Reviewing Magnetic Particle Preparation: Exploring the Viability in Biosensing
Abstract
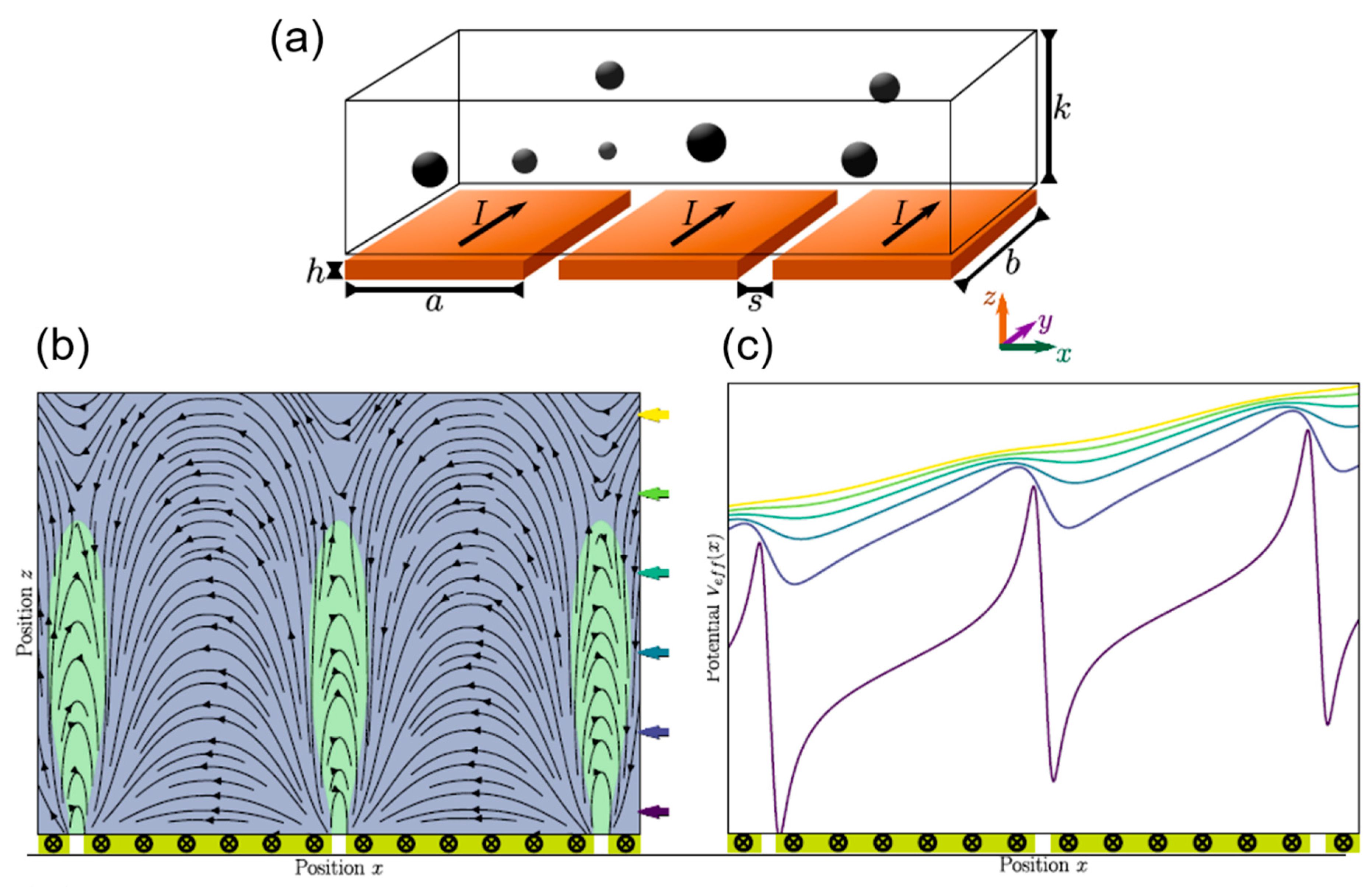
:1. Introduction
2. Intrinsic Magnetic Properties as Decision Support
3. Top-Down Approaches for Realizing Nanoparticles from μm towards nm
3.1. Tunable Perpendicularly Magnetized Synthetic Antiferromagnets
3.2. Martensitic Transformation in a Single Elliptical Disc-Shaped Heusler Ni50Mn32.5Ga17.5 Particle
3.3. Nano-Cylinders from Co2MnSi Heusler Compound
3.4. Validating the Potential of These Particles Employing the Concept of a Magnetic On–Off Ratchet
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baibich, M.N.; Broto, J.M.; Fert, A.; Van Dau, F.N.; Petroff, F.; Etienne, P.; Creuzet, G.; Friederich, A.; Chazelas, J. Giant Magnetoresistance of (001)Fe/(001)Cr Magnetic Superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binasch, G.; Grunberg, P.; Saurenbach, F.; Zinn, W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Phys. Rev. B 1989, 39, 4828–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Tondra, M.; Porter, M.; Lipert, R.J. Model for detection of immobilized superparamagnetic nanosphere assay labels using giant magnetoresistive sensors. J. Vac. Sci. Technol. A 2000, 18, 1125–1129. [Google Scholar] [CrossRef] [Green Version]
- Lagae, L.; Wirix-Speetjens, R.; Das, J.; Graham, D.; Ferreira, H.A.; Freitas, P.; Borghs, G.; De Boeck, J. On-chip manipulation and magnetization assessment of magnetic bead ensembles by integrated spin-valve sensors. J. Appl. Phys. 2002, 91, 7445. [Google Scholar] [CrossRef]
- Graham, D.; Ferreira, H.A.; Bernardo, J.; Freitas, P.; Cabral, J.M.S. Single magnetic microsphere placement and detection on-chip using current line designs with integrated spin valve sensors: Biotechnological applications. J. Appl. Phys. 2002, 91, 7786. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.; Becker, A.; Pühler, A.; Reiss, G.; Brückl, H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.P.; Cheung, Y.-C.; Renneberg, R.; Seydack, M. New Trends in Immunoassays. Adv. Biochem. Eng. Biotechnol. 2007, 109, 123–154. [Google Scholar] [CrossRef]
- Mattiasson, B.; Teeparuksapun, K.; Hedström, M. Immunochemical binding assays for detection and quantification of trace impurities in biotechnological production. Trends Biotechnol. 2010, 28, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Ferreira, H.A.; Freitas, P.; Cabral, J.M.S. High sensitivity detection of molecular recognition using magnetically labelled biomolecules and magnetoresistive sensors. Biosens. Bioelectron. 2003, 18, 483–488. [Google Scholar] [CrossRef]
- Freitas, P.; Cardoso, F.; Martins, V.C.; Martins, S.A.M.; Loureiro, J.; Amaral, J.; Chaves, R.C.; Cardoso, S.; Fonseca, L.P.; Sebastião, A.M.; et al. Spintronic platforms for biomedical applications. Lab Chip 2012, 12, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, S.; Pelaz, B.; Parak, W.J.; Lentijo-Mozo, S.; Soulantica, K.; Dieckhoff, J.; Ludwig, F.; Guenther, A.; Tschöpe, A.; Schotter, J. Homogeneous Biosensing Based on Magnetic Particle Labels. Sensors 2016, 16, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, G.; Brueckl, H.; Hütten, A.; Schotter, J.; Brzeska, M.; Panhorst, M.; Sudfeld, D.; Becker, A.; Kamp, P.B.; Puehler, A.; et al. Magnetoresistive sensors and magnetic nanoparticles for biotechnology. J. Mater. Res. 2005, 20, 3294–3302. [Google Scholar] [CrossRef]
- Hütten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Hachmann, W.; Heinzmann, U.; Wojczykowski, K.; Jutzi, P.; Saikaly, W.; Thomas, G. New magnetic nanoparticles for biotechnology. J. Biotechnol. 2004, 112, 47–63. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schuth, F. Magnetische Nanopartikel: Synthese, Stabilisierung, Funktionalisierung und Anwendung. Angew. Chem. 2007, 119, 1242–1266. [Google Scholar] [CrossRef]
- Van Waeyenberge, B.; Puzic, A.; Stoll, H.; Chou, K.W.; Tyliszczak, T.; Hertel, R.; Faehnle, M.; Brueckl, H.; Rott, K.; Reiss, G.; et al. Magnetic vortex core reversal by excitation with short bursts of an alternating field. Nature 2006, 444, 461–464. [Google Scholar] [CrossRef]
- Mühlbauer, S.; Binz, B.; Jonietz, F.; Pfleiderer, C.; Rosch, A.; Neubauer, A.; Georgii, R.; Boni, P. Skyrmion Lattice in a Chiral Magnet. Science 2009, 323, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Chen, P.; Zhang, Q.; Yu, R.C.; Liu, B.-G. Giant linear anomalous Hall effect in the perpendicular CoFeB thin films. Appl. Phys. Lett. 2014, 104, 202404. [Google Scholar] [CrossRef]
- Kiyohara, N.; Tomita, T.; Nakatsuji, S. Giant Anomalous Hall Effect in the Chiral Antiferromagnet Mn3Ge. Phys. Rev. Appl. 2016, 5, 064009. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Reddy, V.; Torati, S.R.; Hu, X.H.; Sandhu, A.; Kim, C.G. On-chip magnetometer for characterization of superparamagnetic nanoparticles. Lab Chip 2015, 15, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Cubero, D.; Renzoni, F. Brownian Ratchets: From Statistical Physics to Bio and Nano-Motors; Cambridge University Press: Cambridge, UK, 2016; ISBN 9781107063525. [Google Scholar]
- Auge, A.; Weddemann, A.; Wittbracht, F.; Hütten, A. Magnetic ratchet for biotechnological applications. Appl. Phys. Lett. 2009, 94, 183507. [Google Scholar] [CrossRef]
- Holzinger, D.; Lengemann, D.; Göllner, F.; Engel, D.; Ehresmann, A. Controlled movement of superparamagnetic bead rows for microfluid mixing. Appl. Phys. Lett. 2012, 100, 153504. [Google Scholar] [CrossRef]
- Ueltzhöffer, T.; Streubel, R.; Koch, I.; Holzinger, D.; Makarov, D.; Schmidt, O.G.; Ehresmann, A. Magnetically Patterned Rolled-Up Exchange Bias Tubes: A Paternoster for Superparamagnetic Beads. ACS Nano 2016, 10, 8491–8498. [Google Scholar] [CrossRef] [PubMed]
- Reiss, G.; Hütten, A. Magnetic nanoparticles: Applications beyond data storage. Nat. Mater. 2005, 4, 725–726. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Dostmann, W.R.; Herberg, F.W.; Durick, K.; Xuong, N.H.; Eyck, L.T.; Taylor, S.S.; Varughese, K.I. Regulatory subunit of protein kinase A: Structure of deletion mutant with cAMP binding domains. Science 1995, 269, 807–813. [Google Scholar] [CrossRef]
- Wegener, M.; Ennen, I.; Walhorn, V.; Anselmetti, D.; Hütten, A.; Dietz, K.-J. Magnetic Tracking of Protein Synthesis in Microfluidic Environments-Challenges and Perspectives. Nanomaterials 2019, 9, 585. [Google Scholar] [CrossRef] [Green Version]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; pp. 417–424. [Google Scholar]
- Kuang, F.G.; Kuang, X.Y.; Kang, S.Y. A Systematic Investigation on Magnetism and Phase Stability of Cobalt. Z. Nat. A 2014, 69, 254–262. [Google Scholar] [CrossRef]
- De la Peña O’Shea, V.A.; Moreira, I.D.P.; Roldán, A.; Illas, F. Electronic and magnetic structure of bulk cobalt: The α, β, and ε-phases from density functional theory calculations. J. Chem. Phys. 2010, 133, 024701. [Google Scholar] [CrossRef]
- Kämmerer, S.; Thomas, A.; Hütten, A.; Reiss, G. Co 2 Mn Si Heusler alloy as magnetic electrodes in magnetic tunnel junctions. Appl. Phys. Lett. 2004, 85, 79. [Google Scholar] [CrossRef]
- Betancourt-Cantera, J.; Jesús, F.S.-D.; Bolarín-Miró, A.; Torres-Villaseñor, G.; Betancourt-Cantera, L. Magnetic properties and crystal structure of elemental cobalt powder modified by high-energy ball milling. J. Mater. Res. Technol. 2019, 8, 4995–5003. [Google Scholar] [CrossRef]
- Grob, D.T.; Wise, N.; Oduwole, O.; Sheard, S. Magnetic susceptibility characterisation of superparamagnetic microspheres. J. Magn. Magn. Mater. 2018, 452, 134–140. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Stearns, M.B.; Cheng, Y. Determination of para- and ferromagnetic components of magnetization and magnetoresistance of granular Co/Ag films (invited). J. Appl. Phys. 1994, 75, 6894–6899. [Google Scholar] [CrossRef]
- Dreyer, A.; Peter, M.; Mattay, J.; Hütten, A.; Jutzi, P. Ionic Additives and Weak Magnetic Fields in the Thermo-Decomposition of Dicobalt Octacarbonyl: Tools for the Morphology Control of Cobalt Nanoparticles. Eur. J. Inorg. Chem. 2012, 198–202. [Google Scholar] [CrossRef]
- Lavrijsen, R.; Fernández-Pacheco, A.; Petit, D.; Mansell, R.; Lee, J.H.; Cowburn, R. Tuning the interlayer exchange coupling between single perpendicularly magnetized CoFeB layers. Appl. Phys. Lett. 2012, 100, 52411. [Google Scholar] [CrossRef]
- Vemulkar, T.; Mansell, R.; Petit, D.C.M.C.; Cowburn, R.P.; Lesniak, M.S. Highly tunable perpendicularly magnetized synthetic antiferromagnets for biotechnology applications. Appl. Phys. Lett. 2015, 107, 012403. [Google Scholar] [CrossRef]
- Gottschalk, M. Anwendungen der Ionen- & Elektronenmikroskopie im Grenzgebiet Zwischen Nanostrukturphysik und Biologie. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, December 2017. Available online: https://katalogplus.ub.uni-bielefeld.de/title/2522262 (accessed on 16 August 2020).
- Haase, M. Martensitische Nanopartikel. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, March 2018. Available online: https://katalogplus.ub.uni-bielefeld.de/title/2535369 (accessed on 16 August 2020).
- Xu, X.; Nagasako, M.; Ito, W.; Umetsu, R.Y.; Kanomata, T.; Kainuma, R. Magnetic properties and phase diagram of Ni50Mn50−xGax ferromagnetic shape memory alloys. Acta Mater. 2013, 61, 6712–6723. [Google Scholar] [CrossRef]
- Teichert, N. Shape Memory Heusler Alloys for Thin Film Applications. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, April 2016. Available online: https://pub.unibielefeld.de/download/2902807/2902808 (accessed on 16 August 2020).
- Wei, Z.; Liu, E.; Li, Y.; Han, X.L.; Du, Z.W.; Luo, H.; Liu, G.; Xi, X.-K.; Zhang, H.; Wang, W.H.; et al. Magnetostructural martensitic transformations with large volume changes and magneto-strains in all-d-metal Heusler alloys. Appl. Phys. Lett. 2016, 109, 071904. [Google Scholar] [CrossRef] [Green Version]
- Kappe, D. Simulationen der Mikrostruktur und Dynamik von Nanopartikeln im Kontext von Hydrodynamik und Magnetoresistivem Transport. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, February 2020. Available online: http://nbn-resolving.de/urn:nbn:de:0070-pub-29414482 (accessed on 16 August 2020).
- Craik, D.; Brown, R.W. Magnetism: Principles and Applications. Phys. Today 1996, 49, 57. [Google Scholar] [CrossRef] [Green Version]
- Huth, M.; Porrati, F.; Dobrovolskiy, O.V. Focused electron beam induced deposition meets materials science. Microelectron. Eng. 2018, 185, 9–28. [Google Scholar] [CrossRef] [Green Version]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kappe, D.; Bondzio, L.; Swager, J.; Becker, A.; Büker, B.; Ennen, I.; Schröder, C.; Hütten, A. Reviewing Magnetic Particle Preparation: Exploring the Viability in Biosensing. Sensors 2020, 20, 4596. https://doi.org/10.3390/s20164596
Kappe D, Bondzio L, Swager J, Becker A, Büker B, Ennen I, Schröder C, Hütten A. Reviewing Magnetic Particle Preparation: Exploring the Viability in Biosensing. Sensors. 2020; 20(16):4596. https://doi.org/10.3390/s20164596
Chicago/Turabian StyleKappe, Daniel, Laila Bondzio, Joris Swager, Andreas Becker, Björn Büker, Inga Ennen, Christian Schröder, and Andreas Hütten. 2020. "Reviewing Magnetic Particle Preparation: Exploring the Viability in Biosensing" Sensors 20, no. 16: 4596. https://doi.org/10.3390/s20164596




