dismutase |
| SPCE | Screen-printed carbon electrode |
| TMB | 3,5,3′,5′-Tetramethylbenzidine |
| UA | Uric acid |
| WCC | Tungsten carbide decorated by cobalt nanoparticles |
| YSNs | Yolk-shell nanostructures |
| ZIF | Zeolitic imidazolate framework |
| β-CD | β-Cyclodextrin |
References
- Breslow, R.; Overman, L.E. An “Artificial Enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, X.; Wei, H. Artificial enzymes: The next wave. In Encyclopedia of Physical Organic Chemistry, 1st ed.; Wang, Z., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 64. ISBN 978-1-118-46858-6. [Google Scholar]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.; Prieto, T.; Nantes, I.L. Peroxidases in nanostructures. Front. Mol. Biosci. 2015, 2, 50. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Fang, L.; Wu, K.; Yan, X.; Fan, K. Ferritins as natural and artificial nanozymes for theranostics. Theranostics 2020, 10, 687–706. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Hong, J.; Yan, X.; Liang, M. Nanozymes: A New Disease Imaging Strategy. Front. Bioeng. Biotech. 2020, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 5, 2190–2200. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A. Prussian Blue and Its Analogues: Electrochemistry and Analytical Applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Meng, X.; Yu, L.; Deng, D.; Bao, X. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019, 119, 1806–1854. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, A.A.; Das, T.; Ghosh, S.; Mugesh, G. A Remarkably Efficient MnFe2O4-based Oxidase Nanozyme. Chem. Asian, J. 2016, 11, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, K.; Xinghua, S. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Liu, J. Special Topic: Nanozyme-Based Analysis and Testing. J. Anal. Test. 2019, 3, 189–190. [Google Scholar] [CrossRef] [Green Version]
- Golchin, J.; Golchin, K.; Alidadian, N.; Ghaderi, S.; Eslamkhah, S.; Eslamkhah, M.; Akbarzadeh, A. Nanozyme applications in biology and medicine: An overview. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gao, Y.; Chandrawati, R.; Hosta-Rigau, L. Therapeutic applications of multifunctional nanozymes. Nanoscale 2019, 11, 21046–21060. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, Y.; Qiu, Z.; Xu, Z.; Li, D.; Chen, L.; Jiang, J.; Gao, L. Vitamin B2 functionalized iron oxide nanozymes for mouth ulcer healing. Sci. China Life Sci. 2019, 62, 12. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; El-Moghazy, A.Y.; Hussin, M.; Abu-Saied, M.A.; El-Shanshory, A.A.; Soliman, H.M.A. The nanomaterials and recent progress in biosensing systems: A review. Trends Environ. Anal. Chem. 2020, e00087. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, N.F.; Kashaninejad, Z.; Firoz, S.H.; Shim, Y.; Shiddiky, M.J.A. Nanozymes-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Hu, Y.; Wei, H. Nanozymes: Preparation and Characterization. In Nanostructure Science and Technology; Yan, X., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–328. [Google Scholar]
- Luo, L.; Zhang, Y.; Li, F.; Si, X.; Ding, Y.; Wang, T. Enzyme mimics of spinel-type CoxNi1−xFe2O4 magnetic nanomaterial for eletroctrocatalytic oxidation of hydrogen peroxide. Anal. Chim. Acta 2013, 788, 46–51. [Google Scholar] [CrossRef]
- Wu, Q.; He, L.; Jiang, Z.W.; Li, Y.; Zheng, M.C.; Cheng, Z.H.; Li, Y.F. CuO Nanoparticles Derived from Metal-Organic Gel with Excellent Electrocatalytic and Peroxidase-Mimicking Activities for Glucose and Cholesterol Detection. Biosens. Bioelectron. 2019, 145, 111704. [Google Scholar] [CrossRef]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin Nanosheet-Assembled [Ni3(OH)2(PTA)2(H2O)4]·2H2O Hierarchical Flowers for High-Performance Electrocatalysis of Glucose Oxidation Reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Cai, X.; Zhao, H.; Magdassi, S.; Lan, M. Electrochemical detection of superoxide anions in HeLa cells by using two enzyme-free sensors prepared from ZIF-8-derived carbon nanomaterials. Mikrochim. Acta 2019, 186, 370. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Electrospun metal–organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors. Chem. Commun. 2017, 53, 1751–1754. [Google Scholar] [CrossRef]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and Thermal Dewetting of Gold Layer onto Graphene Paper for non-Enzymatic Electrochemical Detection of Glucose and Fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Wang, F.; Liao, M.; Jiang, G. Bimetallic nanoparticles decorated hollow nanoporous carbon framework as nanozyme biosensor for highly sensitive electrochemical sensing of uric acid. Biosens. Bioelectron. 2020, 150, 111869. [Google Scholar] [CrossRef]
- Chou, K.S.; Ren, C.Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Sabu, T. Chapter 5. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials Advances and Key Technologies Micro and Nano Technologies, 1st ed.; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Sabu, T., Eds.; Woodhead Publishing Company: Sawston, UK, 2018; pp. 121–139. [Google Scholar]
- Suriati, G.M.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles bu chemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, X.; Nie, W.; Wang, Y.; Gao, J.; Wen, W.; Wang, S. Hollow copper sulfide nanocubes as multifunctional nanozymes for colorimetric detection of dopamine and electrochemical detection of glucose. Biosens. Bioelectron. 2019, 141, 111450. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, T.M.; Andronescu, C.; Cheong, S.; Wilde, P.; Wordsworth, J.; Kientz, M.; Tilley, R.D.; Schuhmann, W.; Gooding, J.J. Electrocatalytic Nanoparticles That Mimic the Three-Dimensional Geometric Architecture of Enzymes: Nanozymes. J. Am. Chem. Soc. 2018, 140, 13449–13455. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-Modified Metal–Organic Frameworks with Multienzymes Activity as Biomimetic Catalysts and Electrocatalytic Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Liu, W.E.; Guo, Y.; Wu, G.; Qi, W.; Lu, X. Enhanced His@AuNCs Oxidase-Like Activity by Reduced Graphene Oxide and Its Application for Colorimetric and Electrochemical Detection of Nitrite. Anal. Bioanal. Chem. 2019, 411, 2189–2200. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Han, R.; Lu, Y.; Mingjun, L.; Yanbo, W.; Xuan, L.; Chongyang, L.; Kun, L.; Lingxing, Z.; Aihua, L. Green tide biomass templated synthesis of molybdenum oxide nanorods supported on carbon as efficient nanozyme for sensitive glucose colorimetric assay. Sens. Actuators B Chem. 2019, 296, 126517. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Li, F. Bioinspired Nanozymes with pH-Independent and Metal Ions-Controllable Activity: Field-Programmable Logic Conversion of Sole Logic Gate System. Part. Part. Syst. Char. 2018, 1800207. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Chang, H.Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Z.; Zhang, H.; Li, Y.; Chen, K.; Zhao, H.; Lan, M. Carbon-mediated synthesis of shape-controllable manganese phosphate as nanozymes for modulation of superoxide anions in HeLa cells. J. Mater. Chem. B 2019, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Dashtestani, F.; Ghourchian, H.; Eskandari, K.; Rafiee-Pour, H.A. A superoxide dismutase mimic nanocomposite for amperometric sensing of superoxide anions. Microchim. Acta 2015, 82, 1045–1053. [Google Scholar] [CrossRef]
- Sun, D.; Lin, X.; Lu, J.; Wei, P.; Luo, Z.; Lu, X.; Chen, Z.; Zhang, L. DNA Nanotetrahedron-Assisted Electrochemical Aptasensor for Cardiac Troponin I Detection Based on the Co-Catalysis of Hybrid Nanozyme, Natural Enzyme and Artificial DNAzyme. Biosens. Bioelectron. 2019, 42, 111578. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.R.; Weiss, C.K. Nanoparticles from renewable polymers. Front. Chem. 2014, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Guo, P.; Wang, X.; Tu, W.; Bao, J.; Dai, Z. Mesoporous SiO2–(L)-lysine hybrid nanodisks: Direct electron transfer of superoxide dismutase, sensitive detection of superoxide anions and its application in living cell monitoring. RSC Adv. 2013, 3, 20456–20463. [Google Scholar] [CrossRef]
- Manesh, K.M.; Lee, S.H.; Uthayakumar, S.; Gopalan, A.I.; Lee, K.P. Sensitive electrochemical detection of superoxide anion using gold nanoparticles distributed poly(methyl methacrylate)–polyaniline core–shell electrospun composite electrode. Analyst 2011, 136, 1557–1561. [Google Scholar]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [Green Version]
- Al-Bat’hi, S.A.M. Electrodeposition of nanostructure materials. In Electroplating of Nanostructures; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2015; pp. 3–25. [Google Scholar]
- Cioffi, N.; Colaianni, L.; Ieva, E.; Pilolli, R.; Ditaranto, N.; Daniela, M.; Cotrone, S.; Buchholt, K.; Lloyd, A.; Sabbatini, L.; et al. Electrosynthesis and characterization of gold nanoparticles for electronic capacitance sensing of pollutants. Electrochim. Acta 2011, 56, 3713–3720. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Dominguez, S.; Arias-Pardilla, J.; Berenguer-Murcia, A.; Morallon, E.; Cazorla-Amoros, D. Electrochemical deposition of platinum nanoparticles on different carbon supports and conducting polymers. J. Appl. Electrochem. 2008, 38, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Sanchez, L.; Blanco, M.C.; Lopez-Quintela, M.A. Electrochemical Synthesis of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Guangwei, S.; Lixuan, M.; Wensheng, S. Electroplating of nanostructures. Recent Pat. Nanotech. 2009, 3, 182–191. [Google Scholar]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing Electrochemical Interfaces Based on Nanohybrids of Avidin Functionalized-Carbon Nanotubes and Ruthenium Nanoparticles as Peroxidase-Like Nanozyme With Supramolecular Recognition Properties for Site-Specific Anchoring of Biotinylated Residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Zakalskiy, A.; Zakalska, O.; Serkiz, R.; Gonchar, M. Amperometric biosensors based on oxidases and PtRu nanoparticles as artificial peroxidase. Food Chem. 2019, 285, 213–220. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Song, G.; Ran, M.; Lu, X.; Liu, X. An ultrasensitive electrochemical sensor based on cotton carbon fiber composites for the determination of superoxide anion release from cells. Microchim. Acta 2019, 186, 198. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Zhang, J.; Dai, W.; Mo, G.; Ye, J. Bifunctional and highly sensitive electrochemical non-enzymatic glucose and hydrogen peroxide biosensor based on NiCo2O4 nanoflowers decorated 3D nitrogen doped holey graphene hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 708–717. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; Cao, W.; Wang, X.; Qin, L.; Zhou, M.; Wei, H. Nucleobase-mediated synthesis of nitrogen-doped carbon nanozymes as efficient peroxidase mimics. Dalton Trans. 2019, 48, 1993–1999. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, L.; Zhang, J.; Zhou, M.; Shi, G.; Tian, X.; Fan, K.; Hao, C.; Yan, X. Biomineralization Synthesis of the Cobalt Nanozyme in SP94-Ferritin Nanocages for Prognostic Diagnosis of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 9747–9755. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, N.; Zhang, J.; Zhu, J.; Guo, Y.; Xu, L. A novel nanozyme based on selenopeptide-modified gold nanoparticles with a tunable glutathione peroxidase activity. RSC Adv. 2020, 10, 8685–8691. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Zhang, X.; Mao, Y.; Ren, X.; Hu, C.; He, W.; Yin, J. Regulating the Pro- and Anti-Oxidant Capability of Bimetallic Nanozymes for Detection of Fe2+ and Protection of Monascus pigments. Nanoscale 2020, 12, 3068–3075. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kong, L.; Wang, X.; He, J.; Bu, X.H. Engineering Bimetal Synergistic Electrocatalysts Based on Metal–Organic Frameworks for Efficient Oxygen Evolution. Nano Micro. Small 2019, 15, 1903410. [Google Scholar] [CrossRef] [PubMed]
- Cao-Milán, R.; He, L.D.; Shorkey, S.; Tonga, G.Y.; Wang, L.S.; Zhang, X.; Uddin, I.; Das, R.; Sulak, M.; Rotello, V.M. Modulating the Catalytic Activity of Enzyme-like Nanoparticles Through their Surface Functionalization. Mol. Syst. Des. Eng. 2017, 2, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Wang, C. Functionalization of GroEL nanocages with hemin for label-free colorimetric assays. Anal. Bioanal. Chem. 2019, 411, 3819–3827. [Google Scholar] [CrossRef]
- Gao, M.; Lu, X.; Nie, G.; Chi, M.; Wang, C. Hierarchical CNFs/MnCo2O4.5 nanofibers as a highly active oxidase mimetic and its application in biosensing. Nanotechnology 2017, 28, 485708. [Google Scholar] [CrossRef]
- Wu, L.; Wan, G.; Hu, N.; He, Z.; Shi, S.; Suo, Y.; Wang, K.; Xu, X.; Tang, Y.; Wang, G. Synthesis of Porous CoFe2O4 and Its Application as a Peroxidase Mimetic for Colorimetric Detection of H2O2 and Organic Pollutant Degradation. Nanomaterials 2018, 8, 451. [Google Scholar] [CrossRef] [Green Version]
- Shackery, I.; Patil, U.; Pezeshki, A.; Shinde, N.M.; Im, S.; Jun, S.C. Enhanced Non-enzymatic amperometric sensing of glucose using Co(OH)2 nanorods deposited on a three dimensional graphene network as an electrode material. Microchim. Acta 2016, 183, 2473–2479. [Google Scholar] [CrossRef]
- Tyagi, M.; Tomar, M.; Gupta, V. Glad assisted synthesis of NiO nanorods for realization of enzymatic reagentless urea biosensor. Biosens. Bioelectron. 2014, 52, 196–201. [Google Scholar] [CrossRef]
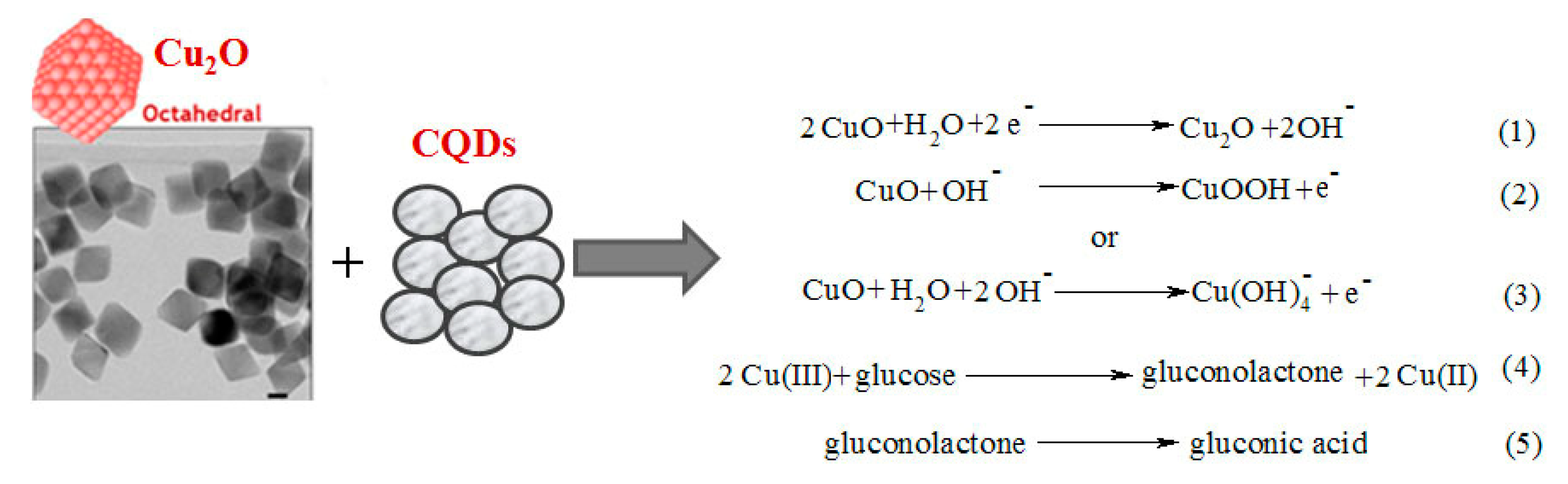
- Li, Y.; Zhong, Y.; Zhang, Y.; Weng, W.; Li, S. Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuators B Chem. 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Wu, H.X.; Cao, W.M.; Li, Y.; Liu, G.; Wen, Y.; Yang, H.F.; Yang, S.P. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta 2010, 55, 3734–3740. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, A.; Cunningham, J.; Bulbula, G.; Andreescu, S. Evaluation of the oxidase like activity of nanoceria and its application in colorimetric assays. Anal. Chim. Acta 2015, 885, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, S.; Wang, L.-S.; Abdullah, M.; Hussain, S.Z.; Iqbal, Z.; Rotello, V.M.; Hussain, I. Facile method to synthesize dopamine-capped mixed ferrite nanoparticles and their peroxidase-like activity. J. Phys. D Appl. Phys. 2017, 50, 11LT02. [Google Scholar] [CrossRef]
- Liu, S.; Lu, F.; Xing, R.; Zhu, J.J. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chem. Eur. J. 2011, 17, 620–625. [Google Scholar] [CrossRef]
- Rauf, S.; Ali, N.; Tayyab, Z.; Shah, M.Y.; Yang, C.P.; Hu, J.F.; Kong, W.; Huang, Q.A.; Hayat, A.; Muhammad, M. Ionic liquid coated zerovalent manganese nanoparticles with stabilized and enhanced peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Mater. Res. Express 2020, 7, 035018. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Wang, Z.; Liu, J.; Li, T.; Wang, B.; Yang, Z. A simple route to CoFe2O4 nanoparticles with shape and size control and their tunable peroxidase-like activity. RSC Adv. 2015, 5, 10632–10640. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, J.; Zhou, Y.; Pan, X.; He, H.; Ye, Z.; Pan, X. Controlled synthesis of spinel ZnFe2O4 decorated ZnO heterostructures as peroxidase mimetics for enhanced colorimetric biosensing. Chem. Commun. 2013, 49, 7656–7658. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, L.; Yang, X.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: A synergistic CeO2/Zeolite Y nanocomposite. J. Colloid Interface Sci. 2019, 535, 425–435. [Google Scholar] [CrossRef]
- Hong, J.; Wang, W.; Huang, K.; Yang, W.; Zhao, Y.; Xiao, B.; Gao, Y.; Moosavi-Movahedi, Z.; Ahmadian, S.; Bohlooli, M.; et al. A self-assembled nano-cluster complex based on cytochrome c and nafion: An efficient nanostructured peroxidise. Biochem. Eng. J. 2012, 65, 16–22. [Google Scholar] [CrossRef]
- Rui, Q.; Liangliang, S.; Aoting, Q.; Ruolin, W.; Yingli, A.; Linqi, S. Artificial Peroxidase/Oxidase Multiple Enzyme System Based on Supramolecular Hydrogel and Its Application as a Biocatalyst for Cascade Reactions. ACS Appl. Mater. Interfaces 2015, 7, 16694–16705. [Google Scholar] [CrossRef]
- Zeng, H.H.; Qiu, W.B.; Zhang, L.; Liang, R.P.; Qiu, J.D. Lanthanide Coordination Polymer Nanoparticles as an Excellent Artificial Peroxidase for Hydrogen Peroxide Detection. Anal. Chem. 2016, 88, 6342–6348. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ding, Y.; Jiang, Y.; Liu, Q. Montmorillonite-loaded ceria nanocomposites with superior peroxidase-like activity for rapid colorimetric detection of H2O2. Sens. Actuators B Chem. 2017, 239, 848–856. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Gao, C.; Zhang, M.; Chen, J.; Qiu, H. Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst 2015, 140, 2857–2863. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. Onestep synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sens. Actuators B Chem. 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Yang, Y.; Tan, Y.; He, Y.; Liu, M.; Liu, X.; Yuan, Q. Peroxidase-mimicking nanozyme with enhanced activity and high stability based on metal-support interaction. Chem. Eur. J. 2018, 24, 409–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.N.; Sun, X.T.; Chen, L.; Xu, Z.R. Boron nitride nanosheet/CuS nanocomposites as mimetic peroxidase for sensitive colorimetric detection of cholesterol. Sens. Actuators B Chem. 2017, 246, 118–126. [Google Scholar] [CrossRef]
- Jampaiah, D.; Reddy, T.S.; Coyle, V.E.; Nafady, A.; Bhargava, S.K. Co3O4@CeO2 hybrid flower-like microspheres: A strong synergistic peroxidase-mimicking artificial enzyme with high sensitivity for glucose detection. J. Mater. Chem. B. 2017, 5, 720–773. [Google Scholar] [CrossRef]
- Vallabani, N.V.; Karakoti, A.S.; Singh, S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surf. B Biointerfaces 2017, 153, 52–60. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, M.; Shu, J.X.; Li, Y. One-step solvothermal synthesis of Fe3O4@Cu@Cu2O nanocomposite as magnetically recyclable mimetic peroxidase. J. Alloys Compd. 2016, 682, 432–440. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhang, L.Y.; Li, H.; Jia, Q.; Jiang, Y.; Yang, Y.; Zhu, R. One-pot synthesis of porphyrin functionalized gamma-Fe2O3 nanocomposites as peroxidase mimics for H2O2 and glucose detection. Mat. Sci. Eng. C Mater. 2015, 55, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahoo, R.; Ray, C.; Dutta, S.; Pa, T. Soft template induced phase selective synthesis of Fe2O3 nanomagnets: One step towards peroxidase-mimic activity allowing colorimetric sensing of thioglycolic acid. RSC Adv. 2016, 6, 32308–32318. [Google Scholar] [CrossRef]
- Nagvenkar, A.P.; Gedanken, A. Cu0.89Zn0.11O, A New Peroxidase-Mimicking Nanozyme with High Sensitivity for Glucose and Antioxidant Detection. ACS Appl. Mater. Interfaces 2016, 8, 22301–22308. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhang, M.; Ding, L.; Zheng, J.; Zeng, C.; Wen, Y.; Liu, G.; Aldalbahi, A.; Shi, J.; Song, S.; et al. Yolk-shell nanostructured Fe3O4@C magnetic nanoparticles with enhanced peroxidase-like activity for label-free colorimetric detection of H2O2 and glucose. Nanoscale 2017, 9, 4508–4515. [Google Scholar] [CrossRef]
- He, W.W.; Zhou, Y.T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z.; Boudreau, M.D.; Yin, J.J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013, 34, 765–773. [Google Scholar] [CrossRef]
- Bustami, Y.; Murray, M.Y.; William, A.A. Manipulation of Fe/Au Peroxidase-Like Activity for Development of a Nanocatalytic-Based Assay. J. Eng. Sci. 2017, 13, 29–52. [Google Scholar] [CrossRef]
- He, W.; Han, X.; Jia, H.; Cai, J.; Zhou, Y.; Zheng, Z. AuPt Alloy Nanostructures with Tunable Composition and Enzyme-like Activities for Colorimetric Detection of Bisulfide. Sci. Rep. 2017, 7, e40103. [Google Scholar] [CrossRef]
- He, S.B.; Chen, R.T.; Wu, Y.Y.; Wu, G.W.; Peng, H.P.; Liu, A.L.; Deng, H.H.; Xia, X.H.; Chen, W. Improved enzymatic assay for hydrogen peroxide and glucose by exploiting the enzyme-mimicking properties of BSA-coated platinum nanoparticles. Mikrochim. Acta 2019, 186, e778. [Google Scholar] [CrossRef]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt Nanoclusters as Robust Peroxidase Mimics for Colorimetric Detection of Glucose in Human Serum. ACS Appl. Mater. Interfaces 2017, 9, 10027–10033. [Google Scholar] [CrossRef]
- Cao, G.; Jiang, X.; Zhang, H.; Croleyb, T.R.; Yin, J. Mimicking horseradish peroxidase and oxidase using ruthenium nanomaterials. RSC Adv. 2017, 7, 52210–52217. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Chen, X.; Shi, S.; Moa, S.; Zheng, N. An investigation of the mimetic enzyme activity of two-dimensional Pd-based nanostructures. Nanoscale 2015, 7, 19018–19026. [Google Scholar] [CrossRef] [PubMed]
- Shkotova, L.; Bohush, A.; Voloshina, I.; Smutok, O.; Dzyadevych, S. Amperometric biosensor modified with platinum and palladium nanoparticles for detection of lactate concentrations in wine. SN Appl. Sci. 2019, 1, 306. [Google Scholar] [CrossRef] [Green Version]
- Kluenker, M.; Tahir, M.N.; Ragg, R.; Korschelt, K.; Simon, P.; Gorelik, T.E.; Barton, B.; Shylin, S.I.; Panthöfer, M.; Herzberger, J.; et al. Pd@Fe2O3 Superparticles with Enhanced Peroxidase Activity by Solution Phase Epitaxial Growth. Chem. Mater. 2017, 29, 1134–1146. [Google Scholar] [CrossRef]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J.; Gong, P.; Wang, C.; Shu, C. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens. Bioelectron. 2013, 47, 502–507. [Google Scholar] [CrossRef]
- Sajjadi, S.; Keihan, A.H.; Norouzi, P.; Habibi, M.M.; Eskandari, K.; Shirazi, N.H. Fabrication of an Amperometric Glucose Biosensor Based on a Prussian Blue/Carbon Nanotube/Ionic Liquid Modified Glassy Carbon Electrode. J. Appl. Biotechnol. Rep. 2017, 4, 603–608. [Google Scholar]
- Guerrero, L.A.; Fernández, L.; González, G.; Montero-Jiménez, M.; Uribe, R.; Díaz Barrios, A.; Espinoza-Montero, P.J. Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials 2020, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yuchi, Q.; Li, T.; Yang, G.; Miao, J.; Huang, C.; Liu, J.; Li, A.; Qin, Y.; Zhang, L. Precise engineering of ultra-thin Fe2O3 decorated Pt-based nanozymes via atomic layer deposition to switch off undesired activity for enhanced sensing performance. Sens. Actuators B Chem. 2020, 305, 127436. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, L.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef]
- Kung, C.C.; Lin, P.Y.; Buse, F.J.; Xue, Y.; Yu, X.; Dai, L.; Liu, C.C. Preparation and characterization of three-dimensional graphene foam supported platinum-ruthenium bimetallic nanocatalysts for hydrogen peroxide based electrochemical biosensors. Biosens. Bioelectron. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Li, X.-R.; Xu, M.; Chen, H.; Xu, J. Bimetallic Au@Pt@Au core–shell nanoparticles on graphene oxide nanosheets for high-performance H2O2 bi-directional sensing. J. Mater. Chem. B 2015, 3, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, H.; Chen, F.; Liu, J.; Zhang, H.; Yang, Z.; Wang, B. Strong coupled palladium nanoparticles decorated on magnetic graphene nanosheets as enhanced peroxidase mimetics for colorimetric detection of H2O2. Dye. Pigment. 2016, 125, 64–71. [Google Scholar] [CrossRef]
- Lv, X.; Weng, J. Ternary composite of hemin, gold nanoparticles and graphene for highly efficient decomposition of hydrogen peroxide. Sci. Rep. 2013, 3, e3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Liu, X.; Wang, X.; Han, Q.; Qi, C.; Li, Y.; Wang, C.; Chen, Y.; Yang, R. Colorimetric determination of ascorbic acid using a polyallylamine-stabilized IrO2/graphene oxide nanozyme as a peroxidase mimic. Microchim. Acta 2020, 187, 110. [Google Scholar] [CrossRef]
- Darabdhara, G.; Sharma, B.; Das, M.R.; Boukherroub, R.; Szunerits, S. Cu-Ag bimetallic nanoparticles on reduced graphene oxidenanosheets as peroxidase mimic for glucose and ascorbic aciddetection. Sens. Actuators B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]
- Song, L.N.; Huang, C.; Zhang, W.; Ma, M.; Chen, Z.; Gu, N.; Zhang, Y. Graphene oxide-based Fe2O3 hybrid enzyme mimetic with enhanced peroxidase and catalase-like activities. Colloid Surf. A 2016, 506, 747–755. [Google Scholar] [CrossRef]
- Dong, W.; Zhuang, Y.; Li, S.; Zhang, X.; Chai, H.; Huang, Y. High peroxidase-like activity of metallic cobalt nanoparticles encapsulated in metal–organic frameworks derived carbon for biosensing. Sens. Actuators B Chem. 2018, 255, 2050–2057. [Google Scholar] [CrossRef]
- Karuppiah, C.; Palanisamy, S.; Chen, S.; Veeramani, V.; Periakaruppan, P. A novel enzymatic glucose biosensor and sensitive non-enzymatic hydrogen peroxide sensor based on graphene and cobalt oxide nanoparticles composite modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 196, 450–456. [Google Scholar] [CrossRef]
- Bahreini, M.; Movahedi, M.; Peyvandi, M.; Nematollahi, F.; Tehrani, H.S. Thermodynamics and kinetic analysis of carbon nanofibers as nanozymes. Nanotechnol. Sci. Appl. 2019, 12, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhou, X.; Wu, X.; Yang, Y.; Wu, H.; Guo, S.; Zhang, J. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 2013, 5, 1816–1819. [Google Scholar] [CrossRef]
- Zhang, L.; Hai, X.; Xia, C.; Chen, X.W.; Wang, J.H. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 248, 374–384. [Google Scholar] [CrossRef]
- Fakhri, N.; Salehnia, F.; Beigi, S.M.; Aghabalazadeh, S.; Hosseini, M.; Ganjali, M.R. Enhanced peroxidase-like activity of platinum nanoparticles decorated on nickel- and nitrogen-doped graphene nanotubes: Colorimetric detection of glucose. Microchim. Acta 2019, 186, e385. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wan, G.; Shi, S.; He, Z.; Xu, X.; Tang, Y.; Hao, C.; Wang, G. Atomic layer deposition-assisted growth of CuAl LDH on carbon fiber as a peroxidase mimic for colorimetric determination of H2O2 and glucose. New J. Chem. 2019, 15, 5826–5832. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, Y.; Li, W.; Ren, J.; Qu, X. Bioinspired Design of Fe3+-doped mesoporous carbon nanospheres for enhanced nanozyme activity. Chemistry 2018, 24, 7259–7263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huo, D.; Bao, J.; Yang, M.; Chen, M.; Hou, J.; Fa, H.; Hou, C. Biosensor based on 3D graphene-supported Fe3O4 quantum dots as biomimetic enzyme for in situ detection of H2O2 released from living cells. Sens. Actuators B Chem. 2017, 244, 1037–1044. [Google Scholar] [CrossRef]
- Song, H.; Zhao, H.; Zhang, X.; Xu, Y.; Cheng, X.; Gao, S.; Huo, L. Ahollow urchin-like α-mno2 as an electrochemical sensor for hydrogen peroxide and dopamine with high selectivity and sensitivity. Microchim. Acta 2019, 186, 210–221. [Google Scholar] [CrossRef]
- Mei, H.; Wang, X.; Zeng, T.; Huang, L.; Wang, Q.; Ru, D.; Huang, T.; Tian, F.; Wu, H.; Gao, J. A nanocomposite consisting of gold nanobipyramids and multiwalled carbon nanotubes for amperometric nonenzymatic sensing of glucose and hydrogen peroxide. Microchim. Acta 2019, 186, 235–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Y.; Shao, Z.; Chen, C.; Yang, M.; Lu, G.; Xu, W.; Liao, X. Amperometric hydrogen peroxide sensor using a glassy carbon electrode modified with a nanocomposite prepared from ferumoxytol and reduced graphene oxide decorated with platinum nanoparticles. Microchim. Acta 2019, 186, 386. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Du, X.; Chen, Y.; Dong, W.; Han, B.; Chen, Q. Facile fabrication of Pt-Ag bimetallic nanoparticles decorated reduced graphene oxide for highly sensitive non-enzymatic hydrogen peroxide sensing. Talanta 2016, 159, 280–286. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Mitra, K.; Singh, R.; Kumar, S.; Gupta, S.; Tiwari, I.; Ray, B. Electrochemical sensing of hydrogen peroxide using brominated graphene as mimetic catalase. Electrochim. Acta 2017, 258, 1435–1444. [Google Scholar] [CrossRef]
- Fazli, G.; Bahabadi, S.E.; Adlnasab, L.; Ahmar, H. A glassy carbon electrode modified with a nanocomposite prepared from Pd/Al layered double hydroxide and carboxymethyl cellulose for voltammetric sensing of hydrogen peroxide. Mikrochim. Acta 2019, 186, 821. [Google Scholar] [CrossRef] [PubMed]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A nanocomposite prepared from platinum particles, polyaniline and a Ti3C2 MXene for amperometric sensing of hydrogen peroxide and lactate. Mikrochim. Acta 2019, 186, 752. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Liu, X.; Chen, L.; Li, D.; Jia, J. A hollow CuOx/NiOy nanocomposite for amperometric and non-enzymatic sensing of glucose and hydrogen peroxide. Microchim. Acta 2019, 186, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Veeramani, V.; Chen, S.M.; Madhu, R.; Liu, S.B. Porous carbon-NiO nanocomposites for amperometric detection of hydrazine and hydrogen peroxide. Microchim. Acta 2019, 186, 59–66. [Google Scholar] [CrossRef]
- Sha, R.; Vishnu, N.; Badhulika, S. Bimetallic Pt-Pd nanostructures supported on MoS2 as an ultra-high performance electrocatalyst for methanol oxidation and nonenzymatic determination of hydrogen peroxide. Microchim. Acta 2018, 185, 399–409. [Google Scholar] [CrossRef]
- Lyu, Y.P.; Wu, Y.S.; Wang, T.P.; Lee, C.L.; Chung, M.Y.; Lo, C.T. Hydrothermal and plasma nitrided electrospun carbon nanofibers for amperometric sensing of hydrogen peroxide. Microchim. Acta 2018, 185, 371–377. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Shang, Y.; Zhang, P.; Liu, J.; Zheng, J. Preparation of a nanocomposite material consisting of cuprous oxide, polyaniline and reduced graphene oxide, and its application to the electrochemical determination of hydrogen peroxide. Microchim. Acta 2018, 185, 172–179. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Balasubramanian, P.; Chen, S.M.; Chen, T.W. Enzyme-free electrocatalytic sensing of hydrogen peroxide using a glassy carbon electrode modified with cobalt nanoparticle-decorated tungsten carbide. Mikrochimica. Acta 2019, 186, 265. [Google Scholar] [CrossRef]
- Davidson, D. The Prussian blue paradox. J. Chem. Educ. 1937, 14, 238–241. [Google Scholar] [CrossRef]
- Guari, Y.; Larionova, J. (Eds.) Prussian Blue-Type Nanoparticles and Nanocomposites: Synthesis, Devices and Applications; Jenny Stanford Publishing: Singapore, 2019; p. 314. ISBN 97898148000510. [Google Scholar]
- Matos-Peralta, Y.; Antuch, M. Review—Prussian Blue and Its Analogs as Appealing Materials for Electrochemical Sensing and Biosensing. J. Electrochem. Soc. 2020, 167, 037510. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ojwang, D.O. Prussian Blue Analogue Copper Hexacyanoferrate: Synthesis, Structure Characterization and Its Applications as Battery Electrode and CO2 Adsorbent. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 13 October 2017. Available online: http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1136799&dswid=8693 (accessed on 7 May 2020).
- Komkova, M.A.; Andreev, E.A.; Ibragimova, O.A.; Karyakin, A.A. Prussian Blue based flow-through (bio)sensors in power generation mode: New horizons for electrochemical analyzers. Sens. Actuators B Chem. 2019, 292, 284–288. [Google Scholar] [CrossRef]
- Ivanov, V.D. Four decades of electrochemical investigation of Prussian blue. Ionics 2020, 26, 531–547. [Google Scholar] [CrossRef]
- Chen, W.; Gao, G.; Jin, Y.; Deng, C. A facile biosensor for Aβ40O based on fluorescence quenching of prussian blue nanoparticles. Talanta 2020, 216, 120390. [Google Scholar] [CrossRef]
- Meng, X.; Gao, L.; Fan, K.; Yan, X. Nanozyme-Based Tumor Theranostics. In Nanostructure Science and Technology; Yan, X., Ed.; Springer: Singapore, 2020; pp. 425–457. ISBN 978-981-15-1489-0. [Google Scholar]
- He, L.; Li, Z.; Guo, C.; Hu, B.; Wang, M.; Zhang, Z.; Du, M. Bifunctional bioplatform based on NiCo Prussian blue analogue: Label-free impedimetric aptasensor for the early detection of carcino-embryonic antigen and living cancer cells. Sens. Actuators B Chem. 2019, 298. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Shamsipur, M.; Saber, R.; Sarkar, S.; Zolfaghari, N. An ultrasensitive sandwich-type electrochemical immunosensor for the determination of SKBR-3 breast cancer cell using rGOTPA/FeHCF-labeld Anti-HCT as a signal tag. Sens. Actuators B Chem. 2017, 243, 823–830. [Google Scholar] [CrossRef]
- Wang, M.; Hu, B.; Ji, H.; Song, Y.; Liu, J.; Peng, D.; He, L.; Zhang, Z. Aptasensor based on hierarchical core-shell nanocomposites of zirconium hexacyanoferrate nanoparticles and mesoporous mFe3O4@mC: Electrochemical quantitation of epithelial tumor marker mucin-1. ACS Omega 2017, 2, 6809–6818. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Zhang, C.; Zhang, S.; Jia, Y.; Dong, Y. An enzyme-free immunosensor for sensitive determination of procalcitonin using NiFe PBA nanocubes@TB as the sensing matrix. Anal. Chim. Acta 2020, 1097, 169–175. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Kang, M.; Song, Y.; He, L.; Zhou, N.; Wang, M.; Zhang, Z.; et al. PEGMA-modified bimetallic NiCo Prussian blue analogue doped with Tb (III) ions: Efficiently pH-responsive and controlled release system for anticancer drug. Chem. Eng. 2020, 389. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, B.; Huang, X.; Mao, Y.; Li, Y.; Yang, F.; Gu, N. Magnetic internal heating-induced high performance Prussian blue nanoparticle preparation and excellent catalytic activity. Dalton. Trans. 2019, 48, 17169–17173. [Google Scholar] [CrossRef] [PubMed]
- Nwamba, O.C.; Echeverria, E.; McIlroy, D.N.; Shreeve, J.M.; Aston, D.R. Electrochemical stability and capacitance of in-situ synthesized Prussian blue on thermally-activated graphite. SN. Appl. Sci. 2019, 1, 731–746. [Google Scholar] [CrossRef] [Green Version]
- Sahar, S.; Zeb, A.; Ling, C.; Raja, A.; Wang, G.; Ullah, N.; Lin, X.M.; Xu, A.-W. A Hybrid VOx Incorporated Hexacyanoferrate Nanostructured Hydrogel as a Multienzyme Mimetic via Cascade Reactions. ACS Nano 2020, 14, 3017–3031. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, T.; Tanaka, M.; Honjo, M.; Fukunaga, Y.; Okamura, T.; Ohba, M. Direct Synthesis of Prussian Blue Nanoparticles in Liposomes Incorporating Natural Ion Channels for Cs+ Adsorption and Particle Size Control. Langmuir 2018, 34, 1666–1672. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Itaya, K.; Uchida, I.; Neff, V.D. Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc. Chem. Res. 1986, 19, 162–168. [Google Scholar] [CrossRef]
- Cui, F.; Deng, Q.; Sun, L. Prussian blue modified metal–organic framework MIL-101(Fe) with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. RSC Adv. 2015, 5, 98215–98221. [Google Scholar] [CrossRef]
- Keihan, A.H.; Karimi, R.R.; Sajjadi, S. Wide dynamic range and ultrasensitive detection of hydrogen peroxide based on beneficial role of gold nanoparticles on the electrochemical properties of prussian blue. J. Electroanal. Chem. 2020, 862, 114001. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Y.; Cheng, T.; Lai, W.Y.; Huang, W. Uniform manganese hexacyanoferrate hydrate nanocubes featuring superior performance for low-cost supercapacitors and nonenzymatic electrochemical sensors. Nanoscale 2015, 7, 16012–16019. [Google Scholar] [CrossRef]
- Wang, C.; Ren, G.; Yuan, B.; Zhang, W.; Lu, M.; Liu, J.; Li, K.; Lin, Y. Enhancing Enzyme-like Activities of Prussian Blue Analog Nanocages by Molybdenum Doping: Toward Cytoprotecting and Online Optical Hydrogen Sulfide Monitoring. Anal. Chem. 2020, 92, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, S.; Yin, J.J.; He, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Zhou, Y.; Zhang, W.; Zhang, Y.; Gu, N. Polystyrene@Au@Prussian Blue Nanocomposites with Enzyme-Like Activity and Their Application in Glucose Detection. Colloids Surf. A 2016, 490, 291–299. [Google Scholar] [CrossRef]
- Zhou, D.; Zeng, K.; Yang, M. Gold Nanoparticle-Loaded Hollow Prussian Blue Nanoparticles with Peroxidase-Like Activity for Colorimetric Determination of L-Lactic Acid. Microchim. Acta 2019, 186, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, D.; Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 2014, 120, 362–367. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Chen, Y.; Li, S.; Gu, N.; Hu, S.; Sun, Y.; Chen, X.; Li, Q. Prussian Blue Modified Ferritin as Peroxidase Mimetics and Its Applications in Biological Detection. J. Nanosci. Nanotechnol. 2013, 13, 60–67. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.; Chai, L.; Chao, L.; Bu, L.; Meng, Y.; Chen, C.; Ma, M.; Xie, Q.; Yao, S. Filling Carbon Nanotubes with Prussian Blue Nanoparticles of High Peroxidase-Like Catalytic Activity for Colorimetric Chemo- And Biosensing. Chem. Eur. J. 2014, 20, 2623–2630. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian Blue-Based First-Generation Biosensor. A Sensitive Amperometric Electrode for Glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Amperometric Biosensor for Glutamate Using Prussian Blue-Based “Artificial Peroxidase” as a Transducer for Hydrogen Peroxide. Anal. Chem. 2000, 72, 1720–1723. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Communication—Accessing Stability of Oxidase-Based Biosensors via Stabilizing the Advanced H2O2 Transducer. J. Electrochem. Soc. 2017, 164, B3056–B3058. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue modified boron-doped diamond interfaces for advanced H2O2 electrochemical sensors. Electrochim. Acta. 2020, 339. [Google Scholar] [CrossRef]
- Vokhmyanina, D.V.; Andreeva, K.D.; Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Artificial peroxidase” nanozyme—Enzyme based lactate biosensor. Talanta 2020, 208, 120393. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, N.A.; Borisova, A.V.; Komkova, M.A.; Karyakin, A.A. Superstable advanced hydrogen peroxide transducer based on transition metal hexacyanoferrates. Anal. Chem. 2011, 83, 2359–2363. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, S.; Xu, H.; Zhao, Q.; Zhang, Z.; Yao, Y. Preparation of poly (N-acetylaniline)–Prussian blue hybrid composite film and its application to hydrogen peroxide sensing. Anal. Methods 2014, 6, 8003–8010. [Google Scholar] [CrossRef]
- Zhao, H.-C.; Zhang, P.; Li, S.-H.; Luo, H.-X. Cobalt hexacyanoferrate-modified graphene platform electrode and its electrochemical sensing toward hydrogen peroxide. Chin. J. Anal. Chem. 2017, 45, 830–836. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.A.; Wang, G.; Zhao, J.; Hu, M.; Qu, L. A novel nonenzymatic H2O2 sensor based on cobalt hexacyanoferrate nanoparticles and graphene composite modified electrode. Sens. Actuators B Chem. 2015, 208, 593. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, J.-H.; Park, H.-K.; Lee, G.-J. A Simple and Facile Glucose Biosensor Based on Prussian Blue Modified Graphite String. J. Sens. 2016, 2016, 1859292. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.C.; Panday, D.; Pandey, A.K. Polyethylenimine mediated synthesis of copper-iron and nickel-iron hexacyanoferrate nanoparticles and their electroanalytical applications. J. Electroanal. Chem. 2016, 780, 90–102. [Google Scholar] [CrossRef]
- Huang, J.; Fang, X.; Liu, X.; Lu, S.; Li, S.; Yang, Z.; Feng, X. High-Linearity Hydrogen Peroxide Sensor Based on Nanoporous Gold Electrode. J. Electrochem. Soc. 2019, 166, B814. [Google Scholar] [CrossRef]
- Kumar, A.V.; Harish, S.; Joseph, J.; Phani, K.L. Nix–Fe(1−x)Fe(CN)6 hybrid thin films electrodeposited on glassy carbon: Effect of tuning of redox potentials on the electrocatalysis of hydrogen peroxide. J. Electroanal. Chem. 2011, 659, 128–133. [Google Scholar] [CrossRef]
- Niu, Q.; Bao, C.; Cao, X.; Liu, C.; Wang, H.; Lu, W. Ni–Fe PBA hollow nanocubes as efficient electrode materials for highly sensitive detection of guanine and hydrogen peroxide in human whole saliva. Biosens. Bioelectron. 2019, 141, 111445. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.S.; Narayanan, S.S. A novel bimediator amperometric sensor for electrocatalytic oxidation of gallic acid and reduction of hydrogen peroxide. Anal. Chim. Acta 2014, 828, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Clausmeyer, J.; Actis, P.; Córdoba, A.L.; Korchev, Y.; Schuhmann, W. Nanosensors for the detection of hydrogen peroxide. Electrochem. Commun. 2014, 40, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Zhang, X.; Wang, J. NanoBiosensing: Principles, Development and Application, Biological and Medical Physics, Biomedical Engineering Series; Springer: New York, NY, USA, 2016; p. 586. ISBN 978-1441996213. [Google Scholar]
- Dimcheva, N. Nanostructures of noble metals as functional materials in biosensors. Curr. Opin. Electrochem. 2020, 19, 35–41. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomater 2011, 32, 1611–1618. [Google Scholar] [CrossRef]
- Shah, K.; Bhagat, S.; Varade, D.; Singh, S. Novel synthesis of polyoxyethylene cholesteryl ether coated Fe-Pt nanoalloys: A multifunctional and cytocompatible bimetallic alloy exhibiting intrinsic chemical catalysis and biological enzyme-like activities. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 50–57. [Google Scholar] [CrossRef]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.; Chen, Z.; Huang, Y. CoFe2O4 Nanoparticles as Oxidase Mimic-Mediated Chemiluminescence of Aqueous Luminol for Sulfite in White Wines. J. Agric. Food Chem. 2013, 61, 840–847. [Google Scholar] [CrossRef]
- Dalapati, R.; Sakthivel, B.; Ghosalya, M.K.; Dhakshinamoorthy, A.; Biswas, S. A cerium-based metal–organic framework having inherent oxidase-like activity applicable for colorimetric sensing of biothiols and aerobic oxidation of thiols. Cryst. Eng. Comm. 2017, 19, 5915–5925. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Stadler, B.; Erlichman, J.S. In vitro analysis of catalase-, oxidase- and SOD-mimetic activity of commercially available and custom synthesized cerium oxide nanoparticles and assessment of neuroprotective effects in a hippocampal brain slice model of ischemia. FASEB J. 2017, 31, 693–695. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, M.; Li, H.; Wang, X.; Cheng, Y.; Ding, L.; Chen, S. Facile preparation of urchin-like NiCo2O4 microspheres as oxidase mimetic for colormetric assay of hydroquinone. Sens. Actuators B Chem. 2018, 255, 1927–1936. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Wu, X.; Zhu, C.; Su, X.; Du, D.; Lin, Y. Oxidase-mimicking activity of ultrathin MnO2 nanosheets in colorimetric assay of acetylcholinesterase activity. Nanoscale 2017, 9, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhao, Y.; Wang, C.; Song, Q. The oxidase-like activity of iridium nanoparticles, and their application to colorimetric determination of dissolved oxygen. Microchim. Acta 2017, 184, 3113–3119. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Kawashima, K.; Okumura, M.; Haruta, M.; Toshima, N. Synthesis and Catalytic Activity of Crown Jewel-Structured (IrPd)/Au Trimetallic Nanoclusters. Adv. Mater. 2015, 27, 1383–1388. [Google Scholar] [CrossRef]
- Deng, H.-H.; Lin, X.-L.; Liu, Y.-H.; Li, K.-L.; Zhuang, Q.-Q.; Peng, H.-P.; Chen, W. Chitosan-stabilized platinum nanoparticles as effective oxidase mimics for colorimetric detection of acid phosphatase. Nanoscale 2017, 9, 10292–10300. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Shen, J.; Yan, W.; He, L.; Thapa, P.S.; Ren, S.; Subramaniam, B.; Chaudhari, R.V. Exceptional performance of bimetallic Pt1Cu3/TiO2 nanocatalysts for oxidation of gluconic acid and glucose with O2 to glucaric acid. J. Catal. 2015, 330, 323–329. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Yuan, J.; Yin, J.J.; Wu, X.; Hu, X.; Zhang, K.; Liu, J.; Chen, C.; Ji, Y.; et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomater 2011, 32, 1139–1147. [Google Scholar] [CrossRef]
- Zhang, H.; Toshima, N. Preparation of novel Au/Pt/Ag trimetallic nanoparticles and their high catalytic activity for aerobic glucose oxidation. Appl. Catal. A Gen. 2011, 400, 9–13. [Google Scholar] [CrossRef]
- Khawaji, M.; Zhang, Y.; Loh, M.; Graça, I.; Ware, E.; Chadwick, D. Composition dependent selectivity of bimetallic Au-Pd NPs immobilised on titanate nanotubes in catalytic oxidation of glucose. Appl. Catal. B Environ. 2019, 256, 117799. [Google Scholar] [CrossRef]
- Guo, S.; Fang, Q.; Li, Z.; Zhang, J.; Zhang, J.; Li, G. Efficient base-free direct oxidation of glucose to gluconic acid over TiO2-supported gold clusters. Nanoscale 2019, 11, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, X.; Wang, H.; Zheng, H.; Huang, Y. Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC Trends Anal. Chem. 2012, 39, 114–129. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Bonet-Aleta, J.; Hueso, J.L.; Santamaria, J. Gold-Based Nanoparticles on Amino-Functionalized Mesoporous Silica Supports as Nanozymes for Glucose Oxidation. Catalysts 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.J.; Zhu, C.F.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C.H. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–74584. [Google Scholar] [CrossRef] [PubMed]
- Kusema, B.T.; Murzin, D.Y. Catalytic oxidation of rare sugars over gold catalysts. Catal. Sci. Technol. 2013, 3, 297–307. [Google Scholar] [CrossRef]
- Benkó, T.; Beck, A.; Geszti, O.; Katona, R.; Tungler, A.; Frey, K.; Guczi, L.; Schay, Z. Selective oxidation of glucose versus CO oxidation over supported gold catalysts. Appl. Catal. A Gen. 2010, 388, 31–36. [Google Scholar] [CrossRef]
- Cao, X.; Wang, N. A novel non-enzymatic glucose sensor modified with Fe2O3 nanowire arrays. Analyst 2011, 136, 4241–4246. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Ivanova, S.; López-Cartes, C.; Centeno, M.A.; Odriozola, J.A. Gold catalysts screening in base-free aerobic oxidation of glucose to gluconic acid. Catal. Today 2017, 279, 148–154. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bobadilla, L.F.; Ivanova, S.; Penkova, A.; Centeno, M.A.; Odriozola, J.A. Gold catalyst recycling study in base-free glucose oxidation reaction. Catal. Today 2018, 301, 72–77. [Google Scholar] [CrossRef]
- Okatsu, H.; Kinoshita, N.; Akita, T.; Ishida, T.; Haruta, M. Deposition of gold nanoparticles on carbons for aerobic glucose oxidation. Appl. Catal. A Gen. 2009, 369, 8–14. [Google Scholar] [CrossRef]
- Lang, N.J.; Liu, B.; Liu, J. Characterization of glucose oxidation by gold nanoparticles using nanoceria. J. Colloid Interface Sci. 2014, 428, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Lou, D.D.; Wu, H.A.; Zhang, X.Z.; Zhu, Y.F.; Gu, N.; Zhang, Y. A Novel AuNP-Based Glucose Oxidase Mimic with Enhanced Activity and Selectivity Constructed by Molecular Imprinting and O2-Containing Nanoemulsion Embedding. Adv. Mater. Interfaces 2018, 5, 1801070. [Google Scholar] [CrossRef]
- Ma, W.; Hafez, M.E.; Ma, H.; Long, Y.-T. Unveiling the Intrinsic Catalytic Activities of Single Gold Nanoparticle-based Enzyme Mimetics. Angew. Chem. 2019, 58, 6327–6332. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Iqbal, S.; Miedziak, P.J.; Edwards, J.K.; Armstrong, R.D.; Morgan, D.J.; Wang, J.; Hutchings, G.J. Base-free oxidation of glucose to gluconic acid using supported gold catalysts. Catal. Sci. Technol. 2016, 6, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. One-Step Electrochemical Synthesis of PtNi Nanoparticle-Graphene Nanocomposites for Nonenzymatic Amperometric Glucose Detection. ACS Appl. Mater. Interfaces 2011, 3, 3049–3057. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Xin, Y.; Zhang, Z. Sensitive electrochemical nonenzymatic glucose sensing based on anodized CuO nanowires on three-dimensional porous copper foam. Sci. Rep. 2015, 5, 16115. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Wu, G.; Lu, B.; Li, X.; Wu, H.; Liu, A. Non-enzymatic Amperometric Glucose Sensor Based on Copper Nanowires Decorated Reduced Graphene Oxide. Electroanalysis 2016, 28, 2543–2551. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, H.; Chi, Q.; Liu, X.; Chen, L. Cellulose-supported Pd nanoparticles: Effective for the selective oxidation of glucose into gluconic acid. Polym. Bull. 2019, 77, 1003–1014. [Google Scholar] [CrossRef]
- Kou, B.; Yuan, Y.; Yuan, R.; Chai, Y. Electrochemical biomolecule detection based on the regeneration of high-efficiency cascade catalysis for bifunctional nanozymes. Chem. Commun. 2020, 56, 2276–2279. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Chen, Z.; Ren, J.; Qu, X. Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis. Biomater 2013, 34, 2600–2610. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Chen, D.; Li, F. Protein-Directed Metal Oxide Nanoflakes with Tandem Enzyme-Like Characteristics: Colorimetric Glucose Sensing Based on One-Pot Enzyme-Free Cascade Catalysis. Adv. Funct. Mater. 2018, 28, e1800018. [Google Scholar] [CrossRef]
- Glucose Oxidase BRENDA. Available online: https://www.brenda-enzymes.org/all_enzymes.php?ecno=1.1.3.4&table=Turnover_%20Number#TABSingh (accessed on 5 May 2020).
- Miao, F.; Tao, B.; Sun, L.; Liu, T.; You, J.; Wang, L.; Chu, P.K. Amperometric glucose sensor based on 3D ordered nickel–palladium nanomaterial supported by silicon MCP array. Sens. Actuators Chem. 2009, 141, 338–342. [Google Scholar] [CrossRef]
- Ye, J.-S.; Hong, B.-D.; Wu, Y.-S.; Chen, H.-R.; Lee, C.-L. Heterostructured palladium-platinum core-shell nanocubes for use in a nonenzymatic amperometric glucose sensor. Microchim. Acta 2016, 183, 3311–3320. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A photoelectrochemical glucose sensor based on gold nanoparticles as a mimic enzyme of glucose oxidase. RSC Adv. 2019, 9, 15307–15313. [Google Scholar] [CrossRef] [Green Version]
- Espro, C.; Marini, S.; Giusi, D.; Ampelli, C.; Neri, G. Non-enzymatic screen printed sensor based on Cu2O nanocubes for glucose determination in bio-fermentation processes. J. Electroanal. Chem. 2020, 873, 114354. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Cho, A.; Weon, S.; Lee, S.; Lee, J.; Choi, W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nat. Commun. 2019, 10, 940. [Google Scholar] [CrossRef] [Green Version]
- Beden, B.; Largeaud, F.; Kokoh, K.B.; Lamy, C. Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of d-glucose: Identification of reactive intermediates and reaction products. Electrochim. Acta 1996, 41, 701–709. [Google Scholar] [CrossRef]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci. Process. Impacts 2015, 17, 326–342. [Google Scholar] [CrossRef]
- Fathali, Z.; Rezaei, S.; Faramarzi, M.A.; Habibi-Rezaei, M. Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int. J. Biol. Macromol. 2019, 122, 359–366. [Google Scholar] [CrossRef]
- Galli, C.; Madzak, C.; Vadalà, R.; Jolivalt, C.; Gentili, P. Concerted electron/proton transfer mechanism in the oxidation of phenols by laccase. Chembiochem 2013, 14, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernekar, M.; Lele, S.S. Laccase: Properties and Applications. BioResources 2009, 4, 1694–1717. [Google Scholar]
- Yesilada, O.; Birhanli, E.; Geckil, H. Bioremediation and Decolorization of Textile Dyes by White Rot Fungi and Laccase Enzymes. In Mycoremediation and Environmental Sustainability. Fungal Biology; Prasad, R., Ed.; Springer: Berlin, Germany, 2018; pp. 121–153. [Google Scholar]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Yan, X. Nanozymes: An emerging field bridging nanotechnology and biology. Sci. China Life Sci. 2016, 59, 400–402. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Tan, L.; Chen, D.; Wu, X.; Ren, X.; Zhang, Y.; Meng, X.; Tang, F. Fe3O4-Au@mesoporous SiO2 microspheres: An ideal artificial enzymatic cascade system. Chem. Commun. 2013, 49, 4643–4645. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Liu, B.W.; Yang, R.H.; Liu, J.W. Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjug. Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hoshino, W.; Shimizu, S.; Youfu, K.; Aratani, N.; Maruyama, N.; Fujita, S.; Osuka, A. Thermal splitting of bis-Cu(II) octaphyrin (1.1.1.1.1.1.1.1) into two Cu(II) porphyrins. J. Am. Chem. Soc. 2004, 126, 3046–3047. [Google Scholar] [CrossRef]
- Zhou, L.; Powell, D.; Nicholas, K.M. Tripodal bis(imidazole) thioether copper(I) complexes: Mimics of the Cu(b) site of hydroxylase enzymes. Inorg. Chem. 2006, 45, 3840–3842. [Google Scholar] [CrossRef]
- Rivas, M.V.; De Leo, L.P.; Hamer, M.; Carballo, R.; Williams, F.J. Self-assembled monolayers of disulfide Cu porphyrins on Au surfaces: Adsorption induced reduction and demetalation. Langmuir 2011, 27, 10714–10721. [Google Scholar] [CrossRef]
- Uhlmann, C.; Swart, I.; Repp, J. Controlling the orbital sequence in individual Cu-phthalocyanine molecules. Nano Lett. 2013, 13, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, D.; Huang, S.; Chen, W.; Tu, G.; Yu, S.; He, Q.; Gong, J.; Li, Y.; Hong, C.; et al. A magnetically recyclable Fe3O4@C@TNCuPc composite catalyst for chromogenic identification of phenolic pollutants. J. Mol. Catal. A Chem. 2015, 410, 193–201. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Fan, K.L. Application of nanozymes in disease diagnosis. Prog. Biochem. Biophys. 2018, 45, 218–236. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, Z.Y.; Xu, Z.B.; Gao, L.Z. Antibacterial mechanism and applications of nanozymes. Prog. Biochem. Biophys. 2018, 45, 118–128. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, M.; Huang, Y.; Xia, Y. Carbon Dot Nanozymes: How to Be Close to Natural Enzymes. Chem. Eur. J. 2019, 25, 954. [Google Scholar] [CrossRef]
- Shams, S.; Ahmad, W.; Memon, A.H.; Wei, Y.; Yuan, Q.; Liang, H. Facile synthesis of laccase mimic Cu/H 3 BTC MOF for efficient dye degradation and detection of phenolic pollutants. RSC Adv. 2019, 9, 40845–40854. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Shi, R.; Yao, J.-F.; Sheng, C.-F.; Li, H. Supramolecular self-assembly of nucleotide–metal coordination complexes: From simple molecules to nanomaterials. Coordin. Chem. Rev. 2015, 292, 107–143. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Z.; Yuan, Q.; Liu, J. Self-healing metal-coordinated hydrogels using nucleotide ligands. Chem. Commun. 2015, 51, 15196–15199. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Liang, H.; Lin, F.; Zhang, Z.; Liu, B.; Jiang, S.; Yuan, Q.; Liu, J. Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl. Mater. Inter. 2017, 9, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, F.; Yuan, Q.; Liu, J.; Li, Y.; Liang, H. Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J. Environ. Sci. (China) 2020, 88, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lei, L.; Bai, J.; Zhang, L.; Song, D.; Zhao, J.; Li, J.; Li, Y. Efficient elimination and detection of phenolic compounds in juice using laccase mimicking nanozymes. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl. Catal. B Environ. 2019, 254, 452–462. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-nanoparticle-based transphosphorylation catalysts. Angew. Chem. Int. Ed. Engl. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, C.; Li, W.; Zhang, J.; Fu, Y. Catalytic Performance of Oligonucleotide-Templated Pt Nanozyme Evaluated by Laccase Substrates. Catal. Lett. 2017, 147, 2144–2152. [Google Scholar] [CrossRef]
- Andrei, V.; Sharpe, E.; Vasilescu, A.; Andreescu, S. A single use electrochemical sensor based on biomimetic nanoceria for the detection of wine antioxidants. Talanta 2016, 156, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortolini, C.; Bollella, P.; Zumpano, R.; Favero, G.; Mazzei, F.; Antiochia, R. Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, J.; Qu, R.; Wang, Z.; Huang, Q. The laccase-like reactivity of manganese oxide nanomaterials for pollutant conversion: Rate analysis and cyclic voltammetry. Sci. Rep. 2017, 7, 7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Delgado, M.; Orona-Navar, C.; García-Morales, R.; Hernandez-Luna, C.; Parra, R.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation kinetics of pharmaceutical and micropollutants in groundwaters by a laccase cocktail from Pycnoporus sanguineus CS43 fungi. Int. Biodeterior. Biodegrad. 2016, 108, 34–41. [Google Scholar]
- Garcia, L.F.; Souza, A.R.; Lobón, G.S.; dos Santos, W.T.P.; Alecrim, M.F.; Santiago, M.F.; de Sotomayor, R.L.Á.; Gil, E.S. Efficient Enzyme-Free Biomimetic Sensors for Natural Phenol Detection. Molecules 2016, 21, 1060. [Google Scholar] [CrossRef] [PubMed]
- Bin, Z.; Yanhong, C.; Jiaojiao, X. Biomimetic oxidase sensor based on functionalized surface of carbon nanotubes and iron prophyrins for catechol detection. Bioprocess Biosyst. Eng. 2018, 42. [Google Scholar] [CrossRef] [PubMed]





| Advantages | Drawbacks |
|---|---|
| An extremely high rate of enzymatic reactions: spontaneous reactions can run for millions of years, while enzymatic ones run for milliseconds. Examples of particularly active enzymes: -Catalase (2H2O2 → 2H2O + O2) 1 molecule of the enzyme catalyzes the decay of 5 mln S per 1 min; -Carbanhydrase (CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3−): 36 mln turnovers per 1 min. | Physicochemical instability to action of environmental (chemical and physical) factors. Biological instability (susceptibility to degradation by proteases). |
| High selectivity | High costs of isolation and purification. |
| (1) | Availability and low preparation costs. |
| (2) | Physicochemical and biological stability. |
| (3) | High surface area. |
| (4) | Self-assembling activity. |
| (5) | Size/composition-dependent activity. Broad possibility for modification and regulation of activity. |
| (6) | Compatibility with biological elements. |
| Mimetic Enzyme | Enzyme-Like Activity | Preparation Method | Pore Diameter, nm | Shape | Particle Size, nm | BET, m2 ·g−1 | Potential, V | Detection | Linear Range, µM | LOD, µM | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Au/Co@HNCF | Urease | Thermal | 1.2 | Dodeca hedron | 300–400 | 7.88 | +0.3 | Uric acid | 0.1–2500 | 0.023 | [10] |
| Co0.5Ni0.5Fe2O4 | PO | Solvothermal | Sphere | 70–130 | +0.5 | H2O2 | 0.01–1000 | 0.001 | [25] | ||
| SOD/mesoporous SiO2-(L)-lysine | SOD | Hydrolyte polycondensation | 3 | disk | 40–70 | 570 | 0.05 | 3.11–177 | 800 | [27] | |
| SOD/PMMA/PANI-Au | SOD | Electrospinning/polymerization | fiber | 400–500 | 0.3 | 0.5–2.4 | 300 | [28] | |||
| CuO-NZs | GOx PO | Thermal | 2.5 | Sphere | ~45 | 20.16 | +0.55 | Glucose Cholesterol | 5–600 1–15 | 0.59 0.43 | [28] |
| GOx/PtNP/PAni/Pt | Sol-gel method | Sphere/fiber | 2/100 | +0.56 | Glucose | 10–8000 | 0.7 | [43] | |||
| HCC/SPCE PCC/SPCE | SOD | Solid statereaction Thermal | Hollow cubic hexahedral | 100–150 | 356 756 | −0.5 | Superoxide-anion O2− | 0–192 192–912 0–112 112–1152 | 0.207 0.140 | [30] | |
| Graphene paper/AuNPs | GOx | Laser Thermal | Sphere Plate | 10–150 200–400 | +0.17 +0.5 +0.19 +0.4 | Glucose Fructose Glucose Fructose | 20–8000 40–4000 15–8000 5–4000 | 2.5 ≥20 2.5 | [32] | ||
| Ox SOD CAT, PO Ox | Glucose | 10–1·104 | [37] | ||||||||
| PCC/SPCE | Thermal | Sphere | 20 ± 3 | +0.4 | P. aeruginosa | 60.0–6.0 × 107 CFU/mL | 60.0 CFU/mL | [38] | |||
| AgNP/NCF/GCE | Thermal/Electrodeposition | sphere/fiber | 15~20/5~8 μm | −0.5 | 6.96·10−11–72 | 1.66·10−4 | [38] | ||||
| ~2 | Sphere | 8.4 | 2.3 | 0.04–1.0 | Oxygen reduction | [39] | |||||
| Pt@PMOF(Fe) | Chemical reduction/Electrodeposition | Ellipsoid | 300 | −0.45 | Glucose | 100–10,000 | 6 | [40] | |||
| His@AuNCs/RGO-GCE | Chemical reduction | Sheet | 1.72 | +0.8 | Nitrite | 1.0–7000 | 0.5 | [41] | |||
| GOx/PtNP/PAni/Pt | Sol-gel method | Sphere/fiber | 2/100 | +0.56 | Glucose | 10–8000 | 0.7 | [43] | |||
| Mn-MPSA-HCC | GOx | Co-precipitation | Hollow cubic | 100–200 | +0.75 | [47] | |||||
| Mn-MPSA-HCS | Hollow sphere | 0–1260 | 0.001 | ||||||||
| AuNPs/Cu-Cys | Chemical reduction/Co-precipitation | sphere | 77 | 0.25 | 3.1–326 | 2.8 | [48] | ||||
| Fe3O4/Au@Pt-HRP-DNAzyme-Tro6 | PO | Co-precipitation | Sphere | 158 | −0.25 | 4.2·10−13–4.2·10−9 | 3.1·10−15 | [49] | |||
| GCE/MWCNTs-Av/RuNPs/biot-GOx | PO | Drop-coating/Electrodeposition | Tubes/sphere | 1000 | −0.05 | Glucose | 20–1230 | 3.3 | [59] | ||
| nPtRu/AO | Electrodeposition | Sphere | 5–150 | −0.1 | Ethanol | 25–200 | 2.5 | [60] | |||
| nPtRu/AMO | Methylamine | 20–600 | 3 |
| Catalyst | Concentration | KMapp, mM | Vmax, μM·s−1 | kcat, s−1 | Reference |
|---|---|---|---|---|---|
| Fe3O4 NPs | 11.4 × 10−13 M | 154.0 | 0.098 | 8.58 × 104 | [12] |
| HRP | 2.5 × 10−11 M | 3.7 | 0.087 | 3.48 × 103 | [12] |
| CoFe2O4 | 20 μg·mL−1 | 8.89 | 0.019 | [71] | |
| CeO2-MMT | 300 μg·mL−1 | 3.4 | 0.010 | [88] | |
| CeO2 NPs | 300 μg·mL−1 | 3.18 | 0.009 | [88] | |
| H/WS2-NSs | 3.2 μg·mL−1 | 0.926 | 0.028 | [89] | |
| CeO2 NPs | 40 μg·mL−1 | 0.28 | 0.009 | [90] | |
| BNNS@CuS | 30 μg·mL−1 | 25 | 0.125 | [92] | |
| Co3O4@CeO2 | 50 μg·mL−1 | 7.09 | 0.430 | [93] | |
| Fe3O4@Cu@Cu2O | 50 μg·mL−1 | 2.3 | 0.119 | [95] | |
| H2TCPP-γ-Fe2O3 | 18.5 μg·mL−1 | 21.1 | 1.3 × 10−3 | [96] | |
| γ-Fe2O3 NPs | 100 μg·mL−1 | 157.2 | 0.013 | [97] | |
| Fe3O4@C YSNs | 20 μg·mL−1 | 0.035 | 0.033 | [99] | |
| CuO | 100 μg·mL−1 | 440 | 0.161 | [98] | |
| Zn-CuO | 100 μg·mL−1 | 71 | 0.003 | [98] | |
| H2TCPP-CeO2 | 40 μg·mL−1 | 0.25 | 0.013 | [90] | |
| Pt/CeO2 NPs | 10 μg·mL−1 | 0.21 | 0.085 | [91] | |
| Pt NCs | 1 × 10−4 M | 3.07 | 0.182 | 1.8 × 10−5 | [104] |
| Ru NPs | 10 μg·mL−1 | 2.2 | 0.580 | [105] | |
| Pd NPs | 5.06 × 10−12 M | 4.4 | 0.065 | 1.3 × 104 | [106] |
| Pd@Pt NPs | 1.9 × 10−12 M | 2.23 | 0.050 | 2.5 × 104 | [106] |
| Pd@γ-Fe2O3 | 1.35 × 10−6 M | 0.25 | 0.128 | 9.4 × 10−2 | [108] |
| C60[C(COOH)2]2 | 2 × 10−5 M | ~50 | 0.003 | 1.6 × 10−4 | [109] |
| Fe2O3/Pt/CNTs | 10 μg·mL−1 | ~0.1 | 6 × 10−5 | [112] | |
| GO-COOH | 40 μg·mL−1 | 3.99 | 0.039 | [115] | |
| H-rGO-Au | 0.5 μg·mL−1 | 3.1 | 0.121 | [118] | |
| IrO2/GO | 2.4 μg·mL−1 | 5.19 | ~0.300 | [119] | |
| Cu-Ag/rGO | 5 μg·mL−1 | 8.63 | 0.070 | [120] | |
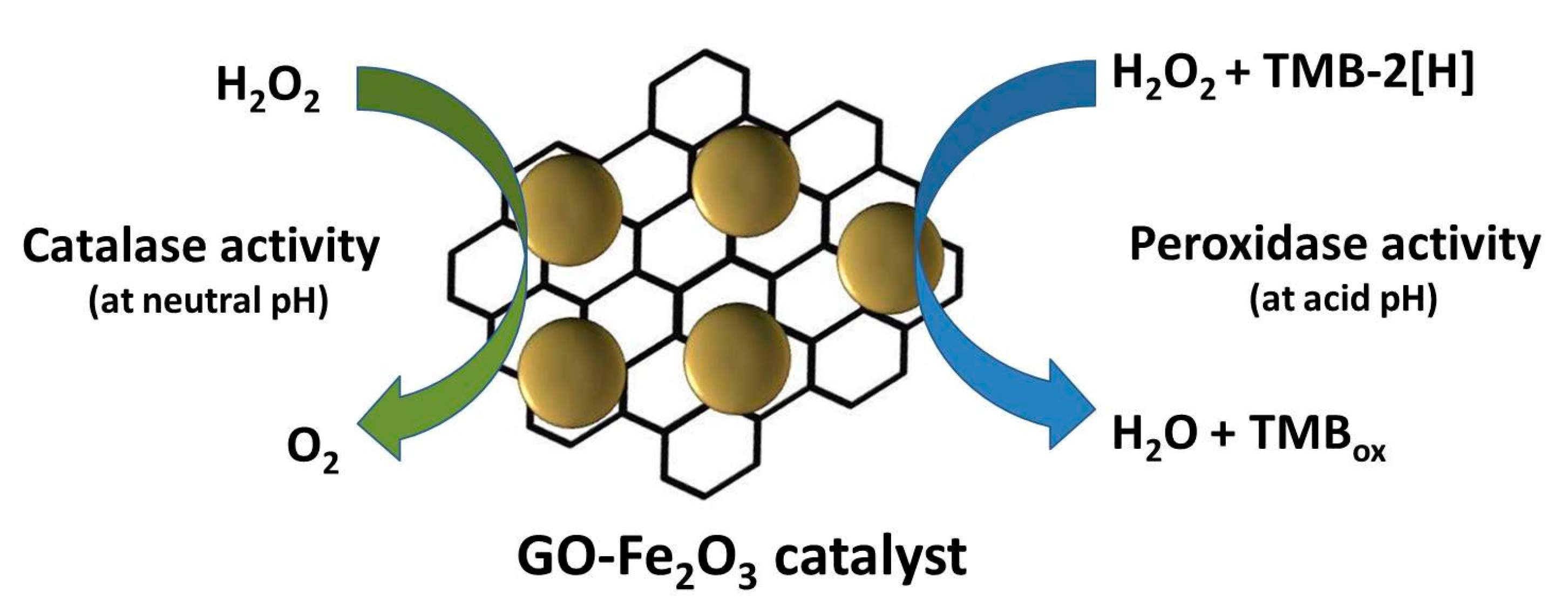
| GO-Fe2O3 | ~1.25 × 10−1 M | 305.0 | 0.101 | 8.1 × 10−7 | [121] |
| MC | 10 μg·mL−1 | 0.74 | 0.028 | [122] | |
| CNFs | 5 μg·mL−1 | 3.0 | 0.390 | 7.8 × 10−2 mmole·g−1·s−1 | [124] |
| CQDs | 15 μg·mL−1 | 26.77 | 0.306 | [125] | |
| CQDs | 0.49 | 0.026 | [126] | ||
| GQDs/CuO | 70 μg·mL−1 | 0.098 | 0.032 | [127] | |
| CF@CuAl-LDH | 50 μg·mL−1 | 0.59 | 0.003 | [129] | |
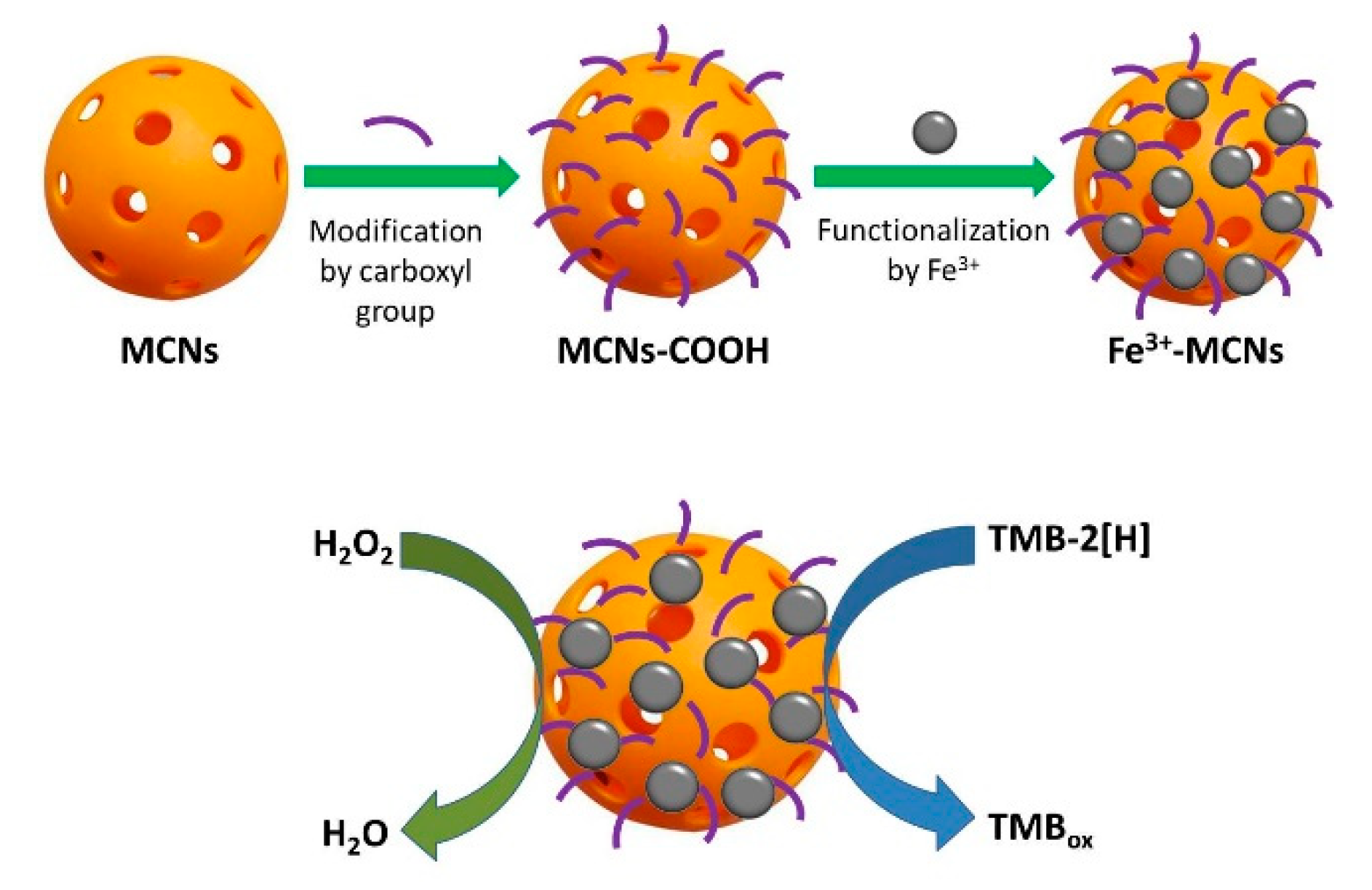
| Fe3+-MCNs | 25 μg·mL−1 | 161.0 | 0.007 | [130] |
| Catalyst/Electrode Type | Working Potential, V | Linearity, mM | LOD, μM | Sensitivity, A·M−1·m−2 | Reference |
|---|---|---|---|---|---|
| Fe3O4/3D GNCs//GCE | −0.2 | 0.0008–0.33 | 0.08 | 2742 | [131] |
| α-MnO2//GCE | −0.4 | 0.0002–0.1 | 0.08 | 5.5 | [132] |
| AuNBP/MWCNTs//GCE | −0.5 | 0.005–47.3 | 1.50 | 1706 | [133] |
| Fer/rGO-Pt//GCE | +0.1 | 0.0075–4.27 | ~0.38 | 3400 | [134] |
| rGO/Pt-Ag//GCE | −0.05 | 0.005–1.5 | 0.04 | 6996 | [135] |
| GBR//GCE | +0.9 | 0.1–10.0 | 48.0 | [136] | |
| CMC@Pd/Al-LDH//GCE | −0.38 | 0.001–0.12 | 0.30 | 163 | [137] |
| Pt/PANI/MXene//SPCE | +0.3 | 0.001–7.0 | 1.00 | [138] | |
| CuOx/NiOy//GCE | −0.35 | 0.0003–9.0 | 0.09 | 2711 | [139] |
| GDCh-NiO//RDE | +0.13 | 0.00001–0.0039 | 0.0015 | 1072 | [140] |
| Pt-Pd/MoS2//GCE | −0.35 | 0.01–0.08 | 3.4 | 764 | [141] |
| N-CNFht//GCE | −0.4 | 0.01–0.71 | 0.62 | 3570 | [142] |
| Cu2O/PANI/rGOn//GCE | −0.2 | 0.0008–12.78 | 0.3 | 394 | [143] |
| WCC//GCE | −0.4 | 0.05–1.0 | 0.006 | 67 | [144] |
| PBA | Concentration | Chromo-Gene | KMapp, mM | Vmax, μM·min−1 | kcat, nmol·µg−1·min−1 | LR, μM | Reference |
|---|---|---|---|---|---|---|---|
| PB/γ-Fe2O3 | 20 µg·mL−1 | TMB | 323.6 | 1.17 | 0.059 | [12] | |
| VOxBG hydrogel | 5 µg·mL−1 | TMB | 20 | 0.045 | 0.009 | [161] | |
| PO | TMB | 3.7 | 0.009 | [161] | |||
| PB-MIL-101 (Fe) | 200 µg·mL−1 | TMB | 0.058 | 1.32 | 0.0066 | 2.4–100 | [166] |
| MoS xNi-Fe | TMB | [169] | |||||
| PB, soluble form | 6 µg·mL−1 | TMB | 14.7 | 0.012 | 0.002 | [170] | |
| PS@Au@PB | 300 µg·mL−1 | TMB | 0.17 | 0.38 | 0.001 | [171] | |
| Au@HMPB (40 °C) | 40 µg·mL−1 | TMB | 88.72 | 2.50 | 0.063 | [172] | |
| PB | 0.2 µM | ABTS | 0.028 | [173] | |||
| PB-Ferritin PB/Fe2O3 | 0.74 nM 0.74 nM 0.31 nM | TMB ABTS TMB | 11.984 0.537 323.6 | 43.2 0.36 70.2 | 58.38 × 103 min−1 0.49 × 103 min−1 226.5 × 103 min−1 | [174] | |
| MWCNTs-PB | 1.33 | 6.6 | 0 | 1–1500 | [175] |
| Electrode | Working Potential, V | Nanozyme | Sensitivity, A·M−1·m−2 | LOD, μM | Linear Range, μM | Reference |
|---|---|---|---|---|---|---|
| GCE | −0.05 | PB/BG AuNPs-PB/BG | 2850 11243 | 4–83,000 9.2–8100 | [167] | |
| GCE | 0.65 | MnPBA | 1472 | 3 | 3–8610 | [168] |
| GCE | −0.3 | MoS xNi-Fe PBA | 0.1–2500 | [169] | ||
| GCE | 0.18 | PB | 10,000/20,000 | 1 | 1–5000 | [176] |
| GCE | 0.05 | PB | 6000 | 0.1 | 0.1–100 | [177] |
| GCE/ | 0.0 | Ni-FePBA | 18,000 | up to 100 | [178] | |
| DBD DBD GE | −0.05 −0.05 −0.05 | PB Ni-FePBA PB/NZ | 2100 1500 4500 | 0.5 | 0.5–1000 | [179] |
| Planar screen-printed | Ni-PB | 3500 | 0.1–1000 | [180] | ||
| Carbon planar screen-printed | 0.0 | PB/film PB/NZ | 6500 8500 | up to 500 up to 500 | [181] | |
| GCE | 0.0 | PNAANI–PB | 5073 | 0.07 | 1–1000 | [182] |
| Graphene | CoPBA | 0.007 | 5–1200 | [183] | ||
| Graphene nanocomposite | CoPBA | 0.1 | 0.6–380 | [184] | ||
| Graphite-string | 0.05 | 6413 | 30–1000 | [185] | ||
| Graphite paste | Cu-FePBA Ni-FePBA | 2030 1130 | 0.2 2 | 0.5–1000 2–1000 | [186] | |
| Nanoporous gold film | PB | 7080 | 0.22 | 1–17,000 | [187] | |
| GCE | Ni-FePBA | 0.192 A/M | 1 | [188] | ||
| GCE | 0.33 | Ni–Fe PBA-HNCs | 361.3 | 0.291 | 0.1–20,000 | [189] |
| GE | Thionine-NiPBA | 0.557 | 1.67–1110 | [190] | ||
| CNE | PB | 500,000 | 10–3000 | [191] |
| Catalyst | Concentration | KMapp, mM | Vmax, μM·s−1 | kcat, s−1 | Reference |
|---|---|---|---|---|---|
| Au/MCM-41 | 0.025 mg·mL−1 | 55.2 | 18.0 | 14.2 | [212] |
| AuNPs | 34 × 10−9 M | 6.97 | 0.63 | 18.52 | [213] |
| GOx | 34 × 10−9 M | 5.0 | 0.69 | 9.7 | [213] |
| AuNPs | 0.05 mg·mL−1 | 0.41 | 0.10 | [221] | |
| AuNPs-MIP | 0.05 mg·mL−1 | 0.18 | 0.42 | 3.76 × 10−7 mmole·g−1·s−1 | [221] |
| AuNPs-PFOP | 0.05 mg·mL−1 | 0.09 | 0.58 | 5.21 × 10−7 mmole·g−1·s−1 | [221] |
| AuNP | 2 × 10−9 M | 4.5 × 107 | [222] | ||
| β-CD@AuNPs | 9.60 | 1.80 × 10−2 | [228] | ||
| EMSN-AuNPs | 562.0 mg·mL−1 | 26.2 | 0.53 | [229] | |
| MnO2NFs | 0.02 mg·mL−1 | 21 | 0.43 | [230] | |
| Electrode Material | Potential, V | Linearity, mM | LOD, μM | Sensitivity, A·M−1·m−2 | Selectivity | Reference |
|---|---|---|---|---|---|---|
| 3DG/Co(OH)2 | +0.6 | 0.1–10 | 0.016 | 36900 | AA, UA, fructose, lactose, urea | [72] |
| Au/MWCNTs | +0.15 | 0.01–36.0 | 3.0 | 1012 | DA, UA, AA, fructose, saccharose, maltose, Ca2+, Cl− | [133] |
| PtNi-ERGO | −0.35 | 0.01–35 | 10 | 204.2 | AA, UA, urea, fructose | [224] |
| CuO NW/CF | +0.35 | 0.001–18.8 | 0.3 | 22174 | AA, UA, DA, lactose, sucrose, maltose | [225] |
| Octahedral Cu2O | +0.6 | 0.3–4.1 | 128 | 2410 | AA, UA, DA, NaCl | [225] |
| CQDs/octahedral Cu2O | +0.6 | 0.02–4.3 | 8.4 | 2980 | AA, UA, DA, NaCl | [225] |
| CuNWs/rGO | +0.58 | 0.01–11 | 0.2 | 16250 | AA, UA, DA, AP, fructose, sucrose | [226] |
| AKCN | +0.7 | 0.8 | fructose, lactose, maltose | [227] | ||
| β-CD@AuNPs | −0.05 | Cu2+, Al3+, UA, AA, guanine, guanosine | [228] | |||
| Ni–Pd/Si-MCP | −0.1 | 0.081 A·M−1 | AA | [232] | ||
| Pd-Pt core-shell NCs | −0.05 | 0.3–6.8 | 41.1 | 1700 | [233] | |
| Pt NPs | −0.05 | 0.3–5.2 | 91.8 | 457 | [233] | |
| Cu2O/GNs | +0.1 | 0.3–3.3 | 3.3 | 2850 | [234] | |
| Cu2O nanocubes | +0.1 | 5.9 | 2000 | [234] | ||
| ITO/PbS/SiO2/AuNPs | −0.2 | 0.001–1 | 0.46 | AA, UA, L-Cys, lactose, maltose, sucrose | [235] |
| Chemo/Biocatalyst | Substrate | KMapp, mM | Vmax, mM·min−1 | NP Concentration, mg·mL−1 | Reference |
|---|---|---|---|---|---|
| CeO2NPs | Dopamine Catechol | 0.25 × 10−3 0.180 | [77] | ||
| Cu/H3BTC MOF | Epinephrine | 0.068 | 94 × 10−3 | 0.1 | [258,262] |
| Laccase Cu/GMP Laccase | Epinephrine 2,4-Dichlorophenol | 0.062 0.59 0.65 | 5.81 × 10−3 0.83 0.15 | 0.1 0.1 0.1 | |
| AMP-Cu | 2,4-Dichlorophenol | 0.09 | 1.3 × 10−3 | 0.1 | [264] |
| Laccase | 2,4-Dichlorophenol | 0.36 | 0.29 × 10−3 | 0.1 | |
| CH-Cu Cys-His dipeptide | Epinephrine/2,4-dichlorophenol | 0.58 0.42 | 2.74 × 10−2 7.32 × 10−3 | 0.1 | [265] |
| Laccase | Epinephrine/2,4-Dichlorophenol | 0.16 0.41 | 3.10 × 10−3 6.41 × 10−3 | 0.1 | |
| PtNP-C10 (cytosine stabilized Pt NPs) | 2,4-Dichlorophenol | 0.12 | 8.49 × 10−3 | 0.01 | [267] |
| Laccase from Trametes versicolor | 2,4-Dichlorophenol | 0.40 | 3.51 × 10−3 | 0.16 | |
| Laccase from Pycnoporus sanguineus sp. CS43 | 2,4-Dichlorophenol | 0.224 | 2.21 × 10−3 | [271] |
| Sensor | Analyte | Linear Range, μM | LOD, μM | Reference |
|---|---|---|---|---|
| CeNPs | Gallic acid | 2–20 | 1.5 | [268] |
| Caffeic acid | 50–200 | 15.3 | ||
| Quercetine | 20–200 | 8.6 | ||
| Ascorbic acid | 0.5–20 | 0.4 | ||
| CeNPs/MWCNTs-COOH/SPE | Gallic acid | 25–50 | 7 | [269] |
| Caffeic acid | 33–100 | 10 | ||
| Quercetin | 25–100 | 8 | ||
| t-Resveratrol | 25–50 | 8 | ||
| Ascorbic acid | 25–100 | 7 | ||
| Laccase/Fc/SPE | Caffeic acid | 2.0–30.0 | 1.6 | [269] |
| Laccase/PAP/SWCNTs/SPE | Gallic acid | 0.53–96 | [269] | |
| Laccase immobilized in polyzetidine prepolymer and SWCNTs | Gallic acid | 0.53–96 | [269] | |
| CuO-C-CPS2 | Rutin Catechol | 1 to 120 10 to 600 | 0.4 2.0 | [272] |
| Laccase on CP | Catechol | 20 to 700 | 4.5 | [272] |
| FePP modified multi-walled carbon nanotubes (OH-MWCNTs/FePP/Nafion/GCE) | Catechol | 65 to 1600 | 3.75 | [273] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors 2020, 20, 4509. https://doi.org/10.3390/s20164509
Stasyuk N, Smutok O, Demkiv O, Prokopiv T, Gayda G, Nisnevitch M, Gonchar M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors. 2020; 20(16):4509. https://doi.org/10.3390/s20164509
Chicago/Turabian StyleStasyuk, Nataliya, Oleh Smutok, Olha Demkiv, Tetiana Prokopiv, Galina Gayda, Marina Nisnevitch, and Mykhailo Gonchar. 2020. "Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review" Sensors 20, no. 16: 4509. https://doi.org/10.3390/s20164509
APA StyleStasyuk, N., Smutok, O., Demkiv, O., Prokopiv, T., Gayda, G., Nisnevitch, M., & Gonchar, M. (2020). Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors, 20(16), 4509. https://doi.org/10.3390/s20164509






