Benefits of Home-Based Solutions for Diagnosis and Treatment of Acute Coronary Syndromes on Health Care Costs: A Systematic Review
Abstract
:1. Introduction
2. Materials & Methods
3. Diagnosing AMI
3.1. Diagnosis and Prognosis of Cut-Off Values in Troponin-Based Assays
3.1.1. Chronic Kidney Disease
3.1.2. Diabetes
3.1.3. Elderly
3.1.4. Heart Failure
3.2. Cost-Effectiveness Analysis of Troponin-Based Strategies
3.2.1. Sensitive vs. High-Sensitive Assays
3.2.2. Point of Care vs. Standard Care
4. Clinical Evidence of Home-Based or Telemonitoring Solutions in High-Risk Patients
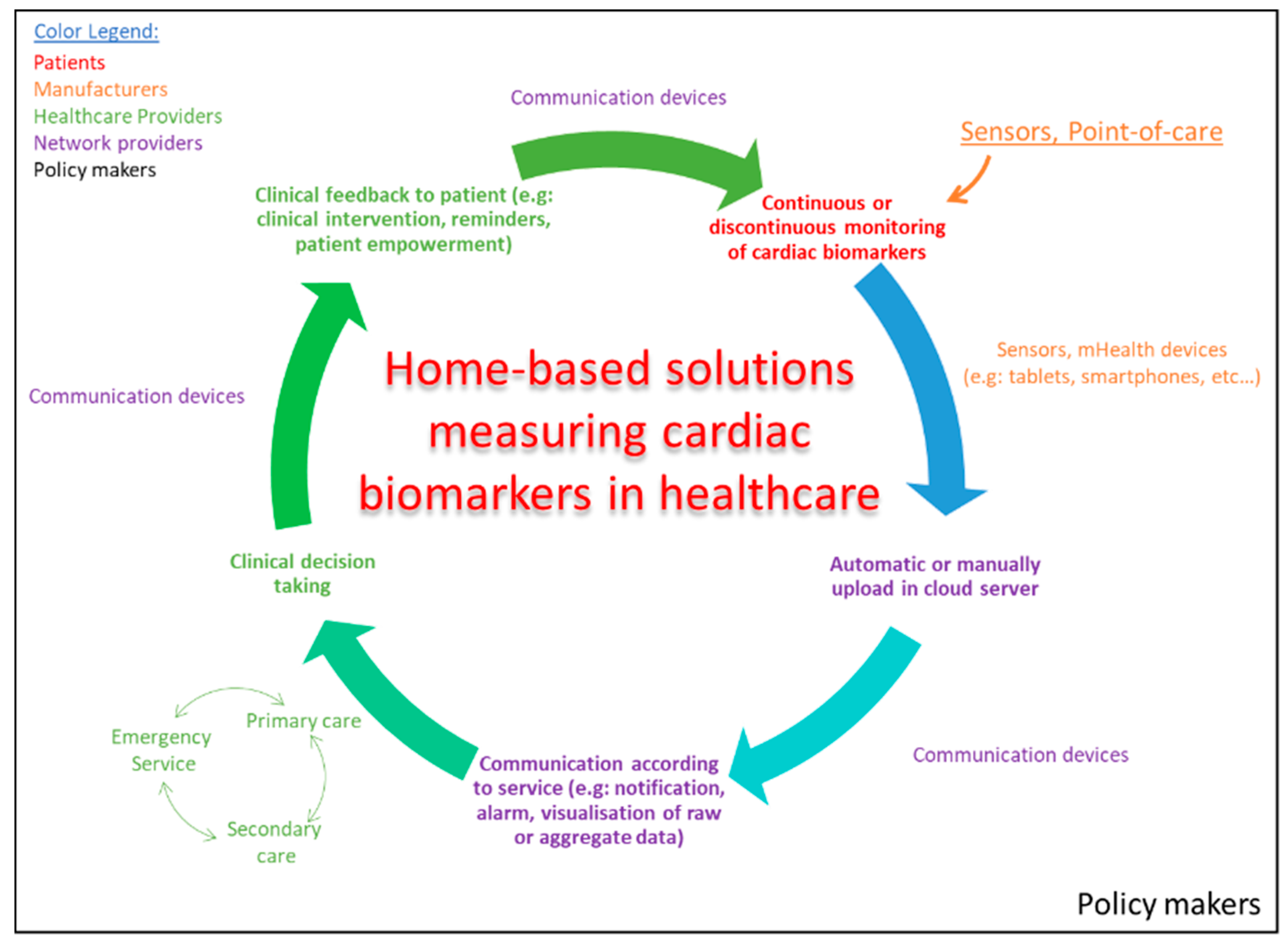
5. Fostering mHealth and eHealth Solutions
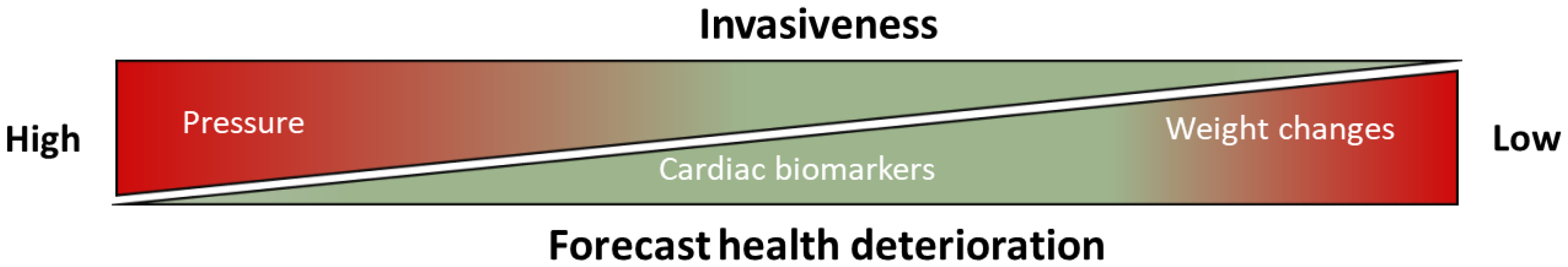
6. mHealth and eHealth Solutions for Detection of Biomarkers in Future Healthcare Systems
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 April 2020).
- Lázár, E.; Sadek, H.A.; Bergmann, O. Cardiomyocyte Renewal in the Human Heart: Insights from the Fall-Out. Eur. Heart J. 2017, 38, 2333–2342. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- World Health Organization. Working Group on the Establishement of Ischemic Heart Disease Registers; Report of the Fifth Working Group; Report No. Eur 8201 (5); World Health Organization: Copenhagen, Denmark, 1971. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal Definition of Myocardial Infarction. Circulation 2007, 116, 2634–2653. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Thygesen, K.; Alpert, J.S.; White, H.D.; Jaffe, A.S.; et al. Third Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twerenbold, R.; Rubini Gimenez, M.; Nestelberger, T.; Boeddinghaus, J.; Wildi, K.; Mueller, C. Optimising the Early Rule-out and Rule-in of Myocardial Infarction Using Biomarkers. Cardiovasc. Med. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Taylor, M.J.; Scuffham, P.A.; McCollam, P.L.; Newby, D.E. Acute Coronary Syndromes in Europe: 1-Year Costs and Outcomes. Curr. Med. Res. Opin. 2007, 23, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.C.; Peterson, E.D.; Chen, A.Y.; Thomas, L.; Doll, J.A.; Fordyce, C.B.; Newby, L.K.; Amsterdam, E.A.; Kosiborod, M.N.; de Lemos, J.A.; et al. Intensive Care Unit Utilization and Mortality Among Medicare Patients Hospitalized With Non-ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2017, 2, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Wasfy, J.H.; Strom, J.B.; Waldo, S.W.; O’Brien, C.; Wimmer, N.J.; Zai, A.H.; Luttrell, J.; Spertus, J.A.; Kennedy, K.F.; Normand, S.-L.T.; et al. Clinical Preventability of 30-Day Readmission after Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2014, 3, e001290. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijnen, B.; Van Mastrigt, G.; Redekop, W.; Majoie, H.; De Kinderen, R.; Evers, S. How to Prepare a Systematic Review of Economic Evaluations for Informing Evidence-Based Healthcare Decisions: Data Extraction, Risk of Bias, and Transferability (Part 3/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 723–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwers, M.; Kho, M.; Browman, G.; Burgers, J.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.; Grimshaw, J.; Hanna, S.; et al. AGREE II: Advancing Guideline Development, Reporting and Evaluation in Healthcare. Can. Med. Assoc. J. 2010, 182, E89–E842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, D.; Neumann, J.T.; Sörensen, N.A.; Blankenberg, S. High-Sensitivity Assays for Troponin in Patients with Cardiac Disease. Nat. Rev. Cardiol. 2017, 14, 472–483. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Kristin Newby, L. High-Sensitivity Troponin Assays: Evidence, Indications, and Reasonable Use. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Michos, E.D.; Berger, Z.; Yeh, H.-C.; Suarez-Cuervo, C.; Wilson, L.M.; Stacy, S.; Bass, E.B. Cardiac Troponins Used as Diagnostic and Prognostic Tests in Patients With Kidney Disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK241525/ (accessed on 2 September 2020).
- Stacy, S.R.; Suarez-Cuervo, C.; Berger, Z.; Wilson, L.M.; Yeh, H.-C.; Bass, E.B.; Michos, E.D. Role of Troponin in Patients With Chronic Kidney Disease and Suspected Acute Coronary Syndrome: A Systematic Review. Ann. Intern. Med. 2014, 161, 502. [Google Scholar] [CrossRef] [Green Version]
- Skadberg, Ø.; Sandberg, S.; Røraas, T.; Petersen, P.H.; Sellevoll, H.; Svarstad, E.; Sæle, K.; Aakre, K.M. The Variation in High Sensitive Cardiac Troponin Concentration during Haemodialysis Treatment Is Not Similar to the Biological Variation Observed in Stable End Stage Renal Disease Patients. Scand. J. Clin. Lab. Investig. 2016, 76, 645–652. [Google Scholar] [CrossRef]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Gimenez, M.R.; Reiter, M.; Reichlin, T.; Walukiewicz, A.; Gugala, M.; Krivoshei, L.; Marti, N.; et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation 2015, 131, 2041–2050. [Google Scholar] [CrossRef]
- Whelton, S.P.; McEvoy, J.W.; Lazo, M.; Coresh, J.; Ballantyne, C.M.; Selvin, E. High-Sensitivity Cardiac Troponin T (Hs-CTnT) as a Predictor of Incident Diabetes in the Atherosclerosis Risk in Communities Study. Diabetes Care 2017, 40, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Ferdinand, K.C.; Igari, M. Defining Cardiovascular Risk in Diabetes: The Emerging Role of Troponin Assays. J. Lab. Precis. Med. 2017, 2. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Katus, H.; Plebani, M.; Venge, P.; Collinson, P.; Lindahl, B.; Giannitsis, E.; Hasin, Y.; Galvani, M.; et al. Recommendations for the Use of Cardiac Troponin Measurement in Acute Cardiac Care. Eur. Heart J. 2010, 31, 2197–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac Troponins: From Myocardial Infarction to Chronic Disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, S.M.; Prud’Homme, P.; Ghachem, A.; Lepage, S.; Nguyen, M.; Fulop, T.; Khalil, A. Increased Level of High-Sensitivity Cardiac Troponin T in a Geriatric Population Is Determined by Comorbidities Compared to Age. Int. J. Cardiol. Heart Vasc. 2019, 22, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Twerenbold, R.; Reichlin, T.; Haaf, P.; Peter, F.; Meissner, J.; Hochholzer, W.; Stelzig, C.; Freese, M.; Heinisch, C.; et al. Early Diagnosis of Acute Myocardial Infarction in the Elderly Using More Sensitive Cardiac Troponin Assays. Eur. Heart J. 2011, 32, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichise, T.; Tada, H.; Sakata, K.; Kawashiri, M.; Yamagishi, M.; Hayashi, K. Impact of Aging on High-Sensitivity Cardiac Troponin T in Patients Suspected of Acute Myocardial Infarction. Intern. Med. 2017, 56, 2097–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.-J.; Wang, Q.; Cui, Y.-J.; Wu, W.; Zhao, Q.-H.; Xu, Y.; Wang, J.-P. High-Sensitivity Cardiac Troponin T in Geriatric Inpatients. Arch. Gerontol. Geriatr. 2016, 65, 111–115. [Google Scholar] [CrossRef]
- Gore, M.O.; Seliger, S.L.; deFilippi, C.R.; Nambi, V.; Christenson, R.H.; Hashim, I.A.; Hoogeveen, R.C.; Ayers, C.R.; Sun, W.; McGuire, D.K.; et al. Age and Sex Dependent Upper Reference Limits for the High Sensitivity Cardiac Troponin T Assay. J. Am. Coll. Cardiol. 2014, 63, 1441–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rains, M.G.; Laney, C.A.; Bailey, A.L.; Campbell, C.L. Biomarkers of Acute Myocardial Infarction in the Elderly: Troponin and Beyond. Clin. Interv. Aging 2014, 9, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjola, V.-P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.-P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ Dysfunction, Injury and Failure in Acute Heart Failure: From Pathophysiology to Diagnosis and Management. A Review on Behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC): Organ Dysfunction and Failure in AHF. Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N.; Maisel, A. Role of Cardiac Troponin Levels in Acute Heart Failure. Card. Fail. Rev. 2015, 1, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): Opportunities to Improve Care of Patients Hospitalized with Acute Decompensated Heart Failure. Rev. Cardiovasc. Med. 2003, 4 (Suppl. 7), S21–S30. [Google Scholar] [PubMed]
- You, J.J.; Austin, P.C.; Alter, D.A.; Ko, D.T.; Tu, J.V. Relation between Cardiac Troponin I and Mortality in Acute Decompensated Heart Failure. Am. Heart J. 2007, 153, 462–470. [Google Scholar] [CrossRef]
- Parenti, N.; Bartolacci, S.; Carle, F.; Angelo, F. Cardiac Troponin I as Prognostic Marker in Heart Failure Patients Discharged from Emergency Department. Intern. Emerg. Med. 2008, 3, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, L.L.; Mezzena, G.; Zanolla, L.; Paccanaro, M.; Varotto, L.; Bonanno, C.; Ometto, R. Cardiac Troponin I as Diagnostic and Prognostic Marker in Severe Heart Failure. J. Heart Lung Transplant. 2000, 19, 644–652. [Google Scholar] [CrossRef]
- Del Carlo, C.H.; Pereira-Barretto, A.C.; Cassaro-Strunz, C.M.; Latorre, M.D.R.D.D.O.; de Oliveira Junior, M.T.; Ramires, J.A.F. Cardiac Troponin T for Risk Stratification in Decompensated Chronic Heart Failure. Arq. Bras. Cardiol. 2009, 92, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Perna, E.R.; Macín, S.M.; Cimbaro Canella, J.P.; Alvarenga, P.M.; Ríos, N.G.; Pantich, R.; Augier, N.; Farías, E.F.; Jantus, E.; Brizuela, M.; et al. Minor Myocardial Damage Detected by Troponin T Is a Powerful Predictor of Long-Term Prognosis in Patients with Acute Decompensated Heart Failure. Int. J. Cardiol. 2005, 99, 253–261. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Fiuzat, M.; Lombardi, C.; Fujita, K.; Jia, G.; Davison, B.A.; Cleland, J.; Bloomfield, D.; Dittrich, H.C.; Delucca, P.; et al. Impact of Serial Troponin Release on Outcomes in Patients with Acute Heart Failure: Analysis from the PROTECT Pilot Study. Circ. Heart Fail. 2011, 4, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Westwood, M.; Van Asselt, T.; Ramaekers, B.; Whiting, P.; Thokala, P.; Joore, M.; Armstrong, N.; Ross, J.; Severens, J.; Kleijnen, J. Chapter 4, Assessment of Cost-Effectiveness. In High-Sensitivity Troponin Assays for the Early Rule-out or Diagnosis of Acute Myocardial Infarction in People with Acute Chest Pain: A Systematic Review and Cost-Effectiveness Analysis; Health Technology Assessment No. 19.44; NIHR Journals Library: Southampton, UK, 2015. [Google Scholar]
- Kip, M.M.A.; Koffijberg, H.; Moesker, M.J.; IJzerman, M.J.; Kusters, R. The Cost-Utility of Point-of-Care Troponin Testing to Diagnose Acute Coronary Syndrome in Primary Care. BMC Cardiovasc. Disord. 2017, 17, 213. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Economic Evidence and Point-of-Care Testing. Clin. Biochem. Rev. 2013, 34, 61–74. [Google Scholar] [PubMed]
- Thokala, P.; Goodacre, S.W.; Collinson, P.O.; Stevens, J.W.; Mills, N.L.; Newby, D.E.; Morris, F.; Kendall, J.; Stevenson, M.D. Cost-Effectiveness of Presentation versus Delayed Troponin Testing for Acute Myocardial Infarction. Heart 2012, 98, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Collinson, P.O.; Gaze, D.C.; Thokala, P.; Goodacre, S. Randomised Assessment of Treatment Using Panel Assay of Cardiac Markers--Contemporary Biomarker Evaluation (RATPAC CBE). Health Technol. Assess. 2013, 17, 1–122. [Google Scholar] [CrossRef]
- Jülicher, P.; Greenslade, J.H.; Parsonage, W.A.; Cullen, L. The Organisational Value of Diagnostic Strategies Using High-Sensitivity Troponin for Patients with Possible Acute Coronary Syndromes: A Trial-Based Cost-Effectiveness Analysis. BMJ Open 2017, 7, e013653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodacre, S.; Bradburn, M.; Fitzgerald, P.; Cross, E.; Collinson, P.; Gray, A.; Hall, A.S. The RATPAC (Randomised Assessment of Treatment Using Panel Assay of Cardiac Markers) Trial: A Randomised Controlled Trial of Point-of-Care Cardiac Markers in the Emergency Department. Health Technol. Assess. 2011, 15, 1–102. [Google Scholar] [CrossRef]
- Goodacre, S.; Thokala, P.; Carroll, C.; Stevens, J.W.; Leaviss, J.; Al Khalaf, M.; Collinson, P.; Morris, F.; Evans, P.; Wang, J. Systematic Review, Meta-Analysis and Economic Modelling of Diagnostic Strategies for Suspected Acute Coronary Syndrome. Health Technol. Assess. 2013, 17, 1–188. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Goodacre, S.W.; Cross, E.; Dixon, S. Cost-Effectiveness of Point-Of-Care Biomarker Assessment for Suspected Myocardial Infarction: The Randomized Assessment of Treatment Using Panel Assay of Cardiac Markers (RATPAC) Trial. Acad. Emerg. Med. 2011, 18, 488–495. [Google Scholar] [CrossRef]
- Vaidya, A.; Severens, J.; Bongaerts, B.; Cleutjens, K.; Nelemans, P.; Hofstra, L. Use of High-Sensitive Troponin T Assay for the Early Diagnosis of Acute Myocardial Infarction in Chest Pain Patients: An Economic Evaluation. Med. Decis. Mak. 2012, 32, E84. [Google Scholar]
- Shojania, K.G.; Jennings, A.; Mayhew, A.; Ramsay, C.; Eccles, M.; Grimshaw, J. Effect of Point-of-Care Computer Reminders on Physician Behaviour: A Systematic Review. CMAJ 2010, 182, E216–E225. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts) Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- National Clinical Guideline Centre (UK). Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease; National Institute for Health and Clinical Excellence: Guidance; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- SIGN. 149 Risk Estimation and the Prevention of Cardiovascular Disease. Available online: https://www.sign.ac.uk/sign-149-risk-estimation-and-the-prevention-of-cardiovascular-disease (accessed on 7 April 2020).
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.-R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; European Association for Cardiovascular Prevention & Rehabilitation; et al. ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.; Sculpher, M.; Philips, Z.; Robinson, M.; Ginnelly, L.; Bakhai, A.; Abrams, K.; Cooper, N.; Packham, C.; Alfakih, K.; et al. Management of Non-ST-Elevation Acute Coronary Syndromes: How Cost-Effective Are Glycoprotein IIb/IIIA Antagonists in the UK National Health Service? Int. J. Cardiol. 2005, 100, 229–240. [Google Scholar] [CrossRef]
- Bosanquet, N.; Jönsson, B.; Fox, K.A.A. Costs and Cost Effectiveness of Low Molecular Weight Heparins and Platelet Glycoprotein IIb/IIIa Inhibitors: In the Management of Acute Coronary Syndromes. Pharmacoeconomics 2003, 21, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Korzeniowska-Kubacka, I.; Chrapowicka, A.; Wolszakiewicz, J.; Dobraszkiewicz-Wasilewska, B.; Batogowski, M.; Piotrowski, W.; Piotrowicz, R. Feasibility of Home-Based Cardiac Telerehabilitation: Results of TeleInterMed Study. Cardiol. J. 2014, 21, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Telehealth for Acute and Chronic Care Consultations—Abstract—Europe PMC. Available online: https://europepmc.org/article/med/31577401 (accessed on 28 January 2020).
- Ware, P.; Ross, H.J.; Cafazzo, J.A.; Boodoo, C.; Munnery, M.; Seto, E. Outcomes of a Heart Failure Telemonitoring Program Implemented as the Standard of Care in an Outpatient Heart Function Clinic: Pretest-Posttest Pragmatic Study. J. Med. Internet Res. 2020, 22, e16538. [Google Scholar] [CrossRef]
- Widmer, R.J.; Collins, N.M.; Collins, C.S.; West, C.P.; Lerman, L.O.; Lerman, A. Digital Health Interventions for the Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2015, 90, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Möckel, M.; Koehler, K.; Anker, S.D.; Vollert, J.; Moeller, V.; Koehler, M.; Gehrig, S.; Wiemer, J.C.; von Haehling, S.; Koehler, F. Biomarker Guidance Allows a More Personalized Allocation of Patients for Remote Patient Management in Heart Failure: Results from the TIM-HF2 Trial. Eur. J. Heart Fail. 2019, 21, 1445–1458. [Google Scholar] [CrossRef]
- Givertz, M.M.; Stevenson, L.W.; Costanzo, M.R.; Bourge, R.C.; Bauman, J.G.; Ginn, G.; Abraham, W.T.; Investigators on behalf of the Champion Trial. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 1875–1886. [Google Scholar] [CrossRef]
- Herman, D.S.; Kavsak, P.A.; Greene, D.N. Variability and Error in Cardiac Troponin Testing. Am. J. Clin. Pathol. 2017, 148, 281–295. [Google Scholar] [CrossRef] [Green Version]
- Adel Serhani, M.; El Kassabi, H.T.; Ismail, H.; Nujum Navaz, A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef] [Green Version]
- Amorim, V.J.P.; Silva, M.C.; Oliveira, R.A.R. Software and Hardware Requirements and Trade-Offs in Operating Systems for Wearables: A Tool to Improve Devices’ Performance. Sensors 2020, 19, 1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial Intelligence and Machine Learning in Clinical Development: A Translational Perspective. NPJ Digit. Med. 2019, 2, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.; Silva Cunha, J.P. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Mondal, T.; Jamal Deen, M. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Zheng, Y.-L.; Lin, W.-H.; Zhang, H.-Y.; Zhou, X.-L. Challenges and Opportunities in Cardiovascular Health Informatics. IEEE Trans. Biomed. Eng. 2013, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Abdorahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Alavi, S.H.; Tayebi, L. Nanomaterials-Based Electrochemical Immunosensors for Cardiac Troponin Recognition: An Illustrated Review. TrAC Trends Anal. Chem. 2016, 82, 337–347. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Electrochemical Biosensors for the Determination of Cardiovascular Markers: A Review. Electroanalysis 2014, 26, 1132–1153. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, Carbon Nanotubes, Zinc Oxide and Gold as Elite Nanomaterials for Fabrication of Biosensors for Healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Upasham, S.; Tanak, A.; Prasad, S. Cardiac Troponin Biosensors: Where Are We Now? Adv. Health Care Technol. 2018, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Diffusion of Ehealth: Making Universal Health Coverage Achievable; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Newsroom. Ehealth Action Plan 2012–2020: Innovative Healthcare for the 21st Century. Available online: https://ec.europa.eu/digital-single-market/en/news/ehealth-action-plan-2012-2020-innovative-healthcare-21st-century (accessed on 7 April 2020).
- REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL—Of 27 April 2016—On the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation). 88. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 31 January 2020).
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. 21. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011L0024 (accessed on 31 January 2020).
- REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL—Of 5 April 2017—On Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 175. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 31 January 2020).
- Frangogiannis, N.G. Biomarkers: Hopes and challenges in the path from discovery to clinical practice. Transl. Res. 2012, 159, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]



| Patient Cohort. | Studies | Key Characteristics | Major Outcomes |
|---|---|---|---|
| Chronic Kidney Disease (CKD) | Michos E.D. et al. [19] | Examined 124 studies to evaluate diagnosis and prognosis of troponins. | Elevated cTnI or cTnT are potent predictors of mortality in CKD patients with and without suspicion of ACS and independent of the condition whether the patient is receiving dialysis or not. |
| Stacy S.R. et al. [20] | Evaluated 23 trials where troponin levels were measured in CKD cohorts. | Identify optimal cut-off points of troponins for patients with CKD and ACS for proper risk stratification. | |
| Skadberg O. et al. [21] | Serum samples were collected from 20 patients before and after 10 consecutive HD treatments using hs-cTnT. | Need to determine specific cut-off points, before and after hemodialysis, when using hs-Tn assays. | |
| Twerenbold R. et al. [22] | Multicenter study with 2813 patients with 16% prevalence of renal dysfunction. | Hs-Tn assays showed slightly lower diagnosis accuracy at presentation in CKD patients versus patients without renal dysfunction, no systematic superiority was found between hs-Tn and s-Tn assays and hs-Tn loses specificity when comparing CKD with normal renal function patients. | |
| Diabetes (T2DM) | Whelton S.P. et al. [23] | ARIC trial where 1500 patients with T2DM were recruited. | Troponin testing using hs-Tn assays could be a good biomarker for personalized medicine. Abnormal troponin levels can predict the CVD risk of T2DM patients 10 years in advance. |
| Ferdinand K.C. et al. [24] | EXAMINE trail where 3808 patients with T2DM were recruited. | Elevated troponins can help to identify patients at extreme risk for cardiac events. This is a new category identified in 2017 by the Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive T2D Management Algorithm. | |
| Thygesen K. et al. [25] | Summary of meta-analyses. | Hs-Tn have comparable diagnostic and prognostic performance as s-Tn in most clinical settings. | |
| Elderly | Sedighi S.M. et al. [27] | 6977 medical records aged ≥65 years without acute coronary events were recruited. | Troponin values in geriatric population is a consequence of non-cardiovascular comorbidities. These results confirm the conclusions of Park KC et al. [26]. |
| Reiter M. et al. [28] | 1098 consecutive patients with symptoms suggestive of AMI where 37% had more than 70 years old. | Elderly NSTEMI patients, using the uniform assay-specific 99th percentile for STEMI diagnosis, were shown to have limited diagnostic capability due to reduced specificity. | |
| Ichise T. et al. [29] | 355 consecutive patients with mean age 66 ± 16.1 years patients attending Kanazawa University Hospital. | When measuring hs-cTnT careful assessment are needed in elderly subjects. | |
| Zhang S. et al. [30] | 679 geriatric inpatients without ACS. | Hs-cTnT elevation caused by non-ischemic acute conditions was very common in geriatric hospitalized patients. Further studies are needed to establish age-specific 99th percentile values of hs-cTnT for elderly individuals. | |
| Gore M.O. et al. [31] | Data included from three well characterized population-based studies: the Dallas Heart Study (DHS), the Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS). | Use of a uniform 14 ng/L cutoff for the hs-cTnT assay may lead to overdiagnosis of myocardial infarction, particularly in men and the elderly. Clinical validation is needed of new age- and sex-specific cutoff values for this assay. | |
| Rains M.G. et al. [32] | Review paper of how different biomarkers were used to diagnose ACS in elderly population evolve. | Desirable to have algorithm specific. | |
| Heart Failure (HF) | Fonarow G.C. et al. [36] | ADHERE study where 65,180 patients were recruited. | Higher troponin levels were associated with higher in-hospital mortality. |
| You J.J et al. [37] | EFFECT study where 2000 patients hospitalized in Ontario (CANADA). | Troponin value greater than 0.5 μg/L during the first 48 h of hospitalization was a predictor of increased 1-year all-cause mortality. | |
| Parenti N. et al. [38] | 99 patients discharged from the department between March and December 2002 with a HF diagnosis and samples of cTnI. Patients with acute coronary syndromes, myocarditis or renal failure were excluded. | Elevated troponin I is associated with lower ejection fraction, higher systolic pulmonary artery pressure and increased length of hospital stay. | |
| Vechia L.L et al. [39] | Thirty-four patients were examined. Upon admission, we measured serum levels of cTnI by conventional immunoenzymatic assay. | cTnI is detected in the blood of 25% to 33% of patients with severe heart failure; its presence may help to identify a high-risk sub-group who faces very poor short-term prognosis. | |
| Del Carlo C.H. et al. [40] | 70 patients with chronic HF worsening that needed hospitalization were studied. | Abnormal troponin T is a predictor of increased risk of HF readmission and mortality. | |
| Perna E.R. et al. [41] | One hundred and eighty-four consecutive patients with ADHF were enrolled in the absence of an acute coronary syndrome. | Troponin T was an independent long-term prognostic marker of morbidity and mortality and it suggests a role of biochemical risk stratification in HF. | |
| O’Connor C.M et al. [42] | PROTECT study. | Increased troponin levels in serial measurements were good predictors of rehospitalization or death at 60 days. |
| References | Key Characteristics | Major Outcomes |
|---|---|---|
| Westwood M. et al. [43] | Review paper including 18 studies including research registers and conference proceedings up to October 2013. | High-sensitivity assays may provide an effective and cost-effective approach to early rule-out AMI patients. Further research is needed to clarify optimal diagnostic thresholds and testing strategies. |
| Kip MMA et al. [44] | Patient-level simulation model representing a hypothetical cohort of Dutch population (>35 years) consulting GP with chest complaints. | Point of care troponin strategy is likely to be cost-effective because it reduces hospital referrals in non-ACS patients. Slight increase in nonreferral among ACS patients but with negligible overall health effects. |
| St. John A. et al. [45] | Review paper on economic assessment of point-of-care testing. | Cost-effectiveness studies in point-of-care testing are limited. There is a need for better understanding of care pathways and how they will change with the introduction of point-of-care testing. |
| Thokala P. et al. [46] | Patients attending acute hospitals in UK with suspect NSTEMI or unstable angina with no major comorbidities requiring admission. | Delayed troponin testing is unlikely to be cost-effective compared with high-sensitivity troponin testing at presentation in most scenarios. |
| Collison P.O et al. [47] | Sub-study of point-of-care arm of the RATPAC trial taking place in the emergency department of six hospitals. | The measurement of high-sensitivity cardiac troponin is the best single marker in patients presenting with chest pain. Additional measurements of myoglobin or CK-MB are not clinically effective or cost-effective. The optimal timing for measurement of cardiac troponin remains to be defined. |
| Jülicher P. et al. [48] | Part of ADAPT trial in Australia-New Zealand of 938 patients who presented at an adult Emergency Department of a tertiary referral hospital with at least 5 min of symptoms suggestive of acute coronary syndrome. | High-sensitivity troponin I algorithms are likely to be cost-effective on a hospital level compared with sensitive troponin protocols. |
| Goodacre S. et al. [49] | Systematic review, meta-analysis and economic modelling of diagnostic strategies suspected of acute coronary syndrome. | Although presentation troponin has suboptimal sensitivity, measurement of a 10-h troponin level is unlikely to be cost-effective in most scenarios compared with a high-sensitivity presentation troponin. |
| Goodacre S. et al. [50] | RATPAC trial. Multicenter pragmatic open randomized controlled trial and economic evaluation of point-of-care testing used in six acute hospital emergency departments in UK. | Point-of-care testing is more expensive than standard care and unlikely to be considered cost-effective. |
| Fitzgerald P. et al. [51] | The RATPAC trial a multicenter individual patient randomized controlled trial comparing diagnostic assessment using a POC biomarker panel (CK-MB, myoglobin and troponin, measured at baseline and 90 min) to standard care without the POC panel in 2243 patients. | Point-of-care panel assessment does not reduce costs despite reducing admissions and may even increase costs. It is unlikely to be considered a cost-effective use of health care resources. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redón, P.; Shahzad, A.; Iqbal, T.; Wijns, W. Benefits of Home-Based Solutions for Diagnosis and Treatment of Acute Coronary Syndromes on Health Care Costs: A Systematic Review. Sensors 2020, 20, 5006. https://doi.org/10.3390/s20175006
Redón P, Shahzad A, Iqbal T, Wijns W. Benefits of Home-Based Solutions for Diagnosis and Treatment of Acute Coronary Syndromes on Health Care Costs: A Systematic Review. Sensors. 2020; 20(17):5006. https://doi.org/10.3390/s20175006
Chicago/Turabian StyleRedón, Pau, Atif Shahzad, Talha Iqbal, and William Wijns. 2020. "Benefits of Home-Based Solutions for Diagnosis and Treatment of Acute Coronary Syndromes on Health Care Costs: A Systematic Review" Sensors 20, no. 17: 5006. https://doi.org/10.3390/s20175006






