An Insulin Bolus Advisor for Type 1 Diabetes Using Deep Reinforcement Learning
Abstract
:1. Introduction
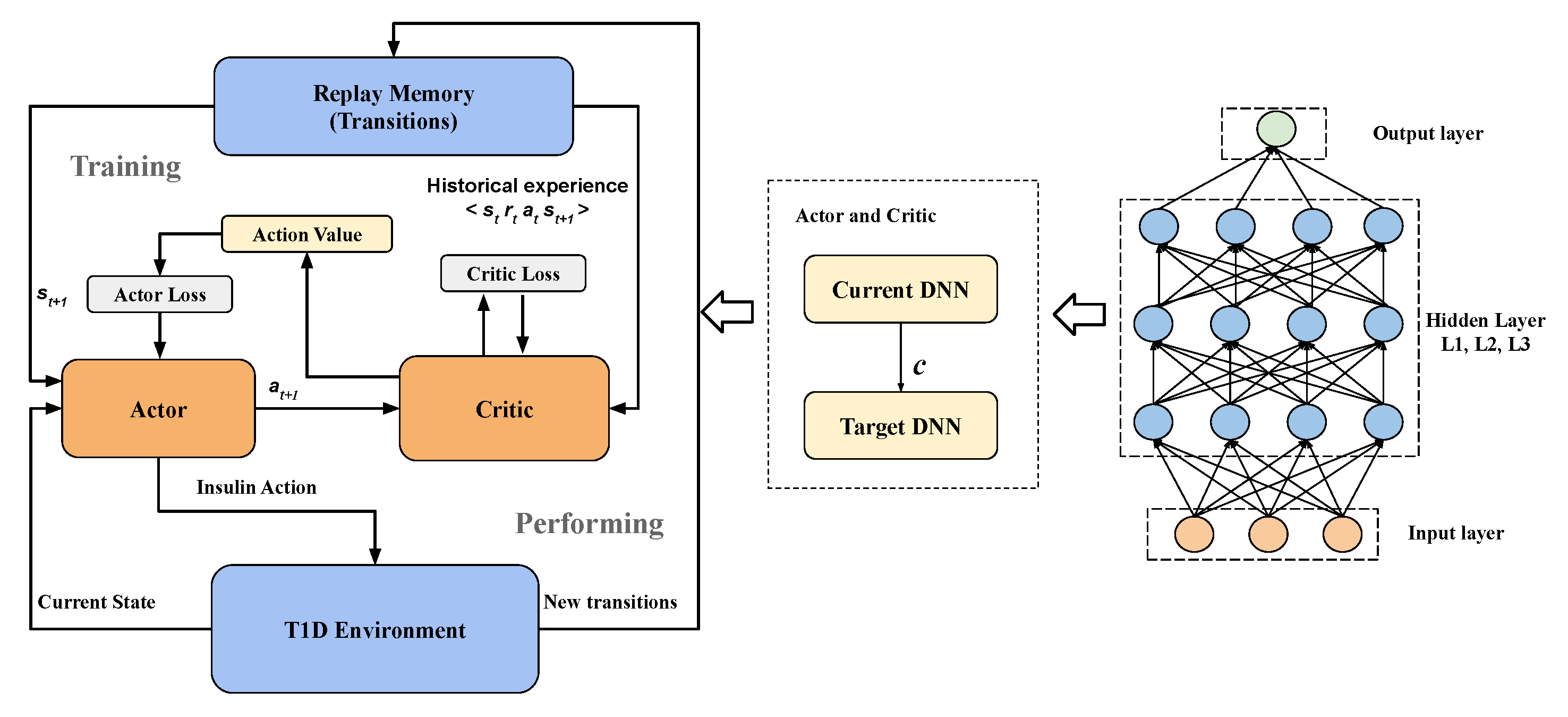
2. Methods
2.1. Problem Formulation
2.1.1. Deep Neural Networks
2.1.2. Agent States And Actions
2.1.3. Reward Function
2.2. Two-Step Learning Framework
| Algorithm 1 DDPG Insulin Bolus Advisor. |
|
2.3. System Architecture
2.4. In Silico Validation
2.5. Performance Metrics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AP | Artificial Pancreas |
| BG | Blood Glucose |
| CBR | Case-based Reasoning |
| CGM | Continuous Glucose Monitoring |
| CSII | Continuous Subcutaneous Insulin Infusion |
| CV | Coefficient of Variation |
| CVGA | Control-variability Grid Analysis |
| DDPG | Deep Deterministic Policy Gradient |
| DNN | Deep Neural Network |
| DRL | Deep Reinforcement Learning |
| HBGI | High Blood Glucose Index |
| ICR | Insulin-to-carbohydrate Ratio |
| IOB | Insulin on Board |
| ISF | Insulin Sensitivity Factor |
| KNN | K-nearest Neighbours |
| LBGI | Low Blood Glucose Index |
| MDI | Multiple Daily Injection |
| MDP | Markov decision process |
| R2R | Run-to-run |
| RL | Reinforcement Learning |
| SBC | Standard Bolus Calculator |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| TAR | Time Above Range |
| TBR | Time Below Range |
| TIR | Time In Range |
Appendix A. Hyper-Parameters
| Parameter | Value |
|---|---|
| The length of CGM measurements L | 6 |
| The hidden units of DNNs | [200, 200, 10] |
| The learning rate of the actor | 0.0001 |
| The learning rate of the critic | 0.0001 |
| The size of replay memory N | 500 |
| Batch size | 32 |
| Soft replacement | 0.01 |
| Target network update period | 100 |
| Discount factor | 0.9 |
| The degree of prioritization | 0.6 |
| Compensation factor | |
| Priority constant | 0.00001 |
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, J.C. Management of diabetes mellitus: Is the pump mightier than the pen? Nat. Rev. Endocrinol. 2012, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Diabetes: Advances in diagnosis and treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Roberts, R.; Bailey, T.S.; Heinemann, L. Bolus advisors: Sources of error, targets for improvement. J. Diabetes Sci. Technol. 2018, 12, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boughton, C.K.; Hovorka, R. Advances in artificial pancreas systems. Sci. Transl. Med. 2019, 11, eaaw4949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, C.; Zisser, H.; Jovanovic, L.; Srinivasan, B.; Bonvin, D.; Doyle, F.J. Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans. Biomed. Eng. 2006, 53, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Palerm, C.C.; Zisser, H.; Bevier, W.C.; Jovanovič, L.; Doyle, F.J. Prandial insulin dosing using run-to-run control: Application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care 2007, 30, 1131–1136. [Google Scholar] [CrossRef] [Green Version]
- Pesl, P.; Herrero, P.; Reddy, M.; Xenou, M.; Oliver, N.; Johnston, D.; Toumazou, C.; Georgiou, P. An advanced bolus calculator for type 1 diabetes: System architecture and usability results. IEEE J. Biomed. Health Inform. 2015, 20, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.; Pesl, P.; Xenou, M.; Toumazou, C.; Johnston, D.; Georgiou, P.; Herrero, P.; Oliver, N. Clinical safety and feasibility of the advanced bolus calculator for type 1 diabetes based on case-based reasoning: A 6-week nonrandomized single-arm pilot study. Diabetes Technol. Ther. 2016, 18, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Contreras, I.; Vehi, J. Artificial intelligence for diabetes management and decision support: Literature review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef]
- Tyler, N.S.; Mosquera-Lopez, C.M.; Wilson, L.M.; Dodier, R.H.; Branigan, D.L.; Gabo, V.B.; Guillot, F.H.; Hilts, W.W.; El Youssef, J.; Castle, J.R.; et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat. Metab. 2020, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Gatton, T.M.; Lee, K.K. A monitoring and advisory system for diabetes patient management using a rule-based method and KNN. Sensors 2010, 10, 3934–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, E.M.; Toffanin, C.; Messori, M.; Cobelli, C.; Magni, L. Postprandial glucose regulation via KNN meal classification in type 1 diabetes. IEEE Control Syst. Lett. 2018, 3, 230–235. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Marturano, F.; Facchinetti, A.; Sparacino, G. A neural-network-based approach to personalize insulin bolus calculation using continuous glucose monitoring. J. Diabetes Sci. Technol. 2018, 12, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jankovic, M.V.; Budzinski, J.; Moore, B.; Diem, P.; Stettler, C.; Mougiakakou, S.G. A dual mode adaptive basal-bolus advisor based on reinforcement learning. IEEE J. Biomed. Health Inf. 2018, 23, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Agianniotis, A.; Anthimopoulos, M.; Daskalaki, E.; Drapela, A.; Stettler, C.; Diem, P.; Mougiakakou, S. GoCARB in the context of an artificial pancreas. J. Diabetes Sci. Technol. 2015, 9, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Bothe, M.K.; Dickens, L.; Reichel, K.; Tellmann, A.; Ellger, B.; Westphal, M.; Faisal, A.A. The use of reinforcement learning algorithms to meet the challenges of an artificial pancreas. Expert Rev. Med. Devices 2013, 10, 661–673. [Google Scholar] [CrossRef]
- Tejedor, M.; Woldaregay, A.Z.; Godtliebsen, F. Reinforcement learning application in diabetes blood glucose control: A systematic review. Artif. Intell. Med. 2020, 104, 101836. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Park, S.W.; Jin, S.M.; Park, S.M. Toward a fully automated artificial pancreas system Using a bioinspired reinforcement learning design: In silico validation. IEEE J. Biomed. Health Inform. 2020. [Google Scholar] [CrossRef]
- Silver, D.; Schrittwieser, J.; Simonyan, K.; Antonoglou, I.; Huang, A.; Guez, A.; Hubert, T.; Baker, L.; Lai, M.; Bolton, A.; et al. Mastering the game of go without human knowledge. Nature 2017, 550, 354–359. [Google Scholar] [CrossRef]
- Sallab, A.E.; Abdou, M.; Perot, E.; Yogamani, S. Deep reinforcement learning framework for autonomous driving. Electron. Imaging 2017, 2017, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Nemati, S.; Ghassemi, M.M.; Clifford, G.D. Optimal medication dosing from suboptimal clinical examples: A deep reinforcement learning approach. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 17 August 2016; pp. 2978–2981. [Google Scholar]
- Zhu, T.; Li, K.; Herrero, P.; Chen, J.; Georgiou, P. A deep learning algorithm for personalized blood glucose prediction. In Proceedings of the 3rd International Workshop on Knowledge Discovery in Healthcare Data, IJCAI-ECAI, Stockholm, Schweden, 13 July 2018; pp. 64–78. [Google Scholar]
- Li, K.; Liu, C.; Zhu, T.; Herrero, P.; Georgiou, P. GluNet: A deep learning framework for accurate glucose forecasting. IEEE J. Biomed. Health Inf. 2019, 24, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mirshekarian, S.; Shen, H.; Bunescu, R.; Marling, C. LSTMs and neural Attention models for blood glucose prediction: Comparative experiments on real and synthetic Data. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 706–712. [Google Scholar]
- Zhu, T.; Li, K.; Herrero, P.; Chen, J.; Georgiou, P. Dilated recurrent neural networks for glucose forecasting in type 1 diabetes. J. Healthc. Inform. Res. 2020, 2020, 1–17. [Google Scholar]
- Dalla Man, C.; Micheletto, F.; Lv, D.; Breton, M.; Kovatchev, B.; Cobelli, C. The UVA/PADOVA type 1 diabetes simulator: New features. J. Diabetes Sci. Technol. 2014, 8, 26–34. [Google Scholar]
- Visentin, R.; Dalla Man, C.; Kovatchev, B.; Cobelli, C. The university of Virginia/Padova type 1 diabetes simulator matches the glucose traces of a clinical trial. Diabetes Technol. Ther. 2014, 16, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Pesl, P.; Reddy, M.; Oliver, N.; Georgiou, P.; Toumazou, C. Advanced insulin bolus advisor based on run-to-run control and case-based reasoning. IEEE J. Biomed. Health Inform. 2014, 19, 1087–1096. [Google Scholar]
- Sun, Q.; Jankovic, M.V.; Bally, L.; Mougiakakou, S.G. Predicting blood glucose with an LSTM and Bi-LSTM based deep neural network. In Proceedings of the IEEE 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21 November 2018; pp. 1–5. [Google Scholar]
- Mnih, V.; Kavukcuoglu, K.; Silver, D.; Rusu, A.A.; Veness, J.; Bellemare, M.G.; Graves, A.; Riedmiller, M.; Fidjeland, A.K.; Ostrovski, G.; et al. Human-level control through deep reinforcement learning. Nature 2015, 518, 529–533. [Google Scholar] [CrossRef]
- Silver, D.; Lever, G.; Heess, N.; Degris, T.; Wierstra, D.; Riedmiller, M. Deterministic policy gradient algorithms. In Proceedings of the 31st ICML, ICML’14, Beijing, China, 21–26 June 2014; Volume 32, pp. I-387–I-395. [Google Scholar]
- Lillicrap, T.P.; Hunt, J.J.; Pritzel, A.; Heess, N.; Erez, T.; Tassa, Y.; Silver, D.; Wierstra, D. Continuous control with deep reinforcement learning. In Proceedings of the 4th International Conference on Learning Representations, ICLR, San Juan, Puerto Rico, 2–4 May 2016; Bengio, Y., LeCun, Y., Eds.; Cornell University: New York, NY, USA, 2016. [Google Scholar]
- Zisser, H.; Robinson, L.; Bevier, W.; Dassau, E.; Ellingsen, C.; Doyle, F.J., III; Jovanovic, L. Bolus calculator: A review of four “smart” insulin pumps. Diabetes Technol. Ther. 2008, 10, 441–444. [Google Scholar] [CrossRef]
- Schmidt, S.; Nørgaard, K. Bolus calculators. J. Diabetes Sci. Technol. 2014, 8, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Bertachi, A.; Biagi, L.; Beneyto, A.; Vehí, J. Dynamic rule-based algorithm to tune insulin-on-board constraints for a hybrid artificial pancreas system. J. Healthc. Eng. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Al-Taee, A.M.; Al-Taee, M.A.; Al-Nuaimy, W.; Muhsin, Z.J.; AlZu’bi, H. Smart bolus estimation taking into account the amount of insulin on board. In Proceedings of the IEEE International Conference on Computer and Information Technology, Liverpool, UK, 26–28 October 2015; pp. 1051–1056. [Google Scholar]
- Toffanin, C.; Zisser, H.; Doyle, F.J., III; Dassau, E. Dynamic insulin on board: Incorporation of circadian insulin sensitivity variation. J. Diabetes Sci. Technol. 2013, 7, 928–940. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; Buckingham, B.A.; Castle, J.R.; Cinar, A.; Damiano, E.R.; Dassau, E.; DeVries, J.H.; Doyle, F.J.; Griffen, S.C.; Haidar, A.; et al. Outcome measures for artificial pancreas clinical trials: A consensus report. Diabetes Care 2016, 39, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yale, J.F.; Paty, B.; Senior, P.A. Hypoglycemia. Can. J. Diabetes 2018, 42, S104–S108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Avari, P.; Leal, Y.; Wos, M.; Sivasithamparam, K.; Georgiou, P.; Reddy, M.; Fernández-Real, J.M.; Martin, C.; Fernández-Balsells, M.; et al. A modular safety system for an insulin dose recommender: A feasibility study. J. Diabetes Sci. Technol. 2020, 14, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Schaul, T.; Quan, J.; Antonoglou, I.; Silver, D. Prioritized experience replay. In Proceedings of the 4th International Conference on Learning Representations, ICLR, San Juan, Puerto Rico, 2–4 May 2016; Bengio, Y., LeCun, Y., Eds.; Cornell University: New York, NY, USA, 2016. [Google Scholar]
- Vehi, J.; Isern, J.R.; Parcerisas, A.; Calm, R.; Contreras, I. Impact of use frequency of a mobile diabetes management app on blood glucose control: Evaluation study. JMIR mHealth uHealth 2019, 7, e11933. [Google Scholar] [CrossRef] [Green Version]
- Keith-Hynes, P.; Guerlain, S.; Mize, B.; Hughes-Karvetski, C.; Khan, M.; McElwee-Malloy, M.; Kovatchev, B.P. DiAs user interface: A patient-centric interface for mobile artificial pancreas systems. J. Diabetes Sci. Technol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.; Pinsker, J.E.; Zavitsanou, S.; Shi, D.; Tompot, R.; Church, M.M.; Andre, C.; Doyle, F.J., III; Dassau, E. Design and clinical evaluation of the interoperable artificial pancreas system (iAPS) smartphone app: Interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol. Ther. 2019, 21, 35–43. [Google Scholar] [CrossRef]
- Spence, R.; Li, K.; Uduku, C.; Zhu, T.; Redmond, L.; Herrero, P.; Oliver, N.; Georgiou, P. A novel hand-held interface supporting the self-management of type 1 diabetes. In Proceedings of the 13th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2020), Madrid, Spain, 19–22 February 2020; p. A58. [Google Scholar]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. Tensorflow: A system for large-scale machine learning. In Proceedings of the 12th {USENIX} Symposium on Operating Systems Design and Implementation ({OSDI} 16), Savannah, GA, USA, 2–4 November 2016; pp. 265–283. [Google Scholar]
- Li, K.; Daniels, J.; Liu, C.; Herrero-Vinas, P.; Georgiou, P. Convolutional recurrent neural networks for glucose prediction. IEEE J. Biomed. Health Inf. 2019, 24, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Herrero, P.; Pesl, P.; Bondia, J.; Reddy, M.; Oliver, N.; Georgiou, P.; Toumazou, C. Method for automatic adjustment of an insulin bolus calculator: In silico robustness evaluation under intra-day variability. Comput. Methods Progr. Biomed. 2015, 119, 1–8. [Google Scholar] [CrossRef]
- Magni, L.; Raimondo, D.M.; Dalla Man, C.; Breton, M.; Patek, S.; De Nicolao, G.; Cobelli, C.; Kovatchev, B.P. Evaluating the efficacy of closed-loop glucose regulation via control-variability grid analysis. J. Diabetes Sci. Technol. 2008, 2, 630–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Li, K.; Herrero, P.; Georgiou, P. Basal Glucose Control in Type 1 Diabetes using Deep Reinforcement Learning: An In Silico Validation. IEEE J. Biomed. Health Inform. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Lillicrap, T.; Sutskever, I.; Levine, S. Continuous deep q-learning with model-based acceleration. In Proceedings of the International Conference on Machine Learning, New York, NY, USA, 20–22 June 2016; pp. 2829–2838. [Google Scholar]
- Munos, R.; Stepleton, T.; Harutyunyan, A.; Bellemare, M. Safe and efficient off-policy reinforcement learning. In Advances in Neural Information Processing Systems; Curran Associates, Inc.: Red Hook, NY, USA, 2016; pp. 1054–1062. [Google Scholar]
- Dulac-Arnold, G.; Mankowitz, D.; Hester, T. Challenges of real-world reinforcement learning. In Proceedings of the Reinforcement Learning for Real Life (RL4RealLife) Workshop in the 36th International Conference on Machine Learning (ICML), Long Beach, CA, USA, 14 June 2019. [Google Scholar]




| Method | TIR (%) | TBR (%) | TAR (%) | Mean (mg/dL) | CV (%) | LBGI | HBGI |
|---|---|---|---|---|---|---|---|
| SBC | |||||||
| DRL |
| Method | TIR (%) | TBR (%) | TAR (%) | Mean (mg/dL) | CV (%) | LBGI | HBGI |
|---|---|---|---|---|---|---|---|
| SBC | |||||||
| DRL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Li, K.; Kuang, L.; Herrero, P.; Georgiou, P. An Insulin Bolus Advisor for Type 1 Diabetes Using Deep Reinforcement Learning. Sensors 2020, 20, 5058. https://doi.org/10.3390/s20185058
Zhu T, Li K, Kuang L, Herrero P, Georgiou P. An Insulin Bolus Advisor for Type 1 Diabetes Using Deep Reinforcement Learning. Sensors. 2020; 20(18):5058. https://doi.org/10.3390/s20185058
Chicago/Turabian StyleZhu, Taiyu, Kezhi Li, Lei Kuang, Pau Herrero, and Pantelis Georgiou. 2020. "An Insulin Bolus Advisor for Type 1 Diabetes Using Deep Reinforcement Learning" Sensors 20, no. 18: 5058. https://doi.org/10.3390/s20185058





