Effect of Tungsten Oxide Nanostructures on Sensitivity and Selectivity of Pollution Gases
Abstract
:1. Introduction
2. Experimental
3. Results and Discussions
3.1. SEM Measurements
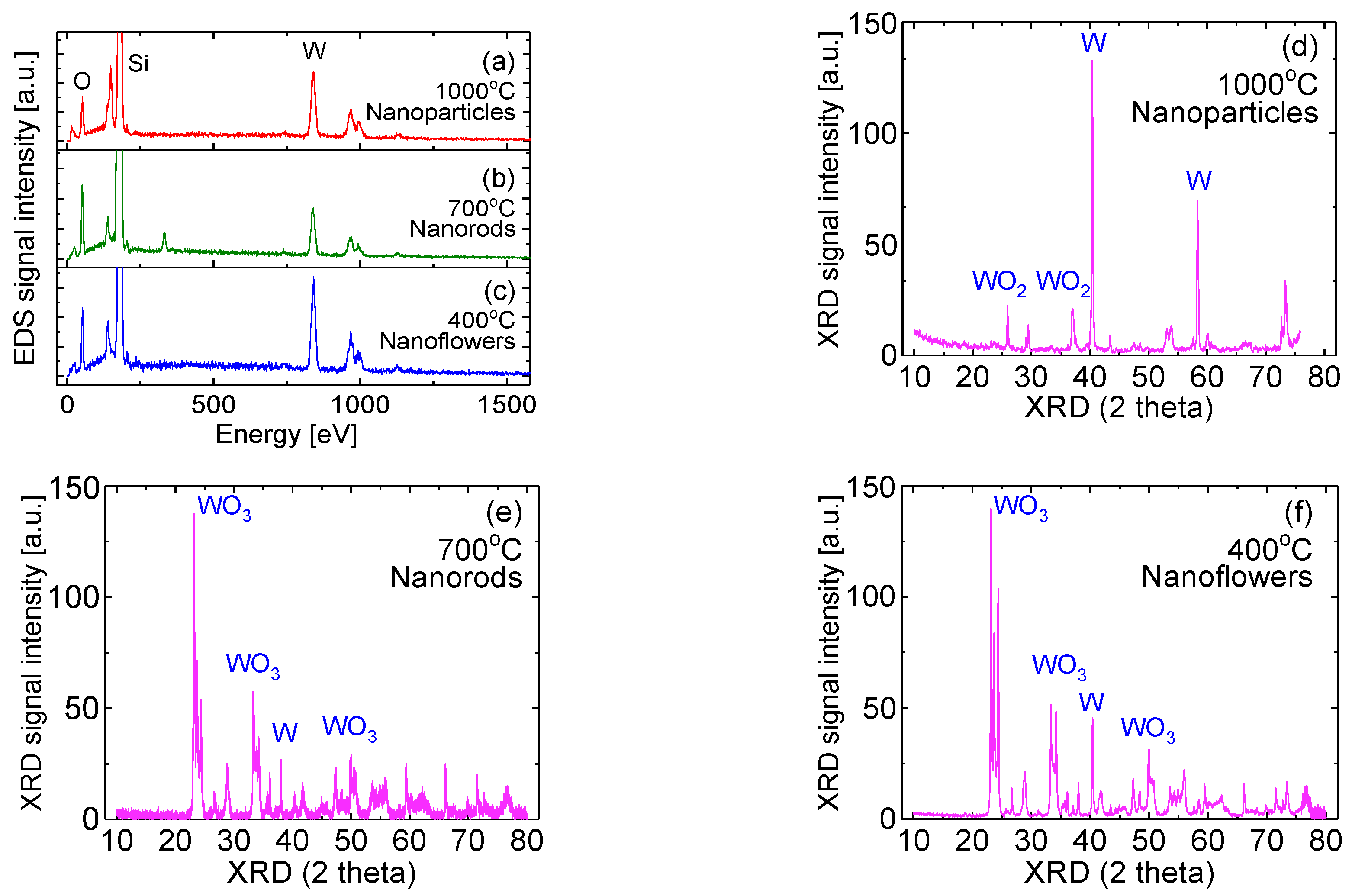
3.2. EDS and XRD Measurements
3.3. Raman Spectral Measurements
3.4. Fabrications of Prototypes
3.5. Electrical Properties and Bias Effect
3.6. Effect of Gas Concentration
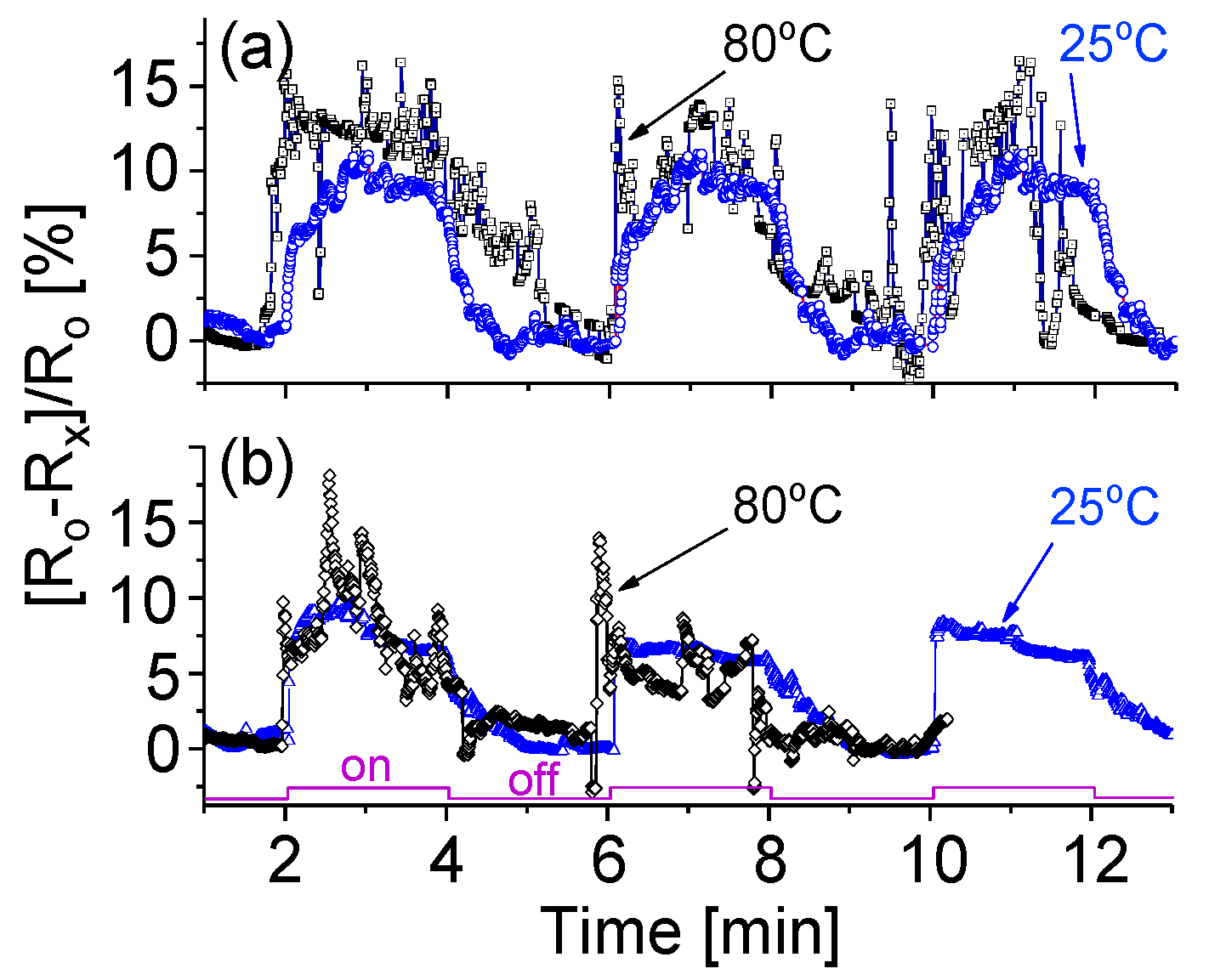
3.7. Temperature Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godbole, R.; Vedpathak, A.; Godbole, V.; Bhagwat, S. Tungsten oxide thin films: Detection and trapping of hazardous gases. Mater. Res. Express 2017, 4, 076401. [Google Scholar] [CrossRef]
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. 2016, 21, 65–76. [Google Scholar] [PubMed]
- Bernardini, S.; Pourcin, F.; Nambiema, N.; Margeat, O.; Aguir, K.; Videlot-Ackermann, C.; Ackermann, J.; Bendahan, M. Ammonia detection at low temperature by tungsten oxide nanowires. Proceedings 2018, 2, 983. [Google Scholar] [CrossRef] [Green Version]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Russell, B. Metal oxide semiconductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Miura, N.; Yamazoe, N. Study of WO3-based sensing material for NH3 and NO detection. Sens. Actuators B 2000, 66, 74–76. [Google Scholar] [CrossRef]
- Basnet, P.; Larsen, G.K.; Jadeja, R.P.; Hung, Y.; Zhao, Y. α-Fe2O3 Nanocolumns and nanorods fabricated by electron beam evaporation for visible light photocatalytic and antimicrobial applications. ACS Appl. Mater. Interfaces 2013, 56, 2085–2095. [Google Scholar] [CrossRef]
- Xiao, Z.; Kisslinger, K. Electron-beam-evaporated thin films of hafnium dioxide for fabricating electronic devices. J. Vac. Sci. Technol. B 2015, 33, 042001. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Shao, M.; Huang, J.; Ding, M.; Deng, X.; Wei, X.; Xu, X. Anodic oxidation synthesis of one-dimensional TiO2 nanostructures for photocatalytic and field emission properties. J. Nanomater. 2014, 2014, 831752. [Google Scholar] [CrossRef] [Green Version]
- Dlugosch, T.; Chnani, A.; Muralidhar, P.; Schirmer, A.; Biskupek, J.; Strehle, S. Thermal oxidation synthesis of crystalline iron-oxide nanowires on low-cost steel substrates for solar water splitting. Semicond. Sci. Technol. 2017, 32, 084001. [Google Scholar] [CrossRef] [Green Version]
- Stumpp, M.; Damtew, D.; Stock, D.; Hess, K.; Schröder, D.; Schlettwein, D. Controlled electrodeposition of zinc oxide on conductive meshes and foams enabling its use as secondary anode. J. Electrochem. Soc. 2018, 165, D461–D466. [Google Scholar] [CrossRef]
- Kim, H.; Bonsu, R.O.; O’Donohue, C.; Korotkov, R.Y.; McElwee-White, L.; Anderson, T.J. Aerosol-assisted chemical vapor deposition of tungsten oxide films and nanorods from oxo tungsten (VI) fluoroalkoxide precursors. ACS Appl. Mater. Interfaces 2015, 74, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Maggio, F.D.; Shujah, T.; Blackman, C. Review: Chemical vapour deposition of gas sensitive metal oxides. Chemosensors 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.N.; Tiwari, N.; Shieh, H.D.; Liu, P. Electrical performance and stability of tungsten indium zinc oxide thin-film transistors. Mater. Lett. 2018, 214, 293–296. [Google Scholar] [CrossRef]
- Firoozbakht, S.; Akbarnejad, E.; Salar Elahi, A.; Ghoranneviss, M. Growth and characterization of tungsten oxide thin films using the reactive magnetron sputtering system. J. Inorg. Organomet. Polym. 2016, 26, 889–894. [Google Scholar] [CrossRef]
- Yadav, P.V.K.; Reddy, Y.A.K.; Ajitha, B.; Reddy, V.R.M. Oxygen partial pressure dependent UV photo detector performance of WO3 sputtered thin films. J. Alloys Compd. 2020, 816, 152565. [Google Scholar] [CrossRef]
- Kawasaki, H.; Namba, J.; Iwatsuji, K.; Suda, Y.; Wada, K.; Ebihara, K.; Ohshima, T. NOx gas sensing properties of tungsten oxide thin films synthesized by pulsed laser deposition method. Appl. Surf. Sci. 2002, 197, 547–551. [Google Scholar] [CrossRef]
- Lethy, K.J.; Beena, D.; Kumar, R.V.; Pillai, V.M.; Ganesan, V.; Sathe, V. Structural, optical and morphological studies on laser ablated nanostructured WO3 thin films. Appl. Surf. Sci. 2008, 254, 2369–2376. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Yin, Y.; Lu, R.; Gao, S.; Han, C.; Wei, D. NO2 sensing properties of WO3 porous films with honeycomb structure. J. Alloys Compd. 2019, 789, 129–138. [Google Scholar] [CrossRef]
- Solis, J.L.; Saukko, S.; Kish, L.; Granqvist, C.G.; Lantto, V. Semiconductor gas sensors based on nanostructured tungsten oxide. Thin Solid Films 2001, 391, 255–260. [Google Scholar] [CrossRef]
- Zheng, H.D.; Qu, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured tungsten oxide-properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Z.; Huang, S.; Wu, R. Preparation of palladium-doped mesoporous WO3 for hydrogen gas sensors. J. Alloys Compd. 2019, 776, 965–973. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. NO and NO2 Sensing Properties of WO3 and Co3O4 Based Gas Sensors. Sensors 2013, 13, 12467–12481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Shen, Y.; Zhong, X.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Wei, K. Effect of noble metal element on microstructure and NO2 sensing properties of WO3 nanoplates prepared from a low-grade scheelite concentrate. J. Alloys Compd. 2020, 818, 152927. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Armelao, L.; Mari, C.; Polizzi, S.; Ruffo, R.; Scotti, R.; Morazzoni, F. Macroporous WO3 thin films active in NH3 sensing: Role of the hosted Cr isolated centers and Pt nanoclusters. J. Am. Chem. Soc. 2011, 133, 5296–5304. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Jain, K. Highly sensitive NH3 sensor using Pt catalyzed silica coating over WO3 thick films. Sens. Actuators B 2008, 133, 46–52. [Google Scholar] [CrossRef]
- Ramgir, N.; Datta, N.; Kaur, M.; Kailasaganapati, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Ammonia sensor based on WO3 thin films. AIP Conf. Proc. 2012, 1451, 185. [Google Scholar]
- Wang, Y.; Liu, J.; Cui, X.; Gao, Y.; Ma, J.; Sun, Y.; Sun, P.; Liu, F.; Liang, X.; Zhang, T.; et al. NH3 gas sensing performance enhanced by Pt-loaded on mesoporous WO3. Sens. Actuators B 2017, 238, 473–481. [Google Scholar] [CrossRef]
- Tao, W.; Tsai, C. H2S sensing properties of noble metal doped WO3 thin film sensor fabricated by micromachining. Sens. Actuators B 2002, 81, 237–247. [Google Scholar] [CrossRef]
- He, M.; Xie, L.; Zhao, X.; Hu, X.; Li, S.; Zhu, Z. Highly sensitive and selective H2S gas sensors based on flower-like WO3/CuO composites operating at low/room temperature. J. Alloys Compd. 2019, 788, 36–43. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Haensch, A.; Barsan, N. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Tian, F.H.; Zhao, L.H.; Xue, X.Y.; Shen, Y.Y.; Jia, X.F.; Chen, S.G.; Wang, Z.H. DFT study of CO sensing mechanism on hexagonal WO3 (001) surface: The role of oxygen vacancy. Appl. Surf. Sci. 2014, 311, 362–368. [Google Scholar] [CrossRef]
- Li, X.-L.; Lou, T.-J.; Sun, X.-M.; Li, Y.-D. Highly Sensitive WO3 Hollow-Sphere Gas Sensors. Inorg. Chem. 2004, 43, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yin, M.; Yan, J.; Liu, S.F. P-type sub-tungsten-oxide based urchin-like nanostructure for superior room temperature alcohol sensor. Appl. Surf. Sci. 2018, 441, 277–284. [Google Scholar] [CrossRef]
- Llobet, E.; Molas, G.; Molinas, P.; Calderer, J.; Vilanova, X.; Brezmes, J.; Sueiras, J.E.; Correig, X. Fabrication of highly selective tungsten oxide ammonia sensors. J. Electrochem. Soc. 2000, 147, 776–779. [Google Scholar] [CrossRef]
- Leng, J.Y.; Xu, X.J.; Lv, N.; Fan, H.T.; Zhang, T. Synthesis and gas-sensing characteristics of WO3 nanofibers via electrospinning. J. Colloid Interface Sci. 2011, 356, 54–57. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Xu, Y.; Li, Y. Nanosheet-assembled hierarchical WO3 flower-like nanostructures: Hydrothermal synthesis and NH3-sensing properties. Mater. Lett. 2019, 250, 155–158. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Mutadak, P.; Maiti, N.; Sonawane, K.M. Synthesis of WO3 nanoflakes by hydrothermal route and its gas sensing application. Sens. Actuators A Phys. 2020, 304, 111877. [Google Scholar] [CrossRef]
- Le, X.V.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Synergistic enhancement of ammonia gas-sensing properties at low temperature by compositing carbon nanotubes with tungsten oxide nanobricks. Vacuum 2019, 168, 108861. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Sun, P.; Liang, X.; Liu, F.; Yan, X.; et al. The room temperature gas sensor based on Polyaniline flower-like WO3 nanocomposites and flexible PET substrate for NH3 detection. Sens. Actuators B 2018, 259, 505–513. [Google Scholar] [CrossRef]
- Feng-Chen, S.; Aldalbahi, A.; Feng, P.X. Nanostructured Tungsten Oxide Composite for High-Performance Gas Sensors. Sensors 2015, 15, 27035–27046. [Google Scholar] [CrossRef]
- Wang, X.P.; Yang, B.Q.; Zhang, H.X.; Feng, P.X. Tungsten oxide nanorods array and nanobundle prepared by using chemical vapor deposition technique. Nanoscale Res. Lett. 2007, 2, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.X.; Wang, X.P.; Zhang, H.X.; Yang, B.Q.; Wang, Z.B.; González-Berríos, A.; Morell, G.; Weiner, B. Study of the structural evolutions of crystalline tungsten oxide films prepared using hot-filament CVD. J. Phys. D 2007, 40, 5239–5245. [Google Scholar] [CrossRef]
- Zhang, H.X.; Yang, B.Q.; Feng, P.X. Ambient pressure synthesis of nanostructured tungsten oxide crystalline films. J. Nanomater. 2008, 2008, 957935. [Google Scholar] [CrossRef]
- Ramana, C.V.; Utsunomiya, S.; Ewing, R.C.; Julien, C.M.; Becker, U. Structural stability and phase transitions in WO3 thin films. J. Phys. Chem. B 2006, 110, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Faudoa-Arzate, A.; Arteaga-Durán, A.; Saenz-Hernández, R.J.; Botello-Zubiate, M.E.; Realyvazquez-Guevara, P.R.; Matutes-Aquino, J.A. HRTEM Microstructural Characterization of β-WO3 Thin Films Deposited by Reactive RF Magnetron Sputtering. Materials 2017, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Shang, D.-S.; Shi, L.; Sun, J.-R.; Shen, B.-G. Local resistance switching at grain and grain boundary surfaces of polycrystalline tungsten oxide films. Nanotechnology 2011, 22, 254008. [Google Scholar] [CrossRef]
- Nguyen, T.-A.; Park, S.; Kim, J.B.; Kim, T.K.; Seong, G.H.; Choo, J.; Kim, Y.S. Polycrystalline tungsten oxide nanofibers for gas-sensing applications. Sens. Actuators B 2011, 160, 549–554. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Commun. 2015, 6, 8064. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.X.; Zhang, H.X.; Peng, X.Y.; Sajjad, M.; Chu, J. A novel compact design of calibration equipment for gas and thermal sensors. Rev. Sci. Instrum. 2011, 82, 043303. [Google Scholar] [CrossRef]
- Kida, T.; Kuroiwa, T.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Study on the response and recovery properties of semiconductor gas sensors using a high-speed gas-switching system. Sens. Actuators B Chem. 2008, 134, 928–933. [Google Scholar] [CrossRef]








| Materials | NH3 (ppm) | Temp. (°C) | Response Formula # | Response | Reference |
|---|---|---|---|---|---|
| Undoped WO3 | 400 | 450 | S = Rg/Ra | 1.04 | [25] |
| Undoped WO3 | 100 | 400 | S = Rg/Ra | 9.8 | [26] |
| Undoped WO3 | 30 | 350 | S = Rg/Ra | 5.40 | [5] |
| Undoped WO3 | 100 | 500 | S = Rg/Ra | 5.5 | [35] |
| Undoped WO3 | 100 | 350 | S = Rg/Ra | 36.3 | [36] |
| Undoped WO3 | 120 | 150 | S = (Ra − Rg) × 100/Ra | 73% | [37] |
| Pt-WO3 | 200 | 125 | S = Rg/Ra | 13.61 | [27] |
| PANI/WO3 | 100 | RT | S = Rg/Ra | 20.1 | [39] |
| 1.0 wt % CNT/WO3 | 10 | RT | S = (Ra − Rg) × 100/Ra | 6.8% | [38] |
| Undoped WO3 | 200 | RT | S = (Ra − Rg) × 100/Ra | 0.4% | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, F.; Zhou, A.F.; Feng, P.X. Effect of Tungsten Oxide Nanostructures on Sensitivity and Selectivity of Pollution Gases. Sensors 2020, 20, 4801. https://doi.org/10.3390/s20174801
An F, Zhou AF, Feng PX. Effect of Tungsten Oxide Nanostructures on Sensitivity and Selectivity of Pollution Gases. Sensors. 2020; 20(17):4801. https://doi.org/10.3390/s20174801
Chicago/Turabian StyleAn, Fenghui, Andrew F. Zhou, and Peter X. Feng. 2020. "Effect of Tungsten Oxide Nanostructures on Sensitivity and Selectivity of Pollution Gases" Sensors 20, no. 17: 4801. https://doi.org/10.3390/s20174801
APA StyleAn, F., Zhou, A. F., & Feng, P. X. (2020). Effect of Tungsten Oxide Nanostructures on Sensitivity and Selectivity of Pollution Gases. Sensors, 20(17), 4801. https://doi.org/10.3390/s20174801






