Development Perspective of Bioelectrocatalysis-Based Biosensors
Abstract
1. Introduction
2. Fundamentals of Bioelectrocatalytic Sensors
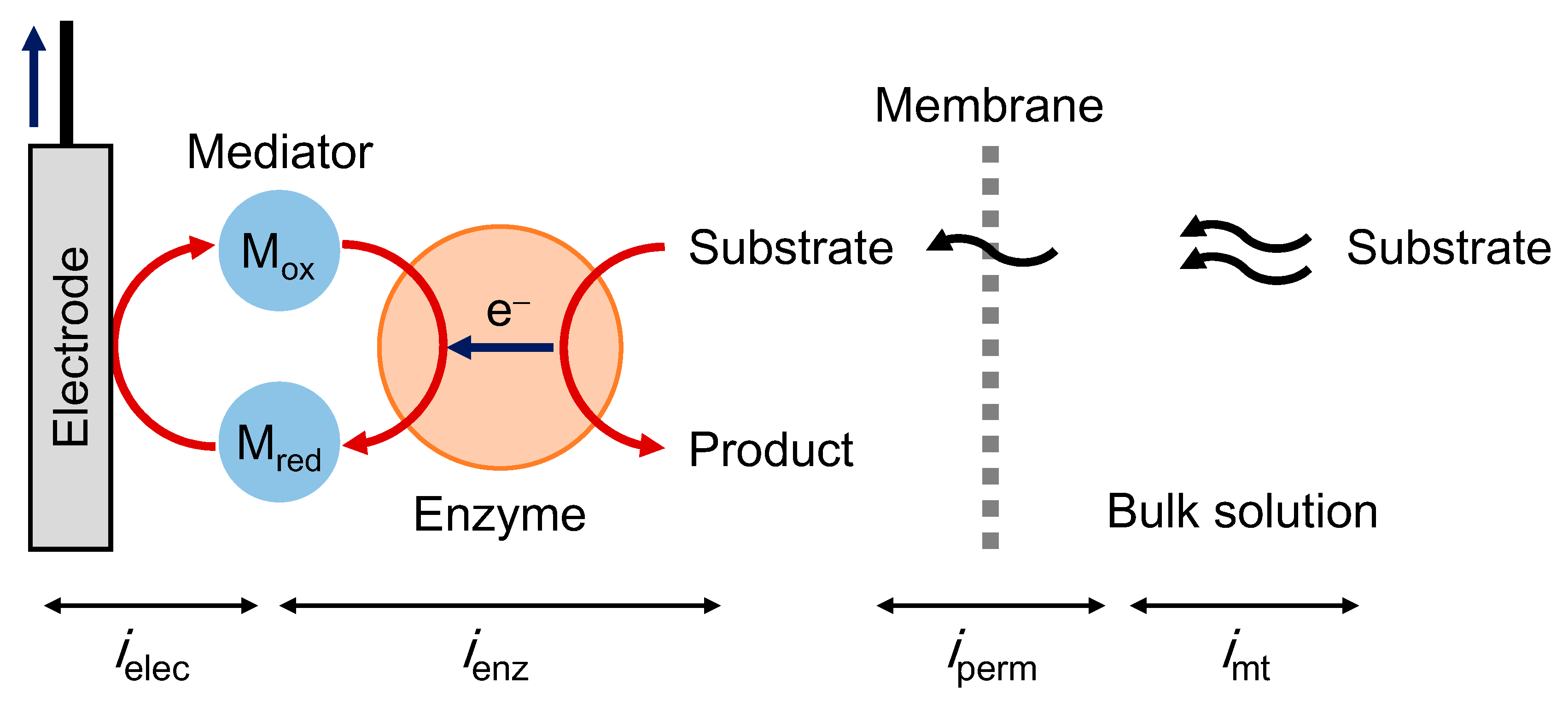
2.1. Theory of Steady-State Catalytic Currents in Met-Type Bioelectrocatalysis
2.1.1. MET-Type Bioelectrocatalysis in Homogeneous System
2.1.2. Reaction-Layer Model at Enzyme/Mediator-Immobilized Electrodes
2.1.3. Serial Resistance Model
2.2. Theory of Steady-State DET-Type Bioelectrocatalysis
2.3. Examples of MET/DET-Type Biosensors
3. Multi-Enzymatic Cascades
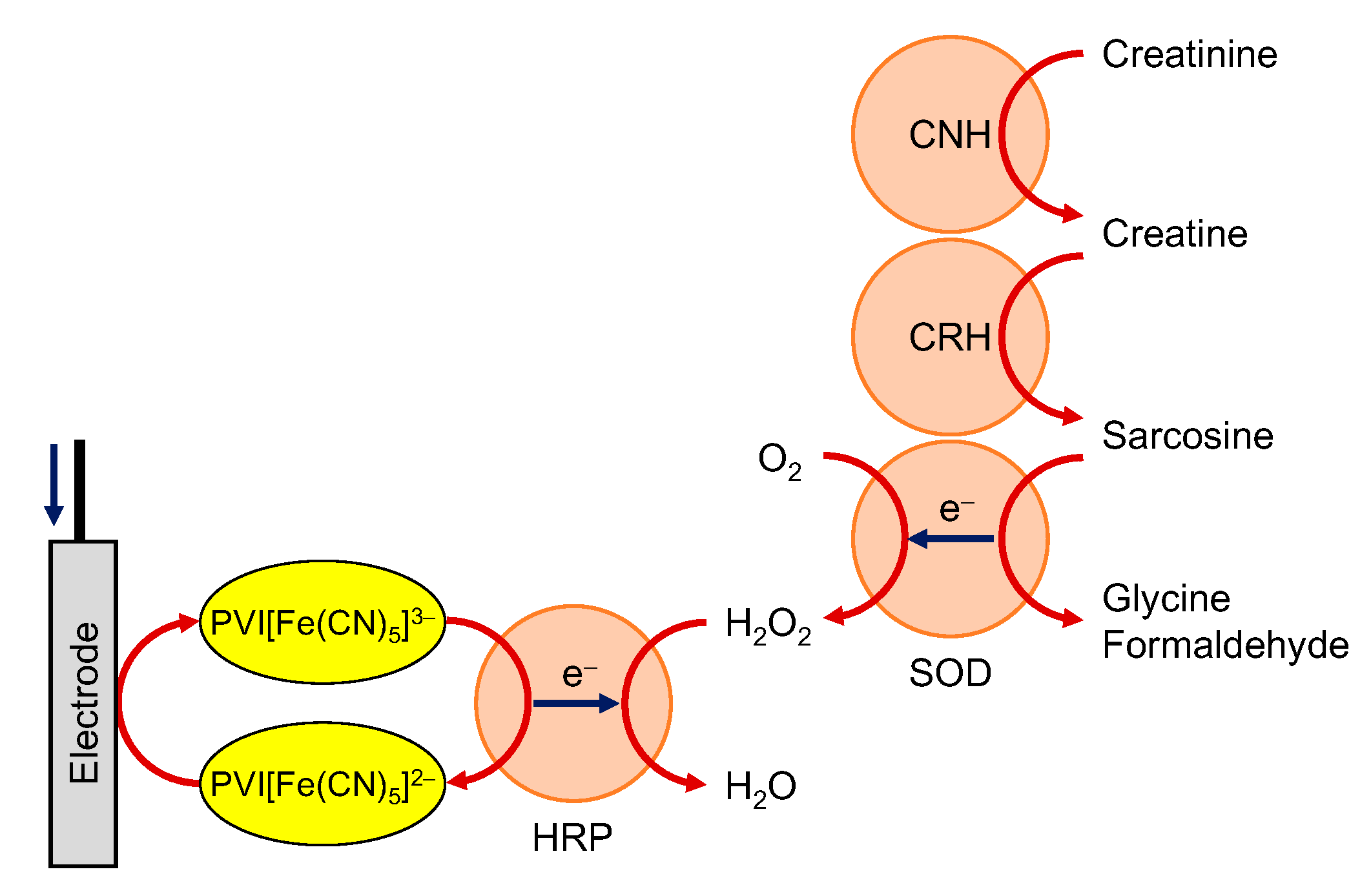
3.1. Diaphorase/NAD(P)+-Dependent Enzymes
3.2. Peroxidase/Oxidases
4. Multianalyte Detection
4.1. Crosstalk among Amperometric Biosensors
4.2. Absolute and Relative Concentration
4.3. Examples of the Internal Standard in Body Fluids
5. Prospective Biosensors without Calibration
5.1. Significance of Mass-Transfer Controlling
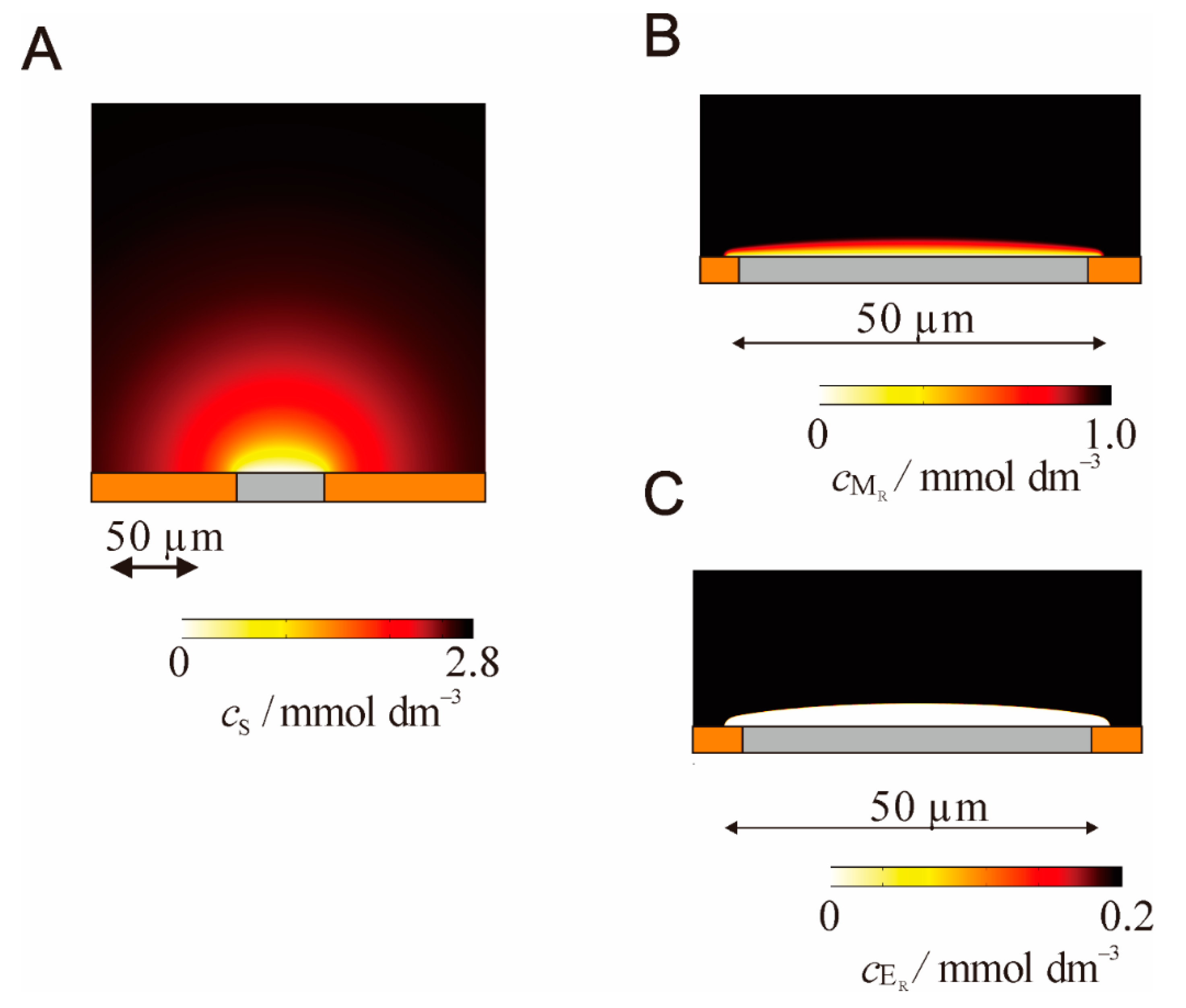
5.2. Bioelectrocatalysis at Microelectrodes
5.3. Pseudo-Steady-State Response
5.4. Potentiometric Coulometry
6. Conclusions
Funding
Conflicts of Interest
References
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Wilson, G.S.; Johnson, M.A. In-vivo Electrochemistry: What Can We Learn about Living Systems? Chem. Rev. 2008, 108, 2462–2481. [Google Scholar] [CrossRef] [PubMed]
- Heller, A. Electrical Connection of Enzyme Redox Centers to Electrodes. J. Phys. Chem. 1992, 96, 3579–3587. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G. Electrochemical Biosensors: Recommended Definitions and Classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Armstrong, F.A.; Hinst, J. Reversibility and Efficiency in Electrocatalytic Energy Conversion and Lessons from Enzymes. Proc. Natl. Acad. Sci. USA 2011, 108, 14049–14054. [Google Scholar] [CrossRef] [PubMed]
- de Poulpiquet, A.; Ranava, D.; Monsalve, K.; Giudici-Orticoni, M.-T.; Lojou, E. Biohydrogen for a New Generation of H2/O2 Biofuel Cells: A Sustainable Energy Perspective. ChemElectroChem 2014, 1, 1724–1750. [Google Scholar] [CrossRef]
- Fourmond, V.; Léger, C. Modelling the Voltammetry of Adsorbed Enzymes and Molecular Catalysts. Curr. Opin. Electrochem. 2017, 1, 110–120. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanska, M. Main Streams in the Construction of Biosensors and Their Applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L. Enzyme Based Amperometric Biosensors. Curr. Opin. Electrochim. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Kano, K. Fundamentals and Applications of Redox Enzyme-Functionalized Electrode Reactions. Electrochemistry 2019, 87, 301–311. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in Nanomaterial Application in Enzyme-Based Electrochemical Biosensors. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef]
- Ngyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Pinyou, P.; Blay, V.; Muresan, L.M.; Noguer, T. Enzyme-Modified Electrodes for Biosensors and Biofuel Cells. Mater. Horiz. 2019, 6, 1336–1358. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Katz, E.; Willner, B. Electrical Contact of Redox Enzyme Layers Associated with Electrodes; Routes to Amperometric Biosensors. Electroanalysis 1997, 9, 965–977. [Google Scholar] [CrossRef]
- Barton, S.C.; Gallaway, J.; Atanassov, P. Enzymatic Biofuel Cells for Implantable and Microscale Devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as Working or Inspirational Electrocatalysts for Fuel Cells and Electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef]
- Meredith, M.T.; Minteer, S.D. Biofuel Cells; Enhanced Enzymatic Bioelectrocatalysis. Annu. Rev. Anal. Chem. 2012, 5, 157–179. [Google Scholar] [CrossRef]
- Mazurenko, I.; de Poulpiquet, A.; Lojou, E. Recent Developments in High Surface Area Bioelectrodes for Enzymatic Fuel Cells. Curr. Opin. Electrochem. 2017, 5, 74–84. [Google Scholar] [CrossRef]
- Mano, N.; de Poulpiquet, A. O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef]
- Krieg, T.; Sydow, A.; Schröder, U.; Schrader, J.; Holtmann, D. Reactor Concepts for Bioelectrochemical Syntheses and Energy Conversion. Trends Biotechnol. 2014, 32, 645–655. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Enzymatic Bioelectrosynthetic Ammonia Production: Recent Electrochemistry of Nitrogenase, Nitrate Reductase, and Nitrite Reductase. ChemPlusChem 2017, 82, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.; González-Arribas, E.; Falk, M. Biosupercapacitors. Curr. Opin. Electrochem. 2017, 5, 226–233. [Google Scholar] [CrossRef]
- Dutton, P.L. Redox Potentiometry: Determination of Midpoint Potentials of Oxidation-Reduction Components of Biological Electron-Transfer Systems. Methods Enzymol. 1978, 54, 411–435. [Google Scholar] [PubMed]
- Scheller, F.; Kirstein, D.; Kirstein, L.; Schubert, F.; Wollenberger, U.; Olsson, B.; Gorton, L.; Johansson, G. Enzyme Electrodes and Their Application. Phil. Trans. R. Lond. B 1987, 316, 85–94. [Google Scholar]
- Scheller, F.; Schubert, F.; Pfeiffer, D.; Hintsche, R.; Dransfeld, I.; Renneberg, R.; Wollenberger, U.; Riedel, K.; Pavlova, M.; Kühn, M.; et al. Research and Development of Biosensors. Analyst 1989, 114, 653–662. [Google Scholar] [CrossRef]
- Freìre, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct Electron Transfer: An Approarch for Electrochemical Biosensors with Higher Selectivity and Sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef]
- Léger, C.; Bertrand, P. Direct Electrochemistry of Redox Enzymes as Tool for Mechanistic Studies. Chem. Rev. 2008, 108, 2379–2438. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Direct Enzymatic Bioelectrocatalysis: Differentiating between Myth and Reality. J. R. Soc. Interface 2017, 14, 20170253. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct Electron Transfer-Type Bioelectrocatalysis of Redox Enzymes at Nanostructured Electrodes. Catalysts 2020, 10, 236. [Google Scholar] [CrossRef]
- Kitazumi, Y.; Shirai, O.; Kano, K. Significance of Nanostructures of an Electrode Surface in Direct Electron Transfer-Type Bioelectrocatalysis of Redox Enzymes, in Novel Catalyst Materials for Bioelectrochemical Systems: Fundamentals and Applications; ACS Publications: Washington, DC, USA, 2020. [Google Scholar]
- Hooda, V.; Gahlaut, A.; Gothwal, A.; Hooda, V. Recent Trends and Perspectives in Enzyme Based Biosensor Development for the Screening of Triglycerides: A Comprehensive Review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 626–635. [Google Scholar] [CrossRef]
- Semenova, D.; Gernaey, K.V.; Morgan, B.; Silina, Y.E. Towards One-Step Design of Tailored Enzymatic Nanobiosensors. Analyst 2020, 145, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Pilas, J.; Yazici, Y.; Selmer, T.; Keusgen, M.; Schöning, M.J. Application of a Portable Multi-Analyte Biosensor for Organic Acid Determination in Silage. Sensors 2018, 18, 1470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, R.; Zhai, J.; Tian, C. Bienzymatic Glucose Biosensor Based on Co-Immobilization of Peroxidase and Glucose Oxidase on a Carbon Nanotubes Electrode. Biosens. Bioelectron. 2007, 23, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Narasimhan, B.; Mallapragada, S. Materials-Based Strategies for Multi-Enzyme Immobilization and Co-Localization. Biotechnol. Bioeng. 2013, 111, 209–222. [Google Scholar] [CrossRef]
- Serafin, V.; Hernández, P.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Biosensor for Creatinine Based on the Immobilization of Creatininase, Creatinase and Sarcosine Oxidase onto a Ferrocene/Horseradish Peroxidase/Gold Nanoparticles/Multi-Walled Carbon Nanotubes/Teflon Composite Electrode. Electrochim. Acta 2013, 97, 175–183. [Google Scholar] [CrossRef]
- Noda, T.; Hamamoto, K.; Tsutumi, M.; Tsujimura, S.; Shirai, O.; Kano, K. Bioelectrocatalytic Endpoint Assays Based on Steady-State Diffusion Current at Microelectrode Array. Electrochem. Commun. 2010, 12, 839–842. [Google Scholar] [CrossRef][Green Version]
- Noda, T.; Wanibuchi, M.; Kitazumi, Y.; Tsujimura, S.; Shirai, O.; Yamamoto, M.; Kano, K. Diffusion-Controlled Detection of Glucose with Microelectrodes in Mediated Bioelectrocatalytic Oxidation. Anal. Sci. 2013, 29, 279–281. [Google Scholar] [CrossRef]
- Kitazumi, Y.; Noda, T.; Shirai, O.; Yamamoto, M.; Kano, K. Characteristics of Fast Mediated Bioelectrocatalytic Reaction near Microelectrodes. Phys. Chem. Chem. Phys. 2014, 16, 8905–8910. [Google Scholar] [CrossRef][Green Version]
- Xia, H.-Q.; Kitazumi, Y.; Shirai, O.; Ohta, H.; Kurihara, S.; Kano, K. Putrescine Oxidase/Peroxidase-Co-Immobilized and Mediator-Less Mesoporous Microelectrode for Diffusion-Controlled Steady-State Amperometric Detection of Putrescine. J. Electroanal. Chem. 2017, 804, 128–132. [Google Scholar] [CrossRef]
- Matsui, Y.; Hamamoto, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Diffusion-Controlled Mediated Electron Transfer-Type Bioelectrocatalysis Using Ultrathin-Ring and Microband Electrodes as Ultimate Amperometric Glucose Sensors. Anal. Sci. 2017, 33, 845–851. [Google Scholar] [CrossRef]
- Matsui, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Simultaneous Detection of Lactate Enantiomers Based on the Diffusion-Controlled Bioelectrocatalysis. Anal. Sci. 2018, 34, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kitazumi, Y.; Shirai, O.; Kano, K. Performance Analysis of an Oxidase/Peroxidase-Based Mediatorless Amperometric Biosensor. J. Electroanal. Chem. 2019, 841, 73–78. [Google Scholar] [CrossRef]
- Nieh, C.-H.; Kitazumi, Y.; Shirai, O.; Yamamoto, M.; Kano, K. Potentiometric Coulometry Based on Charge Accumulation with a Peroxidase/Osmium Polymer-Immobilized Electrode for Sensitive Determination of Hydrogen Peroxide. Electrochem. Commun. 2013, 33, 135–137. [Google Scholar] [CrossRef]
- Katsube, R.; Kitazumi, Y.; Shirai, O.; Yamamoto, M.; Kano, K. Potentiometric Coulometry Using a Liquid-Film-Modified Electrode as a Reversible Surface-Confined System. J. Electroanal. Chem. 2016, 780, 114–118. [Google Scholar] [CrossRef]
- Matsumoto, R.; Kano, K.; Ikeda, T. Theory of Steady-State Catalytic Current of Mediated Bioelectrocatalysis. J. Electroanal. Chem. 2002, 535, 37–40. [Google Scholar] [CrossRef]
- Albery, W.J.; Cass, A.E.G.; Shu, Z.X. Inhibited Enzyme Electrodes. Part 1: Theoretical Model. Biosens. Bioelectron. 1990, 5, 367–378. [Google Scholar] [CrossRef]
- Tsujimura, S.; Nakagawa, T.; Kano, K.; Ikeda, T. Kinetic Study of Direct Bioelectrocatalysis of Dioxygen Reduction with Bilirubin Oxidase at Carbon Electrodes. Electrochemistry 2004, 72, 437–439. [Google Scholar] [CrossRef]
- Moser, C.C.; Keske, J.M.; Warncke, K.; Farid, R.S.; Dutton, P.L. Nature of Biological Electron Transfer. Nature 1992, 355, 796–802. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron Transfers in Chemistry and Biology. BBA Rev. Bioenerg. 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry: Theory and Experiment. Angew. Chem. Int. Ed. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-Based Glucose Biosensor. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef]
- Rahman, G.; Mian, S.A. Recent Trends in the Development of Electrochemical Glucose Biosensors. Int. J. Biosen. Bioelectron. 2017, 3, 210–213. [Google Scholar] [CrossRef]
- Gregg, B.A.; Heller, A. Cross-Linked Redox Gels Containing Glucose Oxidase for Amperometric Biosensor Applications. Anal. Chem. 1990, 62, 258–263. [Google Scholar] [CrossRef]
- Trifonov, A.; Stemmer, A.; Tel-Vered, R. Enzymatic Self-Wiring in Nanopores and Its Application in Direct Electron Transfer Biofuel Cells. Nanoscale Adv. 2019, 1, 347–356. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ferri, S.; Huynh, M.L.; Shimizu, H.; Yamaoka, H.; Sode, K. Direct Electron Transfer Type Disposable Sensor Strip for Glucose Sensing Employing an Engineered FAD Glucose Dehydrogenase. Enzym. Microb. Technol. 2013, 52, 123–128. [Google Scholar] [CrossRef]
- Tsujimura, S.; Kojima, S.; Kano, K.; Ikeda, T.; Sato, M.; Sanada, H.; Omura, H. Novel FAD-Dependent Glucose Dehydrogenase for a Dioxygen-Insensitive Glucose Biosensor. Biosci. Biotechnol. Biochem. 2006, 70, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, A.; Kuzmarskyte, J.; Meskys, R.; Ramanavicius, A. Bioelectrochemical Sensor Based on PQQ-Dependent Glucose Dehydrogenase. Sens. Actuators B 2004, 100, 387–394. [Google Scholar] [CrossRef]
- Flexer, V.; Durand, F.; Tsujimura, S.; Mano, N. Efficient Direct Electron Transfer of PQQ-Glucose Dehydrogenase on Carbon Cryogel Electrodes at Neutral pH. Anal. Chem. 2011, 83, 5721–5727. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Lee, I.; Blaauw, D.; Katz, E. A Microelectronic Sensor Device Powered by a Small Implantable Biofuel Cell. ChemPhysChem 2020, 21, 120–128. [Google Scholar] [CrossRef]
- Mie, Y.; Yasutake, Y.; Ikegami, M.; Tamura, T. Anodized Gold Surface Enables Mediator-Free and Low-Overpotential Electrochemical Oxidation of NADH: A Facile Method for the Development of an NAD+-Dependent Enzyme Biosensor. Sens. Actuators B 2019, 288, 512–518. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef]
- Kakehi, N.; Yamazaki, T.; Tsugawa, W.; Sode, K. A Novel Wireless Glucose Sensor Employing Direct Electron Transfer Principle Based Enzyme Fuel Cell. Biosens. Bioelectron. 2007, 22, 2250–2255. [Google Scholar] [CrossRef]
- Roe, J.H.; Epstein, J.H.; Goldstein, N.P. A Photometric Method for the Determination of Inulin in Plasma and Urine. J. Biol. Chem. 1949, 178, 839–845. [Google Scholar]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly Sensitive Membraneless Fructose Biosensor Based on Fructose Dehydrogenase Immobilized onto Aryl Thiol Modified Highly Porous Gold Electrode: Characterization and Application in Food Samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Bioelectrocatalytic Performance of d-Fructose Dehydrogenase. Bioelectrochemistry 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Bollella, P.; Ludwig, R.; Gorton, L. Cellobiose Dehydrogenase: Insights on the Nanostructuration of Electrodes for Improved Development of Biosensors and Biofuel Cells. Appl. Mater. Today 2017, 9, 319–332. [Google Scholar] [CrossRef]
- Ito, K.; Okuda-Shimazaki, J.; Mori, K.; Kojima, K.; Tsugawa, W.; Ikebukuro, K.; Lin, C.-E.; La Belle, J.; Yoshida, H.; Sode, K. Designer Fungus FAD Glucose Dehydrogenase Capable of Direct Electron Transfer. Biosens. Bioelectron. 2019, 123, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Gorton, L.; Torstensson, A.; Jaegfeldt, H.; Johansson, G. Electrocatalytic Oxidation of Reduced Nicotinamide Coenzymes by Graphite Electrodes Modified with an Adsorbed Phenoxazinium Salt, Meldola Blue. J. Electroanal. Chem. 1984, 161, 103–120. [Google Scholar] [CrossRef]
- Avramescu, A.; Noguer, T.; Magearu, V.; Marty, J.-L. Chronoamperometric Determination of d-Lactate Using Screen-Printed Enzyme Electrodes. Anal. Chim. Acta 2001, 433, 81–88. [Google Scholar] [CrossRef]
- Takagi, K.; Kano, K.; Ikeda, T. Mediated Bioelectrocatalysis Based on NAD-Related Enzymes with Reversible Characteristics. J. Electroanal. Chem. 1998, 445, 211–219. [Google Scholar] [CrossRef]
- Antiochia, R.; Gallina, A.; Lavagnini, I.; Magno, F. Kinetic and Thermodynamic Aspects of NAD-Related Enzyme-Linked Mediated Bioelectrocatalysis. Electroanalysis 2002, 14, 1256–1261. [Google Scholar] [CrossRef]
- Nikitina, O.; Shleev, S.; Gayda, G.; Demkiv, O.; Gonchar, M.; Gorton, L.; Csöregi, E.; Nistor, M. Bi-Enzyme Biosensor Based on NAD+- and Glutathione-Dependent Recombinant Formaldehyde Dehydrogenase and Diaphorase for Formaldehyde Assay. Sens. Actuators B 2007, 125, 1–9. [Google Scholar] [CrossRef]
- Lobo, M.J.; Miranda, A.J.; Tuñón, P. Amperometric Biosensors Based on NAD(P)-Dependent Dehydrogenase Enzymes. Electroanalysis 1997, 9, 191–201. [Google Scholar] [CrossRef]
- Siritanaratkul, B.; Megarity, C.F.; Roberts, T.G.; Samuels, T.O.M.; Winkler, M.; Warner, J.H.; Happe, T.; Armstrong, F.A. Transfer of Photosynthetic NADP+/NADPH Recycling Activity to a Porous Metal Oxide for Highly Specific, Electrochemically-Driven Organic Synthesis. Chem. Sci. 2017, 8, 4579–4586. [Google Scholar] [CrossRef]
- Xu, C.X.; Marzouk, S.A.M.; Cosofret, V.V.; Buck, R.P.; Neuman, M.R.; Sprinkle, R.H. Development of a Diamine Biosensor. Talanta 1997, 44, 1625–1632. [Google Scholar] [CrossRef]
- Carsol, M.-A.; Mascini, M. Diamine Oxidase and Putrescine Oxidase Immobilized Reactors in Flow Injection Analysis: A Comparison in Substrate Specificity. Talanta 1999, 50, 141–148. [Google Scholar] [CrossRef]
- Nagy, L.; Nagy, G.; Gyurcsanyi, R.E.; Neuman, M.R.; Lindner, E. Development and Study of an Amperometric Biosensor for the in Vitro Measurement of Low Concentration of Putrescine in Blood. J. Biochem. Biophys. Methods 2002, 53, 165–175. [Google Scholar] [CrossRef]
- Gorton, L.; Jönsson-Pettersson, G.; Csöregi, E.; Johansson, K.; Domínguez, E.; Marko-Varga, G. Amperometric Biosensors Based on an Apparent Direct Electron Transfer between Electrodes and Immobilized Peroxidases. Plenary Lecture. Analyst 1992, 117, 1235–1241. [Google Scholar] [CrossRef]
- Matsumoto, R.; Mochizuki, M.; Kano, K.; Ikeda, T. Unusual Response in Mediated Biosensors with an Oxidase/Peroxidase Bienzyme System. Anal. Chem. 2002, 74, 3297–3303. [Google Scholar] [CrossRef]
- Tkáč, J.; Gemeiner, P.; Šturdík, E. Rapid and Sensitive Galactose Oxidase-Peroxidase Biosensor for Galactose Detection with Prolonged Stability. Biotechnol. Tech. 1999, 13, 931–936. [Google Scholar] [CrossRef]
- Nieh, C.-H.; Kitazumi, Y.; Shirai, O.; Kano, K. Sensitive d-Amino Acid Biosensor Based on Oxidase/Peroxidase System Mediated by Pentacyanoferrate-Bound Polymer. Biosens. Bioelectron. 2013, 47, 350–355. [Google Scholar] [CrossRef][Green Version]
- Kacaniklic, V.; Johansson, K.; Marko-Varga, G.; Gorton, L.; Jönsson-Pettersson, G.; Csöregi, E. Amperometric Biosensors for Detection of l- and d-Amino Acids Based on Coimmobilized Peroxidase and l- and d-Amino Acid Oxidases in Carbon Paste Electrodes. Electroanalysis 1994, 6, 381–390. [Google Scholar] [CrossRef]
- Yoshida, S.; Kanno, H.; Watanabe, T. Glutamate Sensors Carrying Glutamate Oxidase/Peroxidase Bienzyme System on Tin Oxide Electrode. Anal. Sci. 1995, 11, 251–256. [Google Scholar] [CrossRef][Green Version]
- Castillo, J.; Gáspár, S.; Sakharov, I.; Csöregi, E. Bienzyme Biosensors for Glucose, Ethanol and Putrescine Built on Oxidase and Sweet Potato Peroxidase. Biosens. Bioelectron. 2003, 18, 705–714. [Google Scholar] [CrossRef]
- Vijayakumar, A.R.; Csöregi, E.; Heller, A.; Gorton, L. Alcohol Biosensors Based on Coupled Oxidase-Peroxidase Systems. Anal. Chim. Acta 1996, 327, 223–234. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kuwabata, S.; Fukusaki, E.; Kobayashi, A. Real-Time Quantification of Methanol in Plants Using a Hybrid Alcohol Oxidase−Peroxidase Biosensor. Anal. Chem. 2004, 76, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Jönsson-Pettersson, G.; Gorton, L.; Marko-Varga, G.; Csöregi, E. A Reagentless Amperometric Biosensor for Alcohol Detection in Column Liquid Chromatography Based on Co-Immobilized Peroxidase and Alcohol Oxidase in Carbon Paste. J. Biotechnol. 1993, 31, 301–316. [Google Scholar] [CrossRef]
- Smutok, O.; Ngounou, B.; Pavlishko, H.; Gayda, G.; Gonchar, M.; Schuhmann, W. A Reagentless Bienzyme Amperometric Biosensor Based on Alcohol Oxidase/Peroxidase and an Os-Complex Modified Electrodeposition Paint. Sens. Actuators B 2006, 113, 590–598. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Sezgintürk, M.K.; Dinçkaya, E. A Biosensor Based on Urate Oxidase–Peroxidase Coupled Enzyme System for Uric Acid Determination in Urine. Talanta 2003, 61, 73–79. [Google Scholar] [CrossRef]
- Dharmapandian, P.; Rajesh, S.; Rajasingh, S.; Rajendran, A.; Karunakaran, C. Electrochemical Cysteine Biosensor Based on the Selective Oxidase–Peroxidase Activities of Copper, Zinc Superoxide Dismutase. Sens. Actuators B 2010, 148, 17–22. [Google Scholar] [CrossRef]
- Tkáč, J.; Šturdík, E.; Gemeiner, P. Novel Glucose Non-Interference Biosensor for Lactose Detection Based on Galactose Oxidase–Peroxidase with and without Co-Immobilised β-Galactosidase. Analyst 2000, 125, 1285–1289. [Google Scholar] [CrossRef]
- Crumbliss, A.L.; Stonehuerner, J.G.; Henkens, R.W.; Zhao, J.; O’Daly, J.P. A Carrageenan Hydrogel Stabilized Colloidal Gold Multi-Enzyme Biosensor Electrode Utilizing Immobilized Horseradish Peroxidase and Cholesterol Oxidase/Cholesterol Esterase to Detect Cholesterol in Serum and Whole Blood. Biosens. Bioelectron. 1993, 8, 331–337. [Google Scholar] [CrossRef]
- Li, G.; Liao, J.M.; Hu, G.Q.; Ma, N.Z.; Wu, P.J. Study of Carbon Nanotube Modified Biosensor for Monitoring Total Cholesterol in Blood. Biosens. Bioelectron. 2005, 20, 2140–2144. [Google Scholar] [CrossRef]
- Singh, S.; Solanki, P.R.; Pandey, M.K.; Malhotra, B.D. Cholesterol Biosensor Based on Cholesterol Esterase, Cholesterol Oxidase and Peroxidase Immobilized onto Conducting Polyaniline Films. Sens. Actuators B 2006, 115, 534–541. [Google Scholar] [CrossRef]
- Nieh, C.-H.; Kitazumi, Y.; Shirai, O.; Kano, K. Amperometric Biosensor Based on Reductive H2O2 Detection Using Pentacyanoferrate-Bound Polymer for Creatinine Determination. Anal. Chim. Acta 2013, 767, 128–133. [Google Scholar] [CrossRef]
- Xia, H.-Q.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct Electron Transfer-Type Bioelectrocatalysis of Peroxidase at Mesoporous Carbon Electrodes and Its Application for Glucose Determination Based on Bienzyme System. Anal. Sci. 2017, 33, 839–844. [Google Scholar] [CrossRef]
- Sakai, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Nanostructured Porous Electrodes by the Anodization of Gold for an Application as Scaffolds in Direct-Electron-Transfer-Type Bioelectrocatalysis. Anal. Sci. 2018, 34, 1317–1322. [Google Scholar] [CrossRef]
- Sadik, O.A.; Aluoch, A.O.; Zhou, A. Status of Biomolecular Recognition Using Electrochemical Techniques. Biosens. Bioelectron. 2009, 24, 2749–2765. [Google Scholar] [CrossRef]
- Andreu-Perez, J.; Poon, C.C.Y.; Merrifield, R.D.; Wong, S.T.C.; Yang, G.-Z. Big Data for Health. IEEE J. Biomed. Health 2015, 19, 1193–1208. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The Era of Digital Health; A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef]
- Wang, J.; Katz, E. Digital Biosensors with Built-In Logic for Biomedical Applications–Biosensors Based on a Biocomputing Concept. Anal. Bioanal. Chem. 2010, 398, 1591–1603. [Google Scholar] [CrossRef]
- Guz, N.; Halámek, J.; Rusling, J.F.; Katz, E. A Biocatalytic Cascade with Several Output Signals–Towards Biosensors with Different Levels of Confidence. Anal. Bioanal. Chem. 2014, 406, 3365–3370. [Google Scholar] [CrossRef]
- Katz, E.; Poghossian, A.; Schöning, M.J. Enzyme-Based Logic Gates and Circuits–Analytical Applications and Interfacing with Electronics. Anal. Bioanal. Chem. 2017, 409, 81–94. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of Urinary Biomarkers to Creatinine during Changes in Glomerular Filtration Rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef]
- Ganguly, A.; Rice, P.; Lin, K.-C.; Muthukumar, S.; Prasad, S. A Combinatorial Electrochemical Biosensor for Sweat Biomarker Benchmarking. SLAS Technol. 2020, 25, 25–32. [Google Scholar] [CrossRef]
- Appenzeller, B.M.R.; Schummer, C.; Rodrigues, S.B.; Wennig, R. Determination of the Volume of Sweat Accumulated in a Sweat-Patch Using Sodium and Potassium as Internal Reference. J. Chromatogr. B 2007, 852, 333–337. [Google Scholar] [CrossRef]
- Smith, S.M.; Eng, R.H.K.; Buccini, F. Use of d-Lactic Acid Measurements in the Diagnosis of Bacterial Infections. J. Infect. Dis. 1986, 154, 658–664. [Google Scholar] [CrossRef]
- Shoup, D.; Szabo, A. Chronoamperometric Current at Finite Disk Electrodes. J. Electroanal. Chem. 1982, 140, 237–245. [Google Scholar] [CrossRef]
- Miyata, M.; Kitazumi, Y.; Shirai, O.; Kataoka, K.; Kano, K. Diffusion-Limited Biosensing of Dissolved Oxygen by Direct Electron Transfer-Type Bioelectrocatalysis of Multi-Copper Oxidases Immobilized on Porous Gold Microelectrodes. J. Electroanal. Chem. 2020, 860, 113895. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Takeuchi, R.; Kitazumi, Y.; Shirai, O.; Kano, K. Significance of Mesoporous Electrodes for Noncatalytic Faradaic Process of Randomly Oriented Redox Proteins. J. Phys. Chem. C 2016, 120, 26270–26277. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Effects of Mesoporous Structures on Direct Electron Transfer-Type Bioelectrocatalysis: Facts and Simulation on a Three-Dimensional Model of Random Orientation of Enzymes. Electrochemistry 2017, 85, 82–87. [Google Scholar] [CrossRef]
- Lee, H.J.; Beriet, C.; Ferrigno, R.; Girault, H.H. Cyclic Voltammetry at a Regular Microdisc Electrode Array. J. Electroanal. Chem. 2001, 502, 138–145. [Google Scholar] [CrossRef]
- Ordeig, O.; del Campo, J.; Muñoz, F.X.; Banks, C.E.; Compton, R.G. Electroanalysis Utilizing Amperometric Microdisk Electrode Arrays. Electroanalysis 2007, 19, 1973–1986. [Google Scholar] [CrossRef]
- Guo, J.; Lindner, E. Cyclic Voltammograms at Coplanar and Shallow Recessed Microdisk ElectrodeAarrays: Guidelines for Design and Experiment. Anal. Chem. 2009, 81, 130–138. [Google Scholar] [CrossRef]
- Bixler, J.W.; Fifield, M.; Poler, J.C.; Bond, A.M.; Thormann, W. An Evaluation of Ultrathin Ring and Band Microelectrodes as Amperometric Sensors in Electrochemical Flow Cells. Electroanalysis 1989, 1, 23–33. [Google Scholar] [CrossRef]
- Kitazumi, Y.; Hamamoto, K.; Noda, T.; Shirai, O.; Kano, K. Fabrication and Characterization of Ultrathin-Ring Electrodes for Pseudo-Steady-State Amperometric Detection. Anal. Sci. 2015, 31, 603–607. [Google Scholar] [CrossRef]
- Szabo, A.; Cope, D.K.; Tallman, D.E.; Kovach, P.M.; Wightman, R.M. Chronoamperometric Current at Hemicylinder and Band Microelectrodes: Theory and Experiment. J. Electroanal. Chem. 1987, 217, 417–423. [Google Scholar] [CrossRef]
- Uchiyama, S.; Kato, S.; Suzuki, S.; Hamamoto, O. Biocoulometry of Cholesterol Using Porous Carbon Felt Electrodes. Electroanalysis 1991, 3, 59–62. [Google Scholar] [CrossRef]
- Morris, N.A.; Cardosi, M.F.; Birch, B.J.; Turner, A.P.F. An Electrochemical Capillary Fill Device for the Analysis of Glucose Incorporating Glucose Oxidase and Ruthenium (III) Hexamine as Mediator. Electroanalysis 1992, 4, 1–9. [Google Scholar] [CrossRef]
- Fukaya, M.; Ebisuya, H.; Furukawa, K.; Akita, S.; Kawamura, Y.; Uchiyama, S. Self-Driven Coulometry of Ethanol, d-Glucose and d-Fructose Using Ubiquinone-Dependent Dehydrogenase Reactions. Anal. Chim. Acta 1995, 306, 231–236. [Google Scholar] [CrossRef]
- Tsujimura, S.; Nishina, A.; Kamitaka, Y.; Kano, K. Coulometric d-Fructose Biosensor Based on Direct Electron Transfer Using d-Fructose Dehydrogenase. Anal. Chem. 2009, 81, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, F.; Kato, D.; Kurita, R.; Mie, Y.; Sato, Y.; Niwa, O. Highly-Sensitive Biosensors with Chemically-Amplified Responses. Electrochemistry 2008, 76, 515–521. [Google Scholar] [CrossRef][Green Version]




| Physical Quantities | Stability | Reproducibility | Controllability |
|---|---|---|---|
| Surface area of electrode (A) | Good | good | good |
| Standard redox potential ( | good | good | poor |
| Concentration of mediator (cM) | poor | good | good |
| Electrode potential (E) | poor | poor | good |
| Permeability of membrane (Pm) | good | poor | poor |
| Rotating speed (ω) | good | good | good |
| Enzyme activity ( | poor | poor | poor |
| Type of Bioelectrocatalysis | Advantage | Disadvantage |
|---|---|---|
| MET-type | Easy coupling of enzyme reaction High loading of enzyme and mediator per projected area | Leakage of mediator and/or enzyme Stability of mediator Low thermodynamic efficiency |
| DET-type | Crosstalk-free High thermodynamic efficiency | Limited amounts of effective enzyme per projected area Limited number of enzymes Interference from strongly adsorbing substances |
| Multi-enzymatic cascade | Flexibility in sensor design | Instability due to series reactions |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors 2020, 20, 4826. https://doi.org/10.3390/s20174826
Adachi T, Kitazumi Y, Shirai O, Kano K. Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors. 2020; 20(17):4826. https://doi.org/10.3390/s20174826
Chicago/Turabian StyleAdachi, Taiki, Yuki Kitazumi, Osamu Shirai, and Kenji Kano. 2020. "Development Perspective of Bioelectrocatalysis-Based Biosensors" Sensors 20, no. 17: 4826. https://doi.org/10.3390/s20174826
APA StyleAdachi, T., Kitazumi, Y., Shirai, O., & Kano, K. (2020). Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors, 20(17), 4826. https://doi.org/10.3390/s20174826





