Nanoparticles Produced via Laser Ablation of Porous Silicon and Silicon Nanowires for Optical Bioimaging
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties
3.2. Spectrophotometry and OCT
3.3. Fluorescence
- Laser ablation of microporous silicon layers results in formation of the relatively large SiNPs with sizes more than 10 nm (Figure 4a) in contrast to the initial targets nanostructured at smaller scale [34]. Therefore, the formed SiNPs are characterized by less fluorescence photon energy (larger emission wavelength).
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Focarete, M.L.; Tampieri, A. (Eds.) Core-Shell Nanostructures for Drug Delivery and Theranostics: Challenges, Strategies and Prospects for Novel Carrier Systems; Woodhead Publishing: Duxford, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [Green Version]
- Santi, M.; Mapanao, A.K.; Cassano, D.; Vlamidis, Y.; Cappello, V.; Voliani, V. Endogenously-Activated Ultrasmall-in-Nano Therapeutics: Assessment on 3D Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1063. [Google Scholar] [CrossRef]
- Kurakina, D.; Kirillin, M.Y.; Perekatova, V.V.; Plekhanov, V.I.; Orlova, A.; Sergeeva, E.; Khilov, A.; Nerush, A.; Subochev, P.V.; Mallidi, S.; et al. Towards Bimodal Optical Monitoring of Photodynamic Therapy with Targeted Nanoconstructs: A Phantom Study. Appl. Sci. 2019, 9, 1918. [Google Scholar] [CrossRef] [Green Version]
- Ksenofontova, O.I.; Vasin, A.V.; Egorov, V.; Bobyl, A.; Soldatenkov, F.Y.; Terukov, E.I.; Ulin, V.P.; Ulin, N.V.; Kiselev, O.I. Porous silicon and its applications in biology and medicine. Tech. Phys. 2014, 59, 66–77. [Google Scholar] [CrossRef]
- Stojanovic, V.; Cunin, F.; Durand, J.O.; Garcia, M.; Gary-Bobo, M. Potential of porous silicon nanoparticles as an emerging platform for cancer theranostics. J. Mater. Chem. B 2016, 4, 7050–7059. [Google Scholar] [CrossRef]
- Almeida, P.V.; Shahbazi, M.-A.; Correia, A.; Mäkilä, E.; Kemell, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. A multifunctional nanocomplex for enhanced cell uptake, endosomal escape and improved cancer therapeutic effect. Nanomedicine 2017, 12, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Kafshgari, M.H.; Delalat, B.; Harding, F.J.; Cavallaro, A.A.; Mäkilä, E.; Salonen, J.; Vasilev, K.; Voelcker, N.H. Antibacterial properties of nitric oxide-releasing porous silicon nanoparticles. J. Mater. Chem. B 2016, 4, 2051–2058. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, Q.; Shi, J.; Li, J.; Chen, Z.; Wang, B. Co-loading of photothermal agents and anticancer drugs into porous silicon nanoparticles with enhanced chemo-photothermal therapeutic efficacy to kill multidrug-resistant cancer cells. Colloids Surf. B Biointerfaces 2018, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lia, S.; Zhanga, Y.; Hea, X.-W.; Li, W.-Y.; Zhangab, Y.-K. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; El Cheikh, K.; Bouffard, E.; Maynadier, M.; Aggad, D.; Stojanovic, V.; Knežević, N.Ž.; Garcia, M.; Maillard, P.; Morère, A.; et al. Mesoporous silicon nanoparticles for targeted two-photon theranostics of prostate cancer. J. Mater. Chem. B 2016, 4, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Hsieh, C.-C.; Chen, N.-T.; Chu, C.-H.; Huang, C.-M.; Chou, P.-T.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Well-defined mesoporous nanostructure modulates three-dimensional interface energy transfer for two-photon activated photodynamic therapy. Nano Today 2011, 6, 552–563. [Google Scholar] [CrossRef]
- Sviridov, A.P.; Osminkina, L.A.; Kharin, A.Y.; Gongansky, M.B.; Kargina, J.V.; Kudryavtsev, A.A.; Bezsudnova, Y.; Perova, T.S.; Geloen, A.; Lysenko, V.; et al. Cytotoxicity control of silicon nanoparticles by biopolymer coating and ultrasound irradiation for cancer theranostic applications. Nanotechnology 2017, 28, 105102. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, M.B.; Osminkina, L.A.; Pereira, A.; Manankov, A.A.; Fedorenko, A.A.; Vasiliev, A.; Solovyev, V.V.; Kudryavtsev, A.A.; Sentis, M.; Kabashin, A.V.; et al. Laser-synthesized oxide-passivated bright Si quantum dots for bioimaging. Sci. Rep. 2016, 6, 24732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, G.; Burns, A.; Herz, E.; Hay, A.G.; Houston, P.L.; Wiesner, U.; Lion, L.W. Functional Tomographic Fluorescence Imaging of pH Microenvironments in Microbial Biofilms by Use of Silica Nanoparticle Sensors. Appl. Environ. Microbiol. 2009, 75, 7426–7435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; He, Y.; Chang, J.; Wang, L.; Chen, H.; Tan, Y.-W.; Wang, H.-Y.; Shao, Z. Surface-Modified Silicon Nanoparticles with Ultrabright Photoluminescence and Single-Exponential Decay for Nanoscale Fluorescence Lifetime Imaging of Temperature. J. Am. Chem. Soc. 2013, 135, 14924–14927. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Shuleiko, D.V.; Kirillin, M.Y.; Kashaev, F.V.; Gongalsky, M.B.; Golovan, L.A.; Kashkarov, P.K.; Loginova, D.; Agrba, P.D.; Sergeeva, E. Silicon nanoparticles as contrast agents in the methods of optical biomedical diagnostics. Quantum Electron. 2017, 47, 638–646. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Kurakina, D.; Kashaev, F.V.; Skobelkina, A.V.; Kolchin, A.V.; Kaminskaya, T.P.; Khilov, A.V.; Agrba, P.D.; Sergeeva, E.A.; Kashkarov, P.K.; et al. Structural and optical properties of nanoparticles formed by laser ablation of porous silicon in liquids: Perspectives in biophotonics. Quantum Electron. 2020, 50, 69–75. [Google Scholar] [CrossRef]
- Li, S.; Yuan, Y.; Yang, Y.; Li, C.; McMahon, M.T.; Liu, M.; Chen, S.; Zhou, X. Potential detection of cancer with fluorinated silicon nanoparticles in 19F MR and fluorescence imaging. J. Mater. Chem. B 2018, 6, 4293–4300. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Nirwan, V.P.; Popov, A.; Ryabchikov, Y.V.; Tselikov, G.; Sentis, M.; Fahmi, A.; Kabashin, A.V. Recent Advances in Laser-Ablative Synthesis of Bare Au and Si Nanoparticles and Assessment of Their Prospects for Tissue Engineering Applications. Int. J. Mol. Sci. 2018, 19, 1563. [Google Scholar] [CrossRef] [Green Version]
- Kharin, A.Y.; Lysenko, V.; Rogov, A.; Ryabchikov, Y.V.; Geloen, A.; Tishchenko, I.; Marty, O.; Sennikov, P.G.; Kornev, R.A.; Zavestovskaya, I.N.; et al. Bi-Modal Nonlinear Optical Contrast from Si Nanoparticles for Cancer Theranostics. Adv. Opt. Mater. 2019, 7, 1801728. [Google Scholar] [CrossRef]
- Oleshchenko, V.; Kharin, A.Y.; Alykova, A.; Karpukhina, O.; Karpov, N.; Popov, A.; Bezotosnyi, V.; Klimentov, S.; Zavestovskaya, I.; Kabashin, A.V.; et al. Localized infrared radiation-induced hyperthermia sensitized by laser-ablated silicon nanoparticles for phototherapy applications. Appl. Surf. Sci. 2020, 516, 145661. [Google Scholar] [CrossRef]
- Sviridov, A.P.; Andreev, V.; Ivanova, E.M.; Osminkina, L.A.; Tamarov, K.P.; Timoshenko, V.Y. Porous silicon nanoparticles as sensitizers for ultrasonic hyperthermia. Appl. Phys. Lett. 2013, 103, 193110. [Google Scholar] [CrossRef]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.V.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gu, L.; Howell, S.B.; Sailor, M.J. Porous Silicon Nanoparticle Photosensitizers for Singlet Oxygen and Their Phototoxicity against Cancer Cells. ACS Nano 2011, 5, 3651–3659. [Google Scholar] [CrossRef]
- Secret, E.; Maynadier, M.; Gallud, A.; Gary-Bobo, M.; Chaix, A.; Belamie, E.; Maillard, P.; Sailor, M.J.; Garcia, M.; Durand, J.-O.; et al. Anionic porphyrin-grafted porous silicon nanoparticles for photodynamic therapy. Chem. Commun. 2013, 49, 4202. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.; Lee, J.; Zheng, H.; Hong, S.; Lee, C. Porous silicon nanoparticles for cancer photothermotherapy. Nanoscale Res. Lett. 2011, 6, 321. [Google Scholar] [CrossRef] [Green Version]
- Osminkina, L.A.; Sivakov, V.; Mysov, G.; Georgobiani, V.; Natashina, U.; Talkenberg, F.; Solovyev, V.V.; Kudryavtsev, A.; Timoshenko, V.Y. Nanoparticles prepared from porous silicon nanowires for bio-imaging and sonodynamic therapy. Nanoscale Res. Lett. 2014, 9, 463. [Google Scholar] [CrossRef] [Green Version]
- Tolstik, E.; Osminkina, L.A.; Matthäus, C.; Burkhardt, M.; Tsurikov, K.E.; Natashina, U.; Timoshenko, V.Y.; Heintzmann, R.; Popp, J.; Sivakov, V. Studies of silicon nanoparticles uptake and biodegradation in cancer cells by Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1931–1940. [Google Scholar] [CrossRef]
- Rioux, D.; Laferrieère, M.; Douplik, A.; Shah, D.; Lilge, L.; Kabashin, A.V.; Meunier, M.M. Silicon nanoparticles produced by femtosecond laser ablation in water as novel contamination-free photosensitizers. J. Biomed. Opt. 2009, 14, 021010. [Google Scholar] [CrossRef] [Green Version]
- Skobelkina, A.V.; Kashaev, F.V.; Kolchin, A.V.; Shuleiko, D.V.; Kaminskaya, T.P.; Presnov, D.E.; Golovan, L.A.; Kashkarov, P.K. Silicon nanoparticles formed via pulsed laser ablation of porous silicon in liquids. Tech. Phys. Lett. 2020, 46, 687–690. [Google Scholar] [CrossRef]
- Skobelkina, A.V.; Kashaev, F.V.; Zabotnov, S.V.; Kolchin, A.V.; Kaminskaya, T.P.; Presnov, D.E.; Sergeeva, E.A.; Kirillin, M.Y.; Golovan, L.A.; Kashkarov, P.K. Structural and photoluminescence properties of nanoparticles formed by laser ablation of porous silicon in ethanol and liquid nitrogen. IFMBE Proc. 2019, 77, 101–104. [Google Scholar] [CrossRef]
- Sivakov, V.A.; Brönstrup, G.; Pécz, B.; Berger, A.; Radnoczi, G.Z.; Krause, M.; Christiansen, S. Realization of Vertical and Zigzag Single Crystalline Silicon Nanowire Architectures. J. Phys. Chem. C 2010, 114, 3798–3803. [Google Scholar] [CrossRef]
- Lehmann, V.; Stengl, R.; Luigart, A. On the morphology and the electrochemical formation mechanism of mesoporous silicon. Mater. Sci. Eng. B 2000, 69, 11–22. [Google Scholar] [CrossRef]
- Loni, A. Porous silicon formation by anodization. In Handbook of Porous Silicon, 2nd ed.; Canham, L., Ed.; Springer: Cham, Switzerland, 2018; pp. 13–24. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Kholodov, M.M.; Georgobiani, V.; Presnov, D.; Golovan, L.; Kashkarov, P.K. Photon lifetime correlated increase of Raman scattering and third-harmonic generation in silicon nanowire arrays. Laser Phys. Lett. 2016, 13, 35902. [Google Scholar] [CrossRef]
- Osminkina, L.A.; Tamarov, K.P.; Sviridov, A.P.; Galkin, R.A.; Gongalsky, M.B.; Solovyev, V.V.; Kudryavtsev, A.A.; Timoshenko, V.Y. Photoluminescent biocompatible silicon nanoparticles for cancer theranostic applications. J. Biophotonics 2012, 5, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Khilov, A.V.; Kirillin, M.Y.; Loginova, D.; Turchin, I. Estimation of chlorin-based photosensitizer penetration depth prior to photodynamic therapy procedure with dual-wavelength fluorescence imaging. Laser Phys. Lett. 2018, 15, 126202. [Google Scholar] [CrossRef]
- Besner, S.; Meunier, M. Laser synthesis of nanomaterials. In Laser Precision Microfabrication; Sugioka, K., Meunier, M., Piqué, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Chapter 7; pp. 163–187. [Google Scholar] [CrossRef]
- Bustarret, E.; Hachicha, M.A.; Brunel, M. Experimental determination of the nanocrystalline volume fraction in silicon thin films from Raman spectroscopy. Appl. Phys. Lett. 1988, 52, 1675–1677. [Google Scholar] [CrossRef]
- Shuleiko, D.V.; Potemkin, F.V.; Romanov, I.; Parhomenko, I.N.; Pavlikov, A.V.; Presnov, D.; Zabotnov, S.V.; Kazanskii, A.G.; Kashkarov, P.K. Femtosecond laser pulse modification of amorphous silicon films: Control of surface anisotropy. Laser Phys. Lett. 2018, 15, 056001. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [Green Version]
- Brouillette, M. The Richtmyer-Meshkov instability. Ann. Rev. Fluid Mech. 2002, 34, 445–468. [Google Scholar] [CrossRef] [Green Version]
- Dyachkov, S.; Zhakhovsky, V.V.; Parshikov, A.N.; Inogamov, N. Ablation into water: Fragmentation of metal via Richtmyer–Meshkov instability. J. Phys. Conf. Ser. 2019, 1147, 012064. [Google Scholar] [CrossRef]
- Philipp, H.R.; Taft, E.A. Optical Constants of Silicon in the Region 1 to 10 ev. Phys. Rev. 1960, 120, 37–38. [Google Scholar] [CrossRef]
- Schinke, C.; Peest, C.; Schmidt, J.; Brendel, R.; Bothe, K.; Vogt, M.R.; Kröger, I.; Winter, S.; Schirmacher, A.; Lim, S.; et al. Uncertainty analysis for the coefficient of band-to-band absorption of crystalline silicon. AIP Adv. 2015, 5, 067168. [Google Scholar] [CrossRef] [Green Version]
- Matveev, A.N. Optics; Mir Publishers: Moscow, Russia, 1988; Chapter 9; pp. 365–379. [Google Scholar]
- Gelloz, B. Photoluminscence of porous silicon. In Handbook of Porous Silicon, 2nd ed.; Canham, L., Ed.; Springer: Cham, Switzerland, 2018; pp. 449–462. [Google Scholar] [CrossRef]
- Konstantinova, E.; Demin, V.; Vorontzov, A.; Ryabchikov, Y.V.; Belogorokhov, I.; Osminkina, L.A.; Forsh, P.; Kashkarov, P.; Timoshenko, V.Y. Electron-paramagnetic resonance and photoluminescence study of Si nanocrystals-photosensitizers of singlet oxygen molecules. J. Non Cryst. Solids 2006, 352, 1156–1159. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Lysenko, V.; Nychyporuk, T. Enhanced Thermal Sensitivity of Silicon Nanoparticles Embedded in (Nano-Ag/)SiNx for Luminescent Thermometry. J. Phys. Chem. C 2014, 118, 12515–12519. [Google Scholar] [CrossRef]
- Sivakov, V.A.; Voigt, F.; Berger, A.; Bauer, G.; Christiansen, S. Roughness of silicon nanowire sidewalls and room temperature photoluminescence. Phys. Rev. B 2010, 82, 125446. [Google Scholar] [CrossRef] [Green Version]


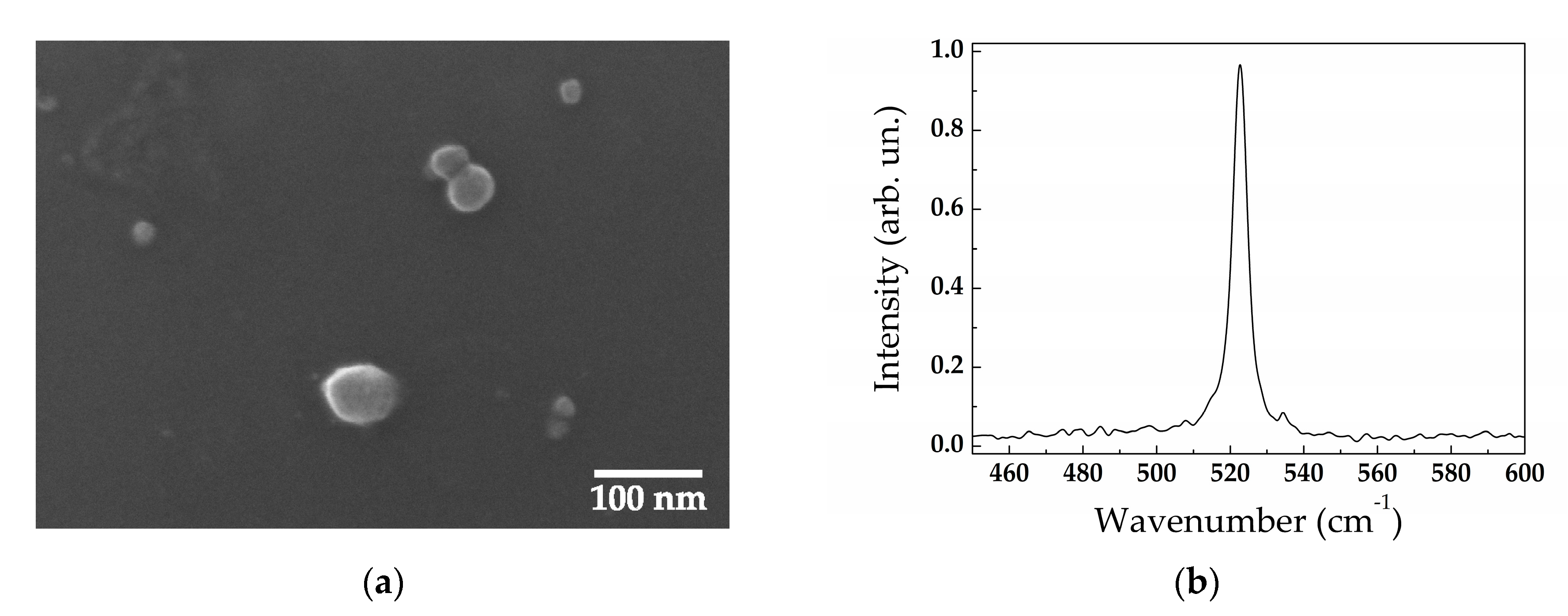
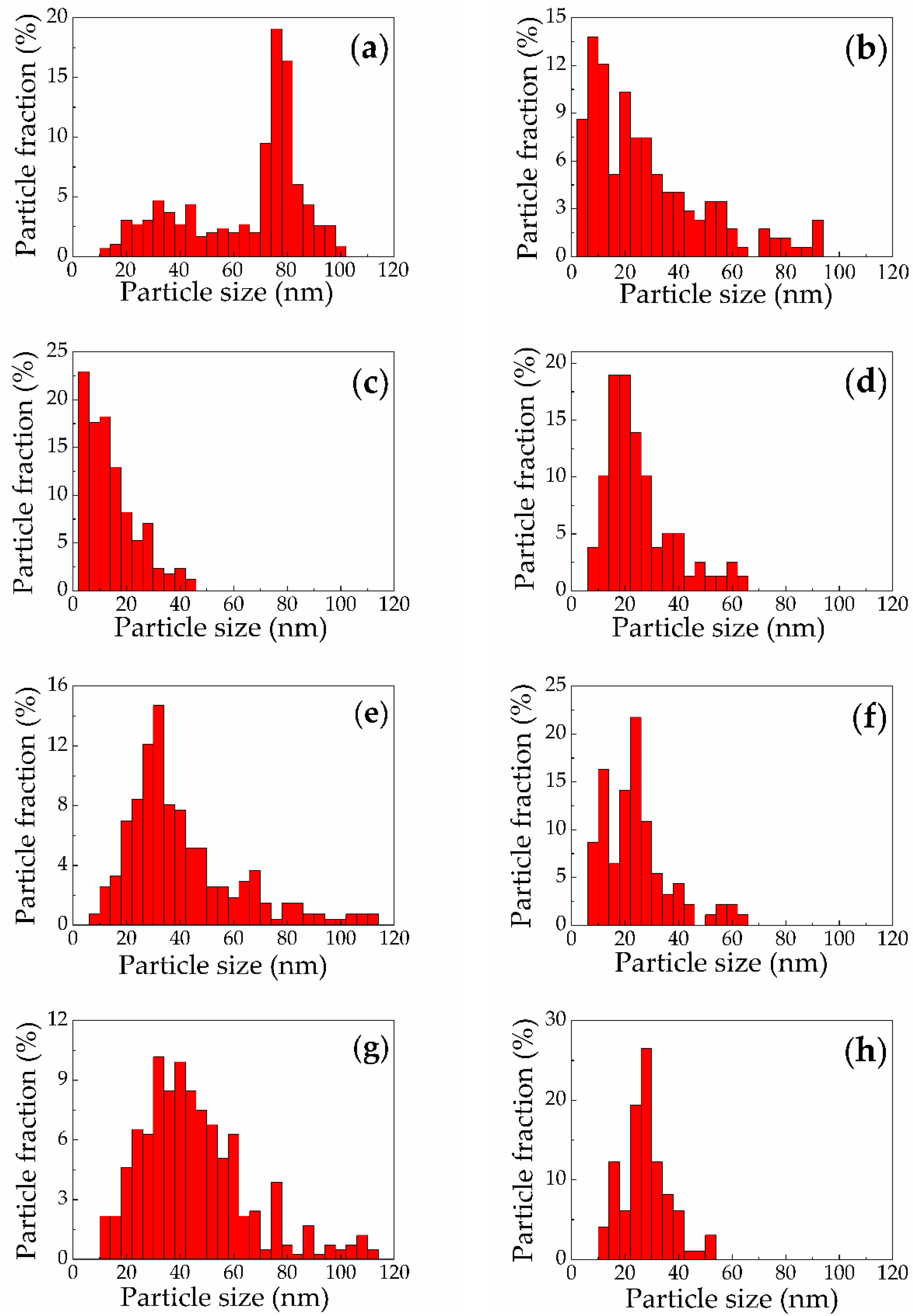
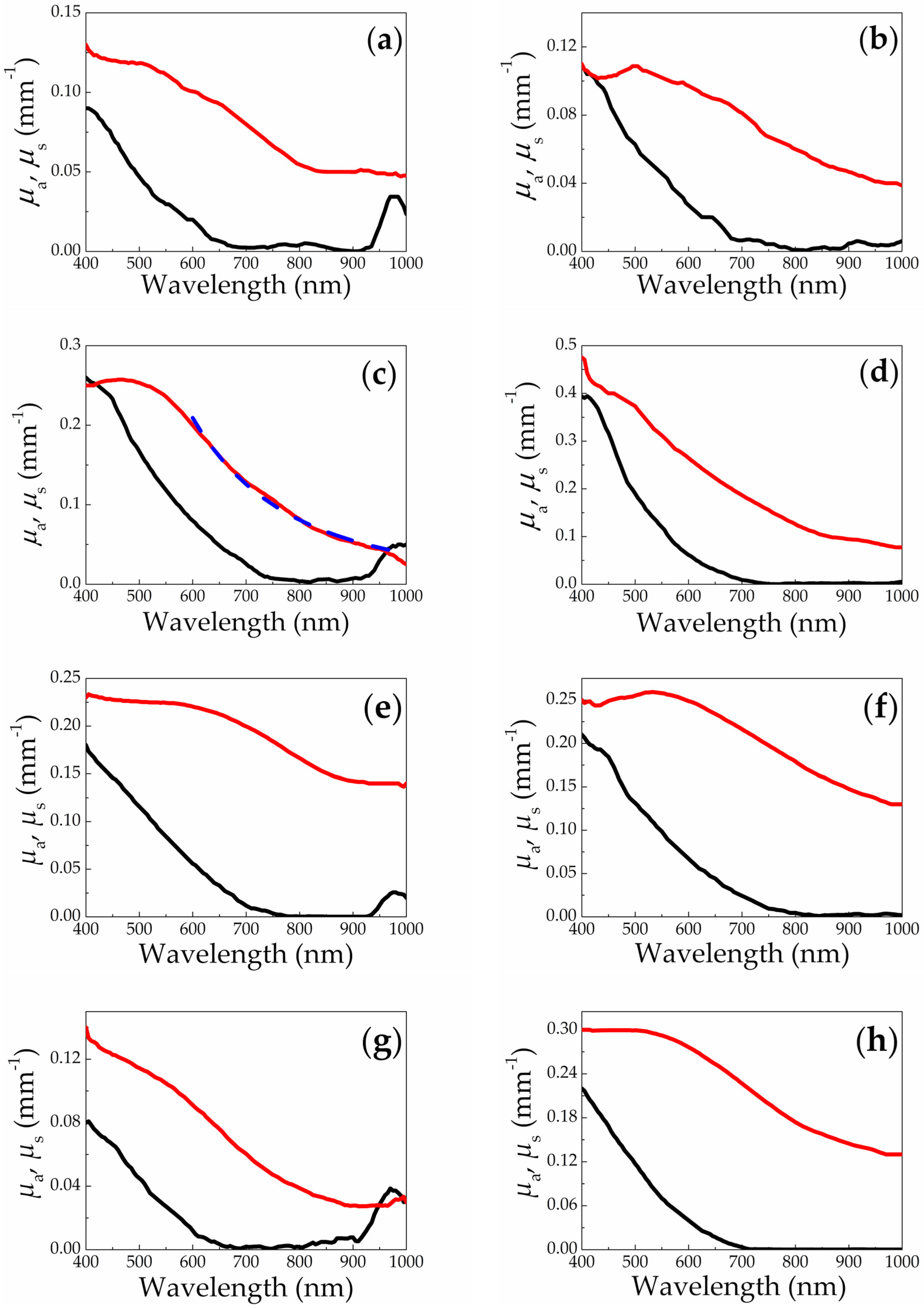




| Target Type | Buffer Liquid | SiNP Mean Size (nm) | Size Standard Deviation (nm) |
|---|---|---|---|
| Microporous silicon | Water | 65 | 22 |
| Microporous silicon | Ethanol | 28 | 22 |
| Mesoporous silicon | Water | 14 | 10 |
| Mesoporous silicon | Ethanol | 25 | 13 |
| Low-doped SiNWs | Water | 42 | 24 |
| Low-doped SiNWs | Ethanol | 24 | 9 |
| Heavily-doped SiNWs | Water | 45 | 20 |
| Heavily-doped SiNWs | Ethanol | 28 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabotnov, S.V.; Skobelkina, A.V.; Sergeeva, E.A.; Kurakina, D.A.; Khilov, A.V.; Kashaev, F.V.; Kaminskaya, T.P.; Presnov, D.E.; Agrba, P.D.; Shuleiko, D.V.; et al. Nanoparticles Produced via Laser Ablation of Porous Silicon and Silicon Nanowires for Optical Bioimaging. Sensors 2020, 20, 4874. https://doi.org/10.3390/s20174874
Zabotnov SV, Skobelkina AV, Sergeeva EA, Kurakina DA, Khilov AV, Kashaev FV, Kaminskaya TP, Presnov DE, Agrba PD, Shuleiko DV, et al. Nanoparticles Produced via Laser Ablation of Porous Silicon and Silicon Nanowires for Optical Bioimaging. Sensors. 2020; 20(17):4874. https://doi.org/10.3390/s20174874
Chicago/Turabian StyleZabotnov, Stanislav V., Anastasiia V. Skobelkina, Ekaterina A. Sergeeva, Daria A. Kurakina, Aleksandr V. Khilov, Fedor V. Kashaev, Tatyana P. Kaminskaya, Denis E. Presnov, Pavel D. Agrba, Dmitrii V. Shuleiko, and et al. 2020. "Nanoparticles Produced via Laser Ablation of Porous Silicon and Silicon Nanowires for Optical Bioimaging" Sensors 20, no. 17: 4874. https://doi.org/10.3390/s20174874







