Non-Invasive PPG-Based System for Continuous Heart Rate Monitoring of Incubated Avian Embryo
Abstract
1. Introduction
2. Photoplethysmographic (PPG)-Based Embryonic Heart Rate Extraction
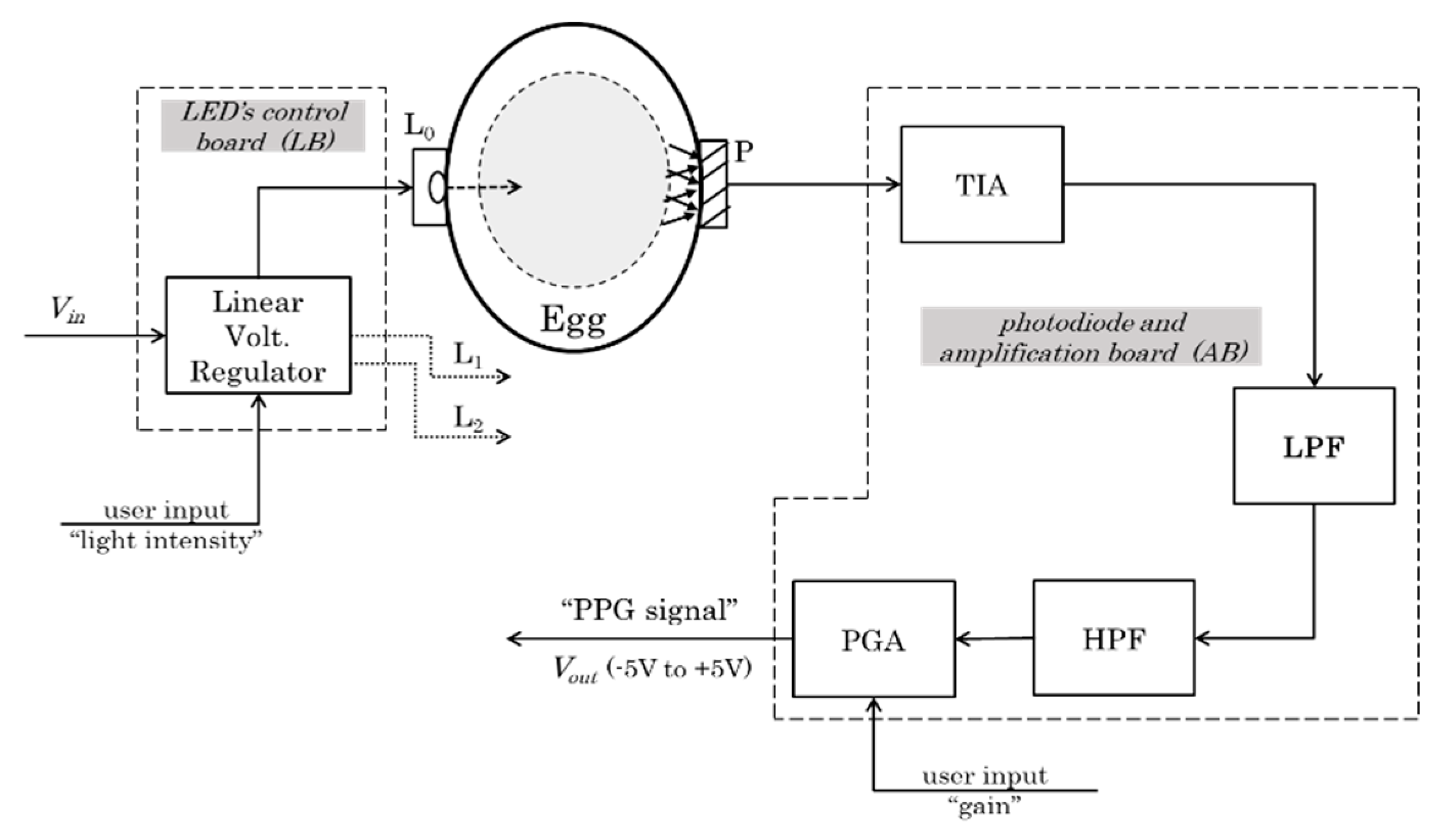
2.1. Hardware Design and Prototype (Egg-PPG)
2.1.1. Light Source System
- (a)
- The light emitting diodes (LEDs)
- (b)
- The LED control board
2.1.2. Photodiode System
- (a)
- Photodiode light sensor
- (b)
- Amplification board
- -
- Transimpedance amplifier
- -
- Analog filters
- -
- Programmable gain amplifier
2.2. Embrypnic Cardiac Wave Extraction Algorithm and Heart Rate Calculation
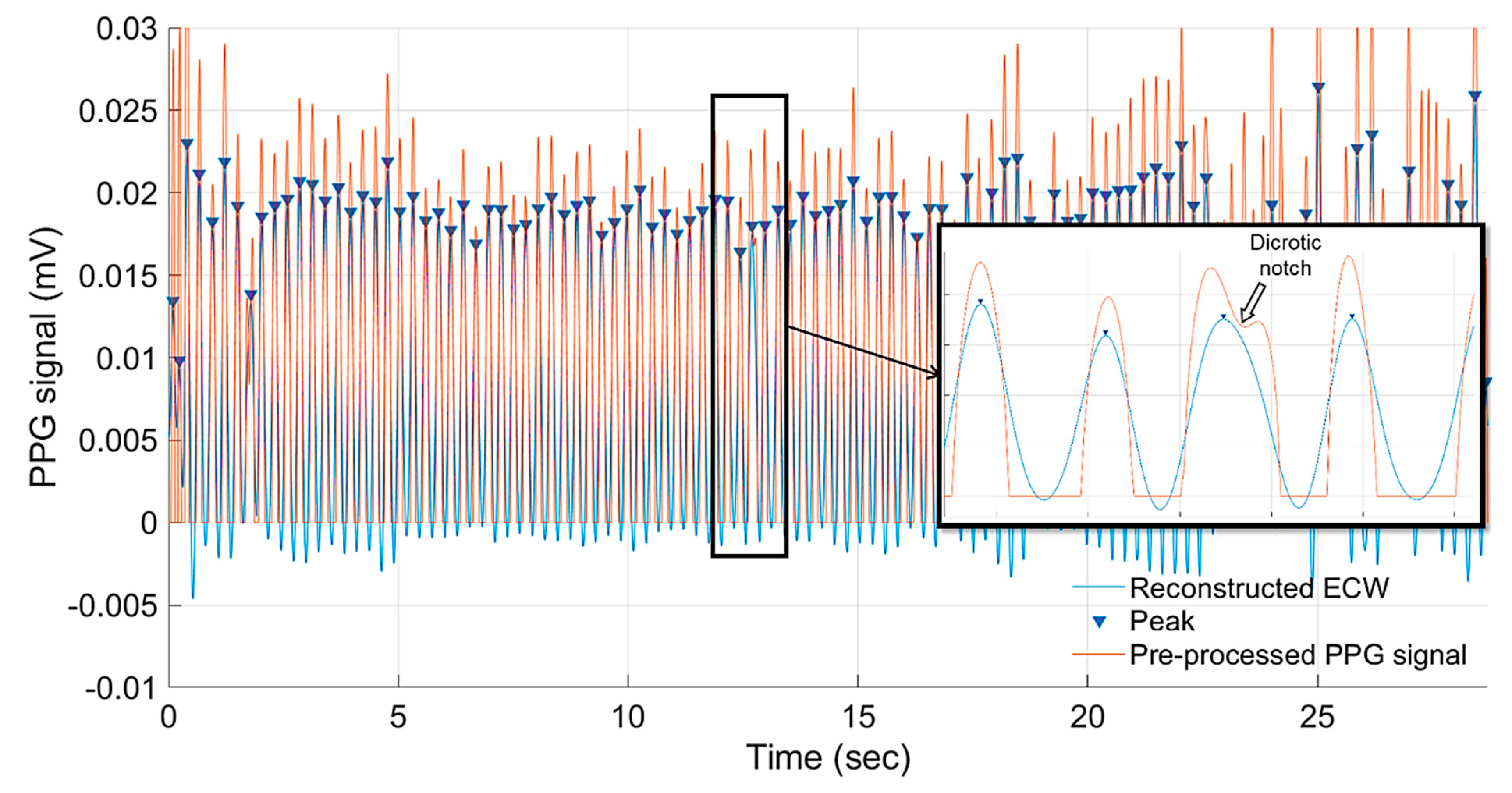
2.2.1. Pre-Processing of PPG Signals
2.2.2. Wavelet Analysis and Peak Detection
- -
- Continuous Wavelet Transform method
- Compute the discrete Fourier transform (DFT) of the analysed signal , including samples, using Fast Fourier Transform (FFT) as follows:where is an index of frequency.
- Obtain the DFT () of the analysed wavelet () at the appropriate angular frequencies as follows:
- Scale the DFT of the analysed wavelet at different scales to ensure different scales are directly comparable.To obtain the unit energy for each scale , the wavelet function is normalized using the following formula:where is the sampling period with is the sampling frequency and .
- Compute the product of the signal DFT and the wavelet DFT over all of the scales. Invert the DFT to obtain the CWT coefficients as follows:
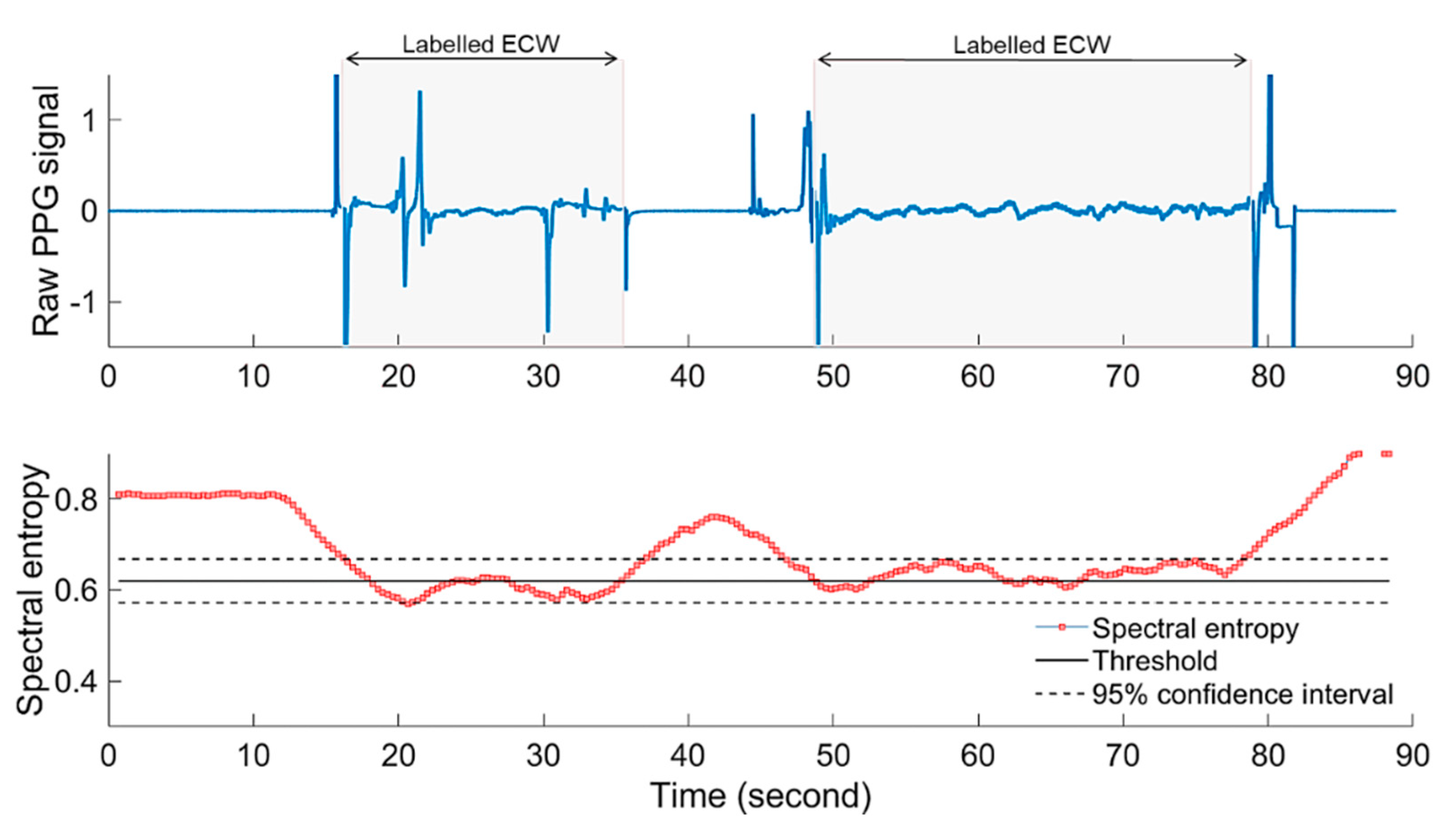
2.2.3. Power Spectral Entropy and Embryonic Cardiac Wave Recognition
- -
- The probability density function (PDF) of the spectrum of the PPG signal can be estimated by normalisation over all of the frequency components:where is the probability density for the , which is the spectral energy of the th frequency component , obtained by fast Fourier transform (FFT) and is the total number of frequency components in the FFT.
- -
- Then, the spectral entropy () of the PPG th segment is calculated as follows:
2.2.4. Peak Detection and Heart Rate Calculation
3. Experiment and Measurements
3.1. Incubation and Incbuated Eggs
3.2. Data Acquesition and PPG Measurments
3.3. Detection of Embryonic Cardiac Wave
4. Results and Discussion
4.1. Detection of Embyonic Cardiac Wave and Signal Quality
4.2. Embryonic Cadiac Wave Extraction and Heart Rate Calculation
4.3. Real-Time Heart Rate Monitoring Algorithm
PPG_signal (Gi, Ij)
INPUT: Gi ∈ {−50, −60, −70, −80, −90, −100} dB; and Ij ∈ {150, 200, 250, 300} mW·Sr−1
LOOP initiation: i = 0 and j = 0; i ∈ {0, 1, 2, 3, 4, 5}; j ∈ {0, 1, 2, 3}
FOR: Gi and Ij
DO: record 15 s of PPG_signal (Gi, Ij)
DO: calculate ES
IF: lower_threshold ≤ ES ≤ upper_threshold
DO: PPG_signal (Gi, Ij) contains ECW
DO: continue recording
ELSE:
DO: continue LOOP
END
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Amplification circuit board |
| ACG | Acoustocardiogram |
| APG | Acceleration Plethysmogram |
| BCG | Ballistocardiogram |
| CAM | Chorioallantoic Membrane |
| CWT | Continuous Wavelet Transform |
| DFT | Discrete Fourier Transform |
| DOG | Derivative of Gaussian |
| ECG | Electrocardiogram |
| ECW | Embryonic Cardiac Wave |
| ED | Embryonic Day |
| FFT | Fast Fourier Transform |
| HPF | High-Pass Filter |
| HR | Heart Rate |
| ICG | Impedance Cardiogram |
| iPPG | image Photoplethysmographic |
| LB | LED’s Control Circuit Board |
| LED | Light-Emitting Diode |
| LPF | Low-Pass Filter |
| LVR | Linear Voltage Regulator |
| PGA | Programmable Gain Amplifier |
| PPG | Photoplethysmography |
| SNR | Signal-to-Noise Ratio |
| TIA | Transimpedance Amplifier |
| WT | Wavelet Transform |
References
- Aubert, A.E.; Beckers, F.; Ramaekers, D.; Verheyden, B.; Leribaux, C.; Berckmans, D.; Aerts, J.-M. Heart rate and heart rate variability in chicken embryos at the end of incubation. Exp. Physiol. 2004, 89, 199–208. [Google Scholar] [CrossRef]
- Tazawa, H. Effect of O2 and CO2 in N2, He, and SF6 on chick embryo blood pressure and heart rate. J. Appl. Physiol. 1981, 51, 1017–1022. [Google Scholar] [CrossRef]
- Tazawa, H.; Hiraguchi, T.; Asakura, T.; Fujii, H.; Whittow, G.C. Noncontact measurements of avian embryo heart rate by means of the laser speckle: Comparison with contact measurements. Med. Biol. Eng. 1989, 27, 580–586. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Lo, C.W. Developmental Biology Protocols; Humana Press: Totowa, NJ, USA, 1999; Volume 135. [Google Scholar]
- Tazawa, H.; Ar, A.; Moriya, K.; Gefen, E.; Pearson, J.T. Embryonic heart rate measurements during artificial incubation of emu eggs. Br. Poult. Sci. 2000, 41, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wittig, J.; Münsterberg, A. The Early Stages of Heart Development: Insights from Chicken Embryos. J. Cardiovasc. Dev. Dis. 2016, 3, 12. [Google Scholar] [CrossRef]
- Lewin, R.; Dörner, M.; Tönhardt, H. Pulse oximetry: A new way of determining the heart rate in chicken embryos. Pflügers Arch. Eur. J. Physiol. 1997, 434, 639–641. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Akiyama, R.; Matsuhisa, A.; Pearson, J.T.; Tazawa, H. Long-term measurement of heart rate in chicken eggs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 124, 483–490. [Google Scholar] [CrossRef]
- Bamelis, F.; Kemps, B.; Mertens, K.; Tona, K.; De Ketelaere, B.; Decuypere, E.; De Baerdemaeker, J. Non-destructive Measurements on Eggs During Incubation. Avian Poult. Biol. Rev. 2004, 15, 150–159. [Google Scholar] [CrossRef]
- Bellville, J.W.; Lazzarini, A.A. Method for Study of Electrocardiogram of Early Chick Embryo within the Shell. Exp. Biol. Med. 1956, 93, 27–30. [Google Scholar]
- Evans, H.E.; Romanoff, A.L. The Avian Embryo: Structural and Functional Development. Avian Dis. 1960, 4, 541. [Google Scholar] [CrossRef]
- Bogue, J.Y. The Heart Rate of the Developing Chick. J. Exp. Biol. 1932, 9, 351–358. [Google Scholar]
- Tazawa, H.; Whittow, G.C. Embryonic heart rate and oxygen pulse in two procellariiform seabirds, Diomedea immutabilis and Puffinus pacificus. J. Comp. Physiol. B 1994, 163, 642–648. [Google Scholar] [CrossRef]
- Aubert, A.; Leribaux, C.; Beckers, F.; Ramaekers, D.; Berckmans, D. Noninvasive measurement of heart rate from chicken embryos in the egg. Comput. Cardiol. 2002, 27, 227–230. [Google Scholar]
- Moriya, K.; Pearson, J.T.; Burggren, W.W.; Ar, A.; Tazawa, H. Continuous measurements of instantaneous heart rate and its fluctuations before and after hatching in chickens. J. Exp. Biol. 2000, 203, 895–903. [Google Scholar]
- Haque, M.; Watanabe, W.; Ono, H.; Sakamoto, Y.; Tazawa, H. Comparisons between invasive and noninvasive determinations of embryonic heart rate in chickens. Comp. Biochem. Physiol. Part A Physiol. 1994, 108, 221–227. [Google Scholar] [CrossRef]
- Cain, J.R.; Abbott, U.K.; Rogallo, V.L. Heart Rate of the Developing Chick Embryo. Exp. Biol. Med. 1967, 126, 507–510. [Google Scholar] [CrossRef]
- Rahn, H.; Poturalski, S.A.; Paganelli, C.V. The acoustocardiogram: A noninvasive method for measuring heart rate of avian embryos in ovo. J. Appl. Physiol. 1990, 69, 1546–1548. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Banzett, R.B. Gas exchange across avian eggshells oscillates in phase with heartbeat. J. Appl. Physiol. 1990, 69, 1549–1552. [Google Scholar] [CrossRef]
- Youssef, A.; Viazzi, S.; Exadaktylos, V.; Berckmans, D. Non-contact, motion-tolerant measurements of chicken (Gallus gallus) embryo heart rate (HR) using video imaging and signal processing. Biosyst. Eng. 2014, 125, 9–16. [Google Scholar] [CrossRef]
- Yu, H.; Wang, G.; Zhao, Z.; Wang, H.; Wang, Z. Chicken embryo fertility detection based on PPG and convolutional neural network. Infrared Phys. Technol. 2019, 103, 103075. [Google Scholar] [CrossRef]
- Phuphanin, A.; Sampanporn, L.; Sutapun, B. Smartphone-Based Device for Non-Invasive Heart-Rate Measurement of Chicken Embryos. Sensors 2019, 19, 4843. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Huang, F.-H.; Yuan, P.-J.; Lin, K.-P.; Chang, H.-H.; Tsai, C.-L. Analysis of Reflectance Photoplethysmograph Sensors. Int. J. Biomed. Biol. Eng. 2011, 5, 622–625. [Google Scholar]
- Maeda, Y.; Sekine, M.; Tamura, T.; Moriya, A.; Suzuki, T.; Kameyama, K. Comparison of reflected green light and infrared photoplethysmography. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Montréal, QC, Canada, 20–25 June 2008; pp. 2270–2272. [Google Scholar]
- Vizbara, V.; Sološenko, A.; Stankevičius, D.; Marozas, V. Comparison of green, blue and infrared light in wristand forehead photoplethysmography. Biomed. Eng. 2013, 17, 78–81. [Google Scholar]
- Tang, S.K.D.; Goh, Y.Y.S.; Wong, M.L.D.; Lew, Y.L.E. PPG signal reconstruction using a combination of discrete wavelet transform and empirical mode decomposition. In Proceedings of the 2016 6th International Conference on Intelligent and Advanced Systems (ICIAS), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 1–4. [Google Scholar]
- Lierz, M.; Gooss, O.; Hafez, H.M.; Ecvph, M.L.O.G.H.M.H. Noninvasive Heart Rate Measurement Using a Digital Egg Monitor in Chicken and Turkey Embryos. J. Avian Med. Surg. 2006, 20, 141–146. [Google Scholar] [CrossRef]
- Elgendi, M.; Liang, Y.; Ward, R. Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases 2018, 6, 20. [Google Scholar] [CrossRef]
- Daubechies, I. The wavelet transform, time-frequency localization and signal analysis. IEEE Trans. Inf. Theory 1990, 36, 961–1005. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Chopra, S.; Marfurt, K.J. Choice of mother wavelets in CWT spectral decomposition. SEG Technical Program Expanded Abstracts. 2015, pp. 2957–2961. Available online: https://doi.org/10.1190/segam2015-5852193.1 (accessed on 19 August 2015).
- Komorowski, D.; Pietraszek, S. The Use of Continuous Wavelet Transform Based on the Fast Fourier Transform in the Analysis of Multi-channel Electrogastrography Recordings. J. Med. Syst. 2015, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Montejo, L.A.; Suarez, L.E. An improved CWT-based algorithm for the generation of spectrum-compatible records. Int. J. Adv. Struct. Eng. 2013, 5, 26. [Google Scholar] [CrossRef]
- Li, L.-C. A New Method of Wavelet Transform Based on FFT for Signal Processing. In Proceedings of the 2010 Second WRI Global Congress on Intelligent Systems, Wuhan, China, 16–17 December 2010; pp. 203–206. [Google Scholar]
- Sahambi, J.S.; Tandon, S.N.; Bhatt, R. Using wavelet transforms for ECG characterization. An on-line digital signal processing system. IEEE Eng. Med. Biol. Mag. 1997, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Zheng, Y.; Xu, B. Robust Speech Endpoint Detection Based on Improved Adaptive Band-Partitioning Spectral Entropy. In Bio-Inspired Computational Intelligence and Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 36–45. [Google Scholar]
- Pan, Y.N.; Chen, J.; Li, X.L. Spectral entropy: A complementary index for rolling element bearing performance degradation assessment. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 223, 1223–1231. [Google Scholar] [CrossRef]
- Huang, L.-S.; Yang, C.-H. A novel approach to robust speech endpoint detection in car environments. In Proceedings of the 2000 IEEE International Conference on Acoustics, Speech, and Signal Processing. Proceedings (Cat. No.00CH37100), Istanbul, Turkey, 5–9 June 2000; Volume 3, pp. 1751–1754. [Google Scholar]
- Van Brecht, A.; Hens, H.; Lemaire, J.-L.; Aerts, J.M.; Degraeve, P.; Berckmans, D. Quantification of the heat exchange of chicken eggs. Poult. Sci. 2005, 84, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Mikawa, T. Avians as a model system of vascular development. Methods Mol. Biol. 2015, 1214, 225–242. [Google Scholar]
- Pardanaud, L.; Luton, D.; Prigent, M.; Bourcheix, L.M.; Catala, M.; Dieterlen-Lievre, F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 1996, 122, 1363–1371. [Google Scholar]
- Reese, D.E.; Hall, C.E.; Mikawa, T. Negative Regulation of Midline Vascular Development by the Notochord. Dev. Cell 2004, 6, 699–708. [Google Scholar] [CrossRef]
- Garriock, R.J.; Czeisler, C.; Ishii, Y.; Navetta, A.M.; Mikawa, T. An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development 2010, 137, 3697–3706. [Google Scholar] [CrossRef]
- Patten, B.M.; Kramer, T.C. The initiation of contraction in the embryonic chick heart. Am. J. Anat. 1933, 53, 349–375. [Google Scholar] [CrossRef]
- Politi, M.T.; Ghigo, A.R.; Fernández, J.M.; Khelifa, I.; Gaudric, J.; Fullana, J.M.; Lagrée, P.-Y. The dicrotic notch analyzed by a numerical model. Comput. Biol. Med. 2016, 72, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Chiarelli, A.M.; Maddiona, L.; Rinella, S.; Bianco, F.; Bucciarelli, V.; Gallina, S.; Perciavalle, V.; Vinciguerra, V.; Merla, A.; et al. Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment. Sensors 2019, 19, 5570. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, A.; Berckmans, D.; Norton, T. Non-Invasive PPG-Based System for Continuous Heart Rate Monitoring of Incubated Avian Embryo. Sensors 2020, 20, 4560. https://doi.org/10.3390/s20164560
Youssef A, Berckmans D, Norton T. Non-Invasive PPG-Based System for Continuous Heart Rate Monitoring of Incubated Avian Embryo. Sensors. 2020; 20(16):4560. https://doi.org/10.3390/s20164560
Chicago/Turabian StyleYoussef, Ali, Daniel Berckmans, and Tomas Norton. 2020. "Non-Invasive PPG-Based System for Continuous Heart Rate Monitoring of Incubated Avian Embryo" Sensors 20, no. 16: 4560. https://doi.org/10.3390/s20164560
APA StyleYoussef, A., Berckmans, D., & Norton, T. (2020). Non-Invasive PPG-Based System for Continuous Heart Rate Monitoring of Incubated Avian Embryo. Sensors, 20(16), 4560. https://doi.org/10.3390/s20164560






