In-Vivo Microsystems: A Review
Abstract
:1. Introduction
2. Biocompatibility and Safety
- Toxicity;
- Injury;
- Inflammation;
- Infection;
- Tumorigenesis.
3. Examples of Important Parameters
4. Surface Devices
5. Short-Medium Term Internal Devices
5.1. Catheter Devices
5.2. Minimally Invasive/Laparoscopic Surgery
5.3. Medium Term Implants for Monitoring
6. Long Term Implants
6.1. Pacemaker
6.2. Cochlear Implant
6.3. Retinal Implant
6.4. Glucose Measurement
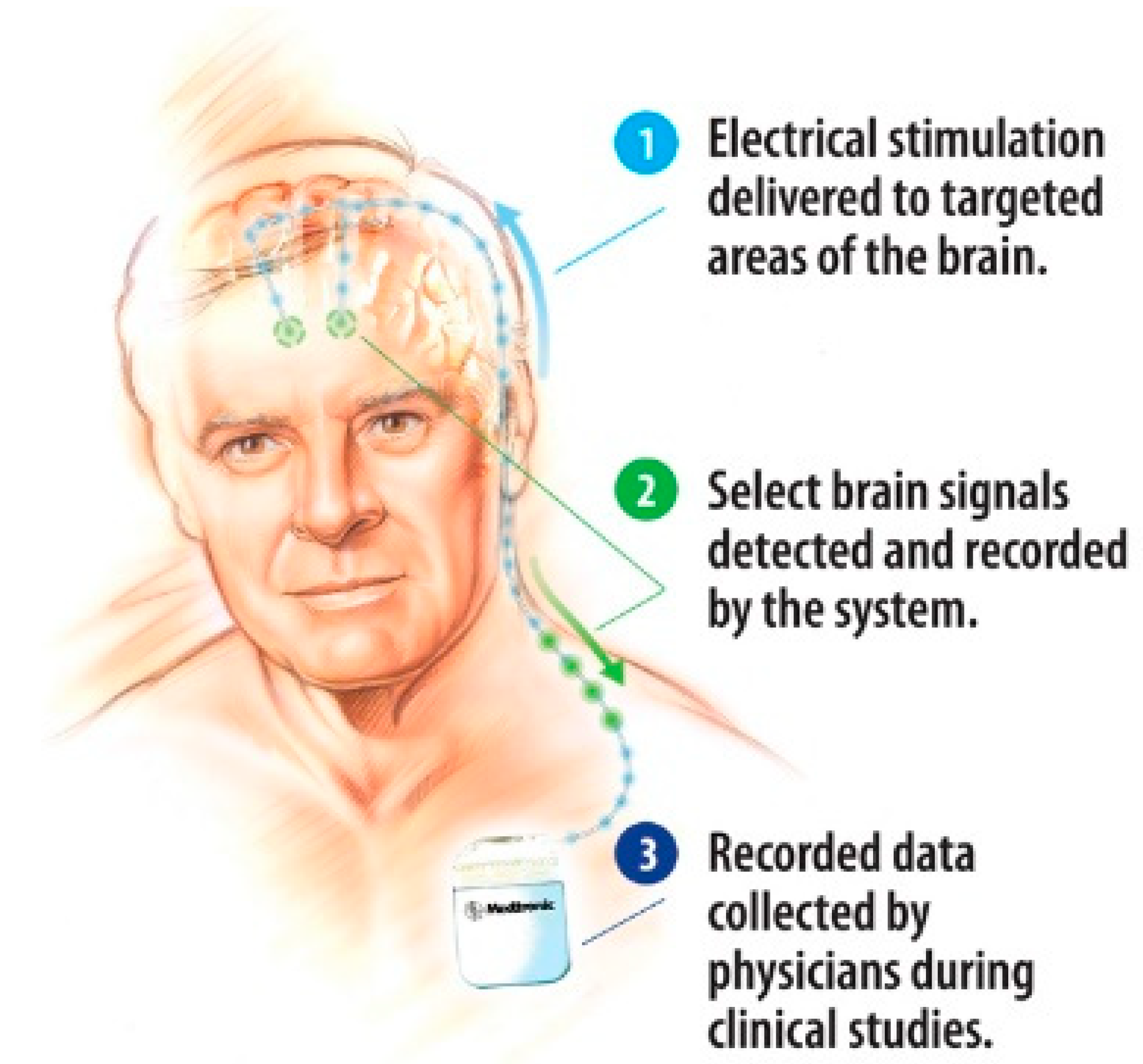
6.5. Brain Implants
7. Micropumps
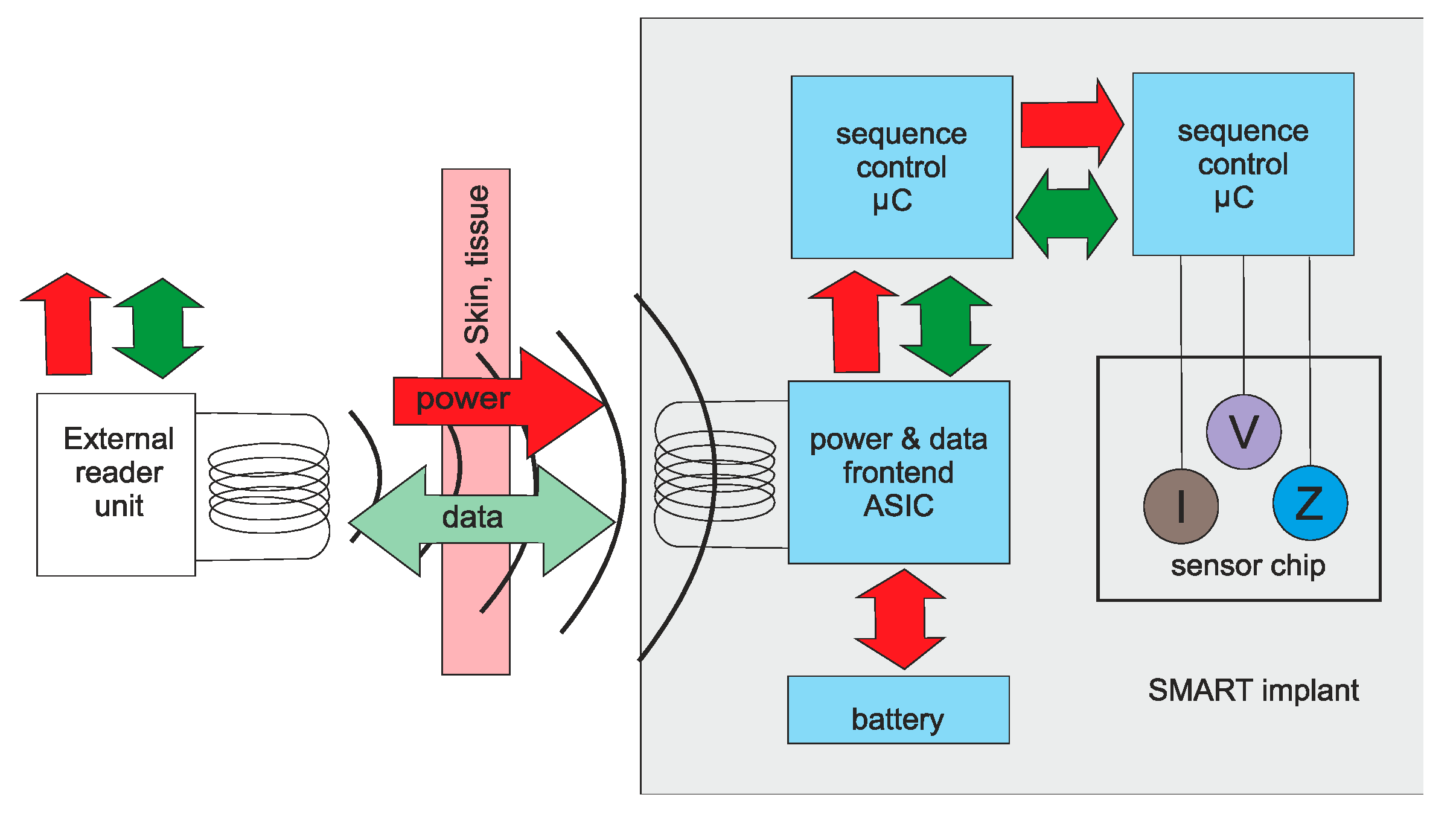
8. Power and Communication
9. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Nims, L.F.; Marshall, C.; Yale, J. Glass electrodes and apparatus for direct recording of pH in vivo. Yale J. Biol. Med. 1938, 10, 445–448, 561–564. [Google Scholar] [PubMed]
- Diamond, D.; Svehla, G. In-vivo sensors, trends in analytical chemistry. TrAC Trends Anal. Chem. 1987, 6, 46–49. [Google Scholar] [CrossRef]
- Waller, A.D. A demonstration on man of electromotive changes accompanying the heart’s beat. J. Physiol. 1887, 8, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Barold, S.S. Willem Einthoven and the birth of clinical electrocardiography a hundred years ago. Card. Electrophysiol. Rev. 2003, 7, 99–104. [Google Scholar] [CrossRef]
- Padmadinata, F.Z.; Veerhoek, J.J.; van Dijk, G.J.A.; Huijsing, J.H. Microelectronic skin electrodes. Sens. Actuators B 1990, 1, 491–494. [Google Scholar] [CrossRef]
- Van Rijn, M.A.C.; Kuiper, A.P.; Dankers, T.E.; Grimbergen, C.A. Low-cost active electrode improves the resolution in biopotential recording. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherland, 31 October–3 November 1996; pp. 101–102. [Google Scholar]
- Fan, Q.; Sebastiano, F.; Huijsing, J.H.; Makinwa, K.A.A. A 2.1 µW area-efficient capacitively-coupled chopper instrumentation amplifier for ECG applications in 65 nm CMOS. In Proceedings of the Asian Solid-State Circuits Conference, Beijing, China, 8–10 November 2010; pp. 1–4. [CrossRef]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Sys. Man Cybern. Part C 2010, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.B.; Omar, M.I.; Fisher, E.; Gillies, K.; MacLennan, S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- West, J.B. The beginnings of cardiac catheterization and the resulting impact on pulmonary medicine. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L651–L658. [Google Scholar] [CrossRef]
- Dotter, C.; Judkins, M. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its applications (PDF). Circulation 1964, 30, 654–670. [Google Scholar] [CrossRef] [Green Version]
- Tanase, D.; Goosen, J.F.L.; Trimp, P.J.; French, P.J. Multi-parameter sensor system with intravascular navigation for catheter/guide wire application. Sens. Actuators A 2002, 97–98, 116–124. [Google Scholar] [CrossRef]
- McWilliam, J.A. Electrical stimulation of the heart in man. Br. Med. J. 1899, 1, 348–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, S.; Schwedel, J.B. An intracardiac pacemaker for Stokes-Adams seizures. N. Engl. J. Med. 1959, 261, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Mond, H.G.; Sloman, J.G.; Edwards, R.H. The first pacemaker. Pacing Clin. Electrophysiol. 1982, 5, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Baxi, M.V. First artificial pacemaker: A milestone in history of cardiac electrostimulation. Asian Stud. Med. J. 2003, 2, 5. [Google Scholar]
- Shi, W.V.; Zhou, M.C. Body sensors applied in pacemakers: A survey. IEEE Sens. J. 2012, 12, 1817–1827. [Google Scholar] [CrossRef]
- Rezaeiyan, Y.; Zamani, M.; Shoaei, O.O.; Serdijn, W.A. mixed-signal ic with pulse width modulation wireless telemetry for implantable cardiac pacemakers in 0.18-µm CMOS. IEEE Trans. Biomed. Circuits Syst. 2018, 12. [Google Scholar] [CrossRef]
- Carlyon, R.P.; Macherey, O.; Frijns, J.H.; Axon, P.R.; Kalkman, R.K.; Boyle, P.; Baguley, D.M.; Briggs, J.; Deeks, J.M.; Briaire, J.J.; et al. Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear. JARO 2010, 11, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Biocompatibility Testing-Medical Devices. Available online: http://www.mbresearch.com/biocompatibility.htm (accessed on 1 August 2020).
- Park, J.; Lakes, R.S. Biomaterials: An Introduction; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-0-387-37879-4. [Google Scholar]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Shaw, G.W.; Claremont, D.J.; Pickup, J.C. In vitro testing of a simply constructed, highly stable glucose sensor suitable for implantation in diabetic patients. Biosens. Electron. 1991, 6, 401–406. [Google Scholar] [CrossRef]
- Garg, A.K.; Schwartz, S.; Edelman, S.V. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with Type 1 diabetes. Diebetes Care 2004, 27, 734–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Du, Y.; Eang, L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Hennig, A.; Lauko, J.; Grabmaier, A.; Wilson, C. Wireless tear glucose sensor. Procedia Eng. 2014, 87, 66–69. [Google Scholar] [CrossRef]
- Hayford, J.T.; Weydert, J.A.; Thompson, R.G. Validity of urine glucose measurements for estimating plasma glucose concentration. Diabetes Care 1983, 6, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Burch, G.E.; de Pasquale, N.P. A History of Electrocardiography; Year Book Medical; Norman Publishing: Chicago, IL, USA, 1964. [Google Scholar]
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Norton, J.J.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016. [Google Scholar] [CrossRef] [Green Version]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 2018. [Google Scholar] [CrossRef] [Green Version]
- Promphet, A.N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.S.; et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A self-powered sensor patch for glucose monitoring in sweat. In Proceedings of the IEEE MEMS 2017, Las Vegas, NV, USA, 22–26 January 2017; pp. 366–369. [Google Scholar]
- Acar, G.; Ozturk, O.; Golparvar1, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and flexible textile electrodes for biopotential signal monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Grittith, J.; Cluff, K.; Eckerman, B.; Aldrich, J.; Becker, R.; Moore-Jansen, P.; Patterson, J. Non-invasive electromagnetic skin patch sensor to measure intracranial fluid–volume shifts. Sensors 2018, 18, 1022. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Pastorin, G.; Lee, C. Toward self-powered wearable adhesive skin patch with bendable microneedle array for transdermal drug delivery. Adv. Sci. 2016, 3, 1500441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhao, J.; Ding, Y.; Wang, W. Dry electrodes for human bioelectrical signal monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Cuartero, M.; Sánchez, S.P.; Rajabi, M.; Roxhed, N.; Niklaus, F.; Crespo, G.A. Wearable all-solid-state potentiometric microneedle patch from intradermal potassium detection. Anal. Chem. 2019, 91, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W.; et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Bimicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [Green Version]
- Sydney, J.J. Werner Forssmann. Notable Twentieth-Century Scientists; Gale Research Inc.: Detroit, MI, USA, 1995; ISBN 978-0810391819. [Google Scholar]
- Forssmann, W.G.; Hirsch, J.R. 50 years Nobel Prize: Werner Forssmann and the issue of commemorative stamps. Eur. J. Med. Res. 2006, 11, 406–408. [Google Scholar]
- Ohta Laboratory. Available online: http://www.ifs.tohoku.ac.jp/bfc/html/English/Keywords_stent.html (accessed on 12 June 2019).
- Loschak, P.M.; Tschabrunn, A.D.C.M.; anter, E.; Howe, R.D. Automatic steering catheters in vivo with respiratory motion compensation. Int. J. Robot. Res. 2020, 35, 586–597. [Google Scholar] [CrossRef]
- Chang, Z.-Y.; Pop, G.A.P.; Meijer, G.C.M. A comparison of two- and four-electrode techniques to characterize blood impedance for the frequency range of 100 Hz to 100 MHz. IEEE Trans. Biomed. Eng. 2008, 55, 1247–1249. [Google Scholar] [CrossRef] [Green Version]
- Dekker, R.; Braam, S.; Henneken, V.; van der Horst, A.; Pakazad, S.K.; Louwerse, M.; van Meer, B.; Mimoun, B.; Savov, A.; van de Stolpe, A. Living chips and chips for the living. In Proceedings of the IEEE Bipolar/BiCMOS Circuits and Technology Meeting, Portland, OR, USA, 30 September–3 October 2012. [Google Scholar] [CrossRef]
- The Next Generation Smart Catheters and Implants. Available online: http://position-2.eu/ (accessed on 2 June 2019).
- CMUT Breakthrough Ultrasound Technology. Available online: www.innovationservices.philips.com/cmut (accessed on 2 June 2019).
- Tan, M.; Chen, C.; Chen, Z.; Janjic, J.; Daeichin, V.; Chang, Z.-Y.; Noothout, E.; van Soest, G.; Verweij, M.D.; de Jong, N.; et al. A front-end ASIC with high-voltage transmit switching and receive digitization for 3-D forward-looking intravascular ultrasound imaging. IEEE JSSC 2018, 53, 2284–2297. [Google Scholar] [CrossRef] [Green Version]
- Bera, D.; van de Nadel, F.; Radeljic-Jakic, N.; Lippe, B.; Soozande, M.; Pertijs, M.A.P.; Verweij, M.D.; Kruizinga, P.; Daeichin, V.; Vos, H.J.; et al. Fast volumetric imaging using a matrix transesophageal echocardiography probe with partioned transmit-receive array. Ultrasound Med. Biol. 2018, 9, 2025–2042. [Google Scholar] [CrossRef] [Green Version]
- Hatzinger, M.; Kwon, S.T.; Lanbein, S.; Kamp, S.; Häcker, A.; Alken, P. Hans Christian Jacobaeus: Inventor of human laparoscopic and thoracoscopy. J. Endourol. 2006. [Google Scholar] [CrossRef]
- Bera, D.; Raghunathan, S.B.; Chen, C.; Chen, Z.; Pertijs, M.A.; Verweij, M.D.; Daeichin, V.; Vos, H.J.; van der Steen, A.F.; de Jong, N.; et al. Multiline 3D beamforming using micro-beamformed datasets for pediatric transesophageal echocardiography. Phys. Med. Biol. 2018, 63, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Yamamoto, T.; Maeda1, Y.; Terao, K.; Shimokawa1, F.; Fujiwara, M.; Takao1, H. Highly sensitive silicon slip sensing imager for forceps grippers used under low friction condition. In Proceedings of the 2019 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 7 December 2019; pp. 18–28. [Google Scholar]
- Ju, F.; Wang, Y.; Zhang, Z.; Wang, Y.; Yun, Y.; Gao, H.; Chen, B. A miniature piezoelectric spiral tactile sensor for tissue harness palpatatin with catheter robot in minimally invasive surgery. Smart Mater. Struct. 2019. [Google Scholar] [CrossRef]
- Overtoom, E.M.; Hareman, T.; Jansen, F.-W.; Dakelman, J.; Schreuder, H.W.R. Haptic feedback, force feedback and forece-sensing in simulation training for laparoscopy: A systematic overview. J. Surg. Educ. 2018. [Google Scholar] [CrossRef]
- Tanase, D.; French, P.J.; Komen, N.; Kleinrensink, G.J.; Jeekel, J.; Lange, J.F.; Draaijer, A. Oxygen-tension measurements—The first step towards prevention and early detection of anastomotic leakage. In Proceedings of the IEEE Sensors 2007, Atlanta, GA, USA, 28–31 October 2007; pp. 68–71. [Google Scholar]
- Cha, G.D.; Kang, D.; Lee, J.; Kim, D.-H. Bioresorbable electronic implants: History, materials, fabrication, devices, and clinical applications. Adv. Healthc. Mater. 2019. [Google Scholar] [CrossRef]
- De Santis, M.; Cacciotti, I. Wireless implantable and biodegradable sensors for postsurgery monitoring: Current status and future perspectives. Nanotechnology 2020, 31, 252001. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tam, H.; Pfattner, A.C.H.R.; Niu, S.; Li, J.; Claverie, J.; Wang, Z.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nature Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Lidwell, M.C. Cardiac disease in relation to anaesthesia. In Transactions of the Third Session; Australasian Medical Congress: Sydney, Australia, 1929; p. 160. [Google Scholar]
- Tjong, F.V.Y.; Reddy, V.Y. Permanent leadless cardiac pacemaker therapy. Circulation 2017, 135, 1458–1470. [Google Scholar] [CrossRef]
- Aquilina, O. A brief history of cardiac pacing. Images Paediatr. Cardiol. 2006, 27, 17–81. [Google Scholar]
- Furman, S.; Szarka, G.; Layvand, D. Reconstruction of Hyman’s second pacemaker. Pacing Clin. Electrophysiol. 2005, 28, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.F. Physical Layer Security for in-Body Wireless Cardiac Sensor Network. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, May 2020. [Google Scholar]
- Dong, L.; Closson, A.B.; Jin, C.; Nie, Y.; Cabe, A.; Escobedo, D.; Huang, S.; Trase, I.; Xu, Z.; Chen, Z.; et al. Multifunctional pacemaker lead for cardiac energy harvesting and pressure sensing. Adv. Healthc. Mater. 2020, 9, 2000053. [Google Scholar] [CrossRef] [PubMed]
- Fishler, M.G.; Paspa, P. Pacemaker Systems and Methods Using Multiple Sensors for Rate Response Pacing. US Patent No. US20200054880A1, 20 February 2020. [Google Scholar]
- Svirsky, M. Cochlear implants and electronic hearing. Phys. Today 2017, 70, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Lawand, N. Micromachining Technologies for Future Cochlear Implants. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2015. [Google Scholar]
- Yüksel, M.B.; İlik, B.; Koyuncuoğlu, A.; Külah, H. Multi-channel thin film piezoelectric acoustic transducer for cochlear implant applications. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Dieter, A.; Keppeler, D.; Moser, T. Towards the optical cochlear implant: Optogenetic approaches for hearing restoration. EMBO Mol. Med. 2020, 12, e11618. [Google Scholar] [CrossRef] [PubMed]
- Sawigun, C.; Ngamkham, W.; Serdijn, W.A. An ultra low-power peak-instant detector for a peak picking cochlear implant processor. In Proceedings of the 2010 Biomedical Circuits and Systems Conference (BioCAS), Paphos, Cyprus, 3–5 November 2010; pp. 222–225. [Google Scholar] [CrossRef]
- Learning Retinal Implant System. Available online: https://www.medgadget.com/2006/01/learning_retina.html (accessed on 3 March 2019).
- Laube, G.R.T.; Brockmann, C.; Kirschkamp, T.; Mazinani, B.; Goertz, M.; Koch, C.; Krisch, I.; Sellhaus, B.; Trieu, H.K.; Weis, J.; et al. Implantation and explanation of a wireless epiretinal retina implant device: Observations during the EPIRET3 prospective clinical trial. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3003–3008. [Google Scholar]
- Bloch, E.; Luo, Y.; de Cruz, L. Advances in retinal prosthesis systems. Ther. Adv. Ophthalmol. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.J.; Kameneva, T.; Grayden, D.B.; Meffin, H.; Burkitt, A.N. Global activity shaping strategies for a retinal implant. J. Neural Eng. 2019. [Google Scholar] [CrossRef]
- Lohmann, T.B.; Haiss, F.; Schaffrath, K.; Schnitzler, A.-C.; Waschkowski, F.; Barz, C.; van der Meer, A.-M.; Werner, C.; Johnen, S.; Laube, T.; et al. The very large electrode array for retinalstimulation (VLARS)—A concept study. J. Neural Eng. 2019. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Zhao, S.; Yang, H.; Zhang, Y.; Wu, T. Micro/nano technologies for high-density retinal implant. Micromachines 2019, 10, 419. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.M.; Ackland, H.M.; Lowery, A.J.; Rosenfeld, J.V. Restoration of vision in blind individuals using bionic devices: A review with a focus on cortical visual prostheses. Brain Res. 2015, 1595, 51–73. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, T.; Takahashi, M.; Uejima, K.; Masuda, K.; Kawamura, T.; Ohta, Y.; Motoyama, M.; Noda, T.; Sasagawa, K.; Okitsu, T.; et al. CMOS image sensor-based implantable glucose sensor using glucose-responsive fluorescent hydrogel. Biomed. Opt. Express 2014, 5, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.J.; Knuth, M.; Nikolaus, K.S.; Herbrechtsmeier, P. First clinical evaluation of a new long-term subconjunctival glucose sensor. J. Diabetes Sci. Technol. 2012, 6, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.J.; Knuth, M.; Nikolaus, K.S.; Krivánek, R.; Küster, F.; Hasslacher, C.; Auffarth, G.U. Blood glucose self-monitoring with a long-term subconjunctival glucose sensor. J. Diabetes Sci. Technol. 2013, 7, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, S.-Y.; Lee, J.H. Human-urine diabetes assay and in vivo rat bladder assay using a fluorine-doped carbon nanotube catheter sensor. Ann. Biomed. Eng. 2009, 37, 2028–2033. [Google Scholar] [CrossRef]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing a biopolymer coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jeon, H.-J.; Park, S.; Yun, D.; Chung, E. Tear Glucose Measurement by Reflectance Spectrum of a Nanoparticle Embedded Contact Lens. Sci. Rep. 2020, 10, 8254. [Google Scholar] [CrossRef]
- Medtronic’s Deep Brain Stimulators with Recording Function Implanted in First U.S. Patients. Available online: https://www.medgadget.com/2013/12/medtronics-deep-brain-stimulators-with-recording-function-implanted-in-first-u-s-patients.html?utm_source=feedburner&utm_medium=email&utm_campaign=Feed:+Medgadget+ (accessed on 30 June 2020).
- Schwerdt, H.N.; Zhang, E.; Kim, M.-J.; Yoshida, T.; Stanwicks, L.; Amemori, S.; Dagdeviren, H.E.; Langer, R.; Cima, M.J.; Graybiel, A.M. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun. Biol. 2018, 1, 144. [Google Scholar] [CrossRef] [Green Version]
- Pezaris, J.S.; Eskandar, E.N. Getting signals into the brain: Visual prosthetics through thalamic microstimulation. Neurosurg. Focus 2009. [Google Scholar] [CrossRef]
- Liu, Y.; Urso, A.; da Ponte, R.M.; Costa, T.; Valente, V.; Giagka, V.; Serdijn, W.A.; Condtandinou, T.G.; Dennison, T. Bidirectional bioelectronic interfaces: System design and circuit implications. IEEE Solid-State Circuits Mag. 2020. [Google Scholar] [CrossRef]
- Thomas, L.J.; Bessman, S.P. Micropump Powered by Piezoelectric Disk Benders. U.S. Patent 3,963,380, 15 June 1975. [Google Scholar]
- Woias, P. Micropumps—Past progress and future prospects. Sens. Actuators B 2005, 105, 28–38. [Google Scholar] [CrossRef]
- Abhari, F.; Jaafar, H.; Yunus, N.A.M. A Comprehensive Study of Micropumps Technologies. Int. J. Electrochem. Sci. 2010, 7, 9765–9780. [Google Scholar]
- Johnson, D.G.; Borkholder, D.A. Towards an implantable, low flow micropump that uses no power in the blocked-flow state. Micromachines 2016, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saggere, L. Membrane actuation for micropumps. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008; p. 1079. [Google Scholar] [CrossRef]
- Humayun, M.; Santos, A.; Altamirano, J.C.; Ribeiro, R.; Gonzalez, R.; de la Rosa, A.; Shih, J.; Pang, C.; Jiang, F.; Calvillo, P.; et al. Implantable micropump for drug delivery in patients with diabetic macular edema. Transl. Vis. Sci. Technol. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Gensler, H.; Sheybani, R.; Li, P.-T.; Mann, R.L.; Meng, E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices 2012, 14, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Park, J. Ultra-thin magnetic micropump for on-demand drug delivery. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 27–31 January 2019; pp. 445–447. [Google Scholar] [CrossRef]
- Yan, B.; An, D.; Wang, X.; DeLong, B.J.; Kiourti, A.; Dungan, K.; Volakis, J.L.; Ma, M.; Guo, L. Battery-free implantable insulin micropump operating at transcutaneously radio frequency-transmittable power. Med. Devices Sens. 2019, 2. [Google Scholar] [CrossRef]
- Iacovacci, V.; Tamadon, I.; Rocchi, M.; Dario, P.; Menciassi, A. Toward dosing precision and insulin stability in an artificial pancreas system. J. Med. Devices 2019, 13, MED-18-1176. [Google Scholar] [CrossRef]
- SMART IMPLANT—Sensor Capsule for Medical In Vivo Biosensors. Available online: https://www.express-ca.eu/public/ecosystem-knowledge-gateway/ssi-showcases/mnbs-smartimplant (accessed on 20 June 2019).
- Li, P.; Bashirullah, R. A wireless power interface for rechargeable battery operated medical implants. IEEE Trans. Circuits Syst. II Express Briefs 2007. [Google Scholar] [CrossRef] [Green Version]
- Reilly, E.K.; Carleton, E.; Wright, P.K. Thin Film Piezoelectric Energy Scavenging Systems for Long Term Medical Monitoring. In Proceeding of the International Workshop on Wearable and Implantable Body Sensor Networks (BSN’06), Cambridge, MA, USA, 3–5 April 2006; pp. 4–41. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for waste water treatment and bioenergy. Biotecchnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Rapoport, B.I.; Kedzierski, J.T.; Sarpeshkar, R. A glucose fuel cell for implantable brain–machine interfaces. PLoS ONE 2012, 7, e38436. [Google Scholar] [CrossRef] [Green Version]
- Parkash, A. Microbial fuel cells: A source of bioenergy. J. Microb. Biochem. Technol. 2016, 8, 247–255. [Google Scholar] [CrossRef]
- Islam, M.N.; Yuce, M.R. Review of Medical Implant Communication System (MICS) band and network. ICT Express 2016, 2, 188–194. [Google Scholar] [CrossRef] [Green Version]




















© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
French, P. In-Vivo Microsystems: A Review. Sensors 2020, 20, 4953. https://doi.org/10.3390/s20174953
French P. In-Vivo Microsystems: A Review. Sensors. 2020; 20(17):4953. https://doi.org/10.3390/s20174953
Chicago/Turabian StyleFrench, Paddy. 2020. "In-Vivo Microsystems: A Review" Sensors 20, no. 17: 4953. https://doi.org/10.3390/s20174953



