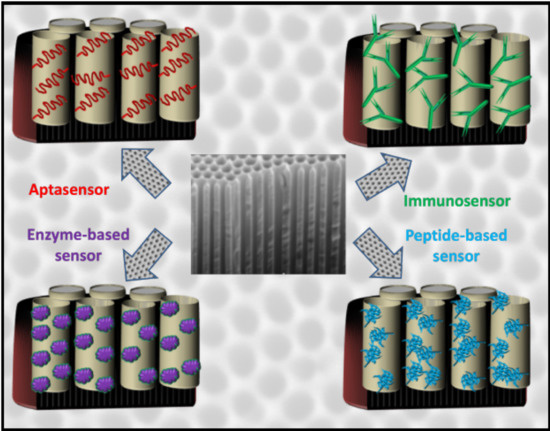
Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina
Abstract
1. Introduction
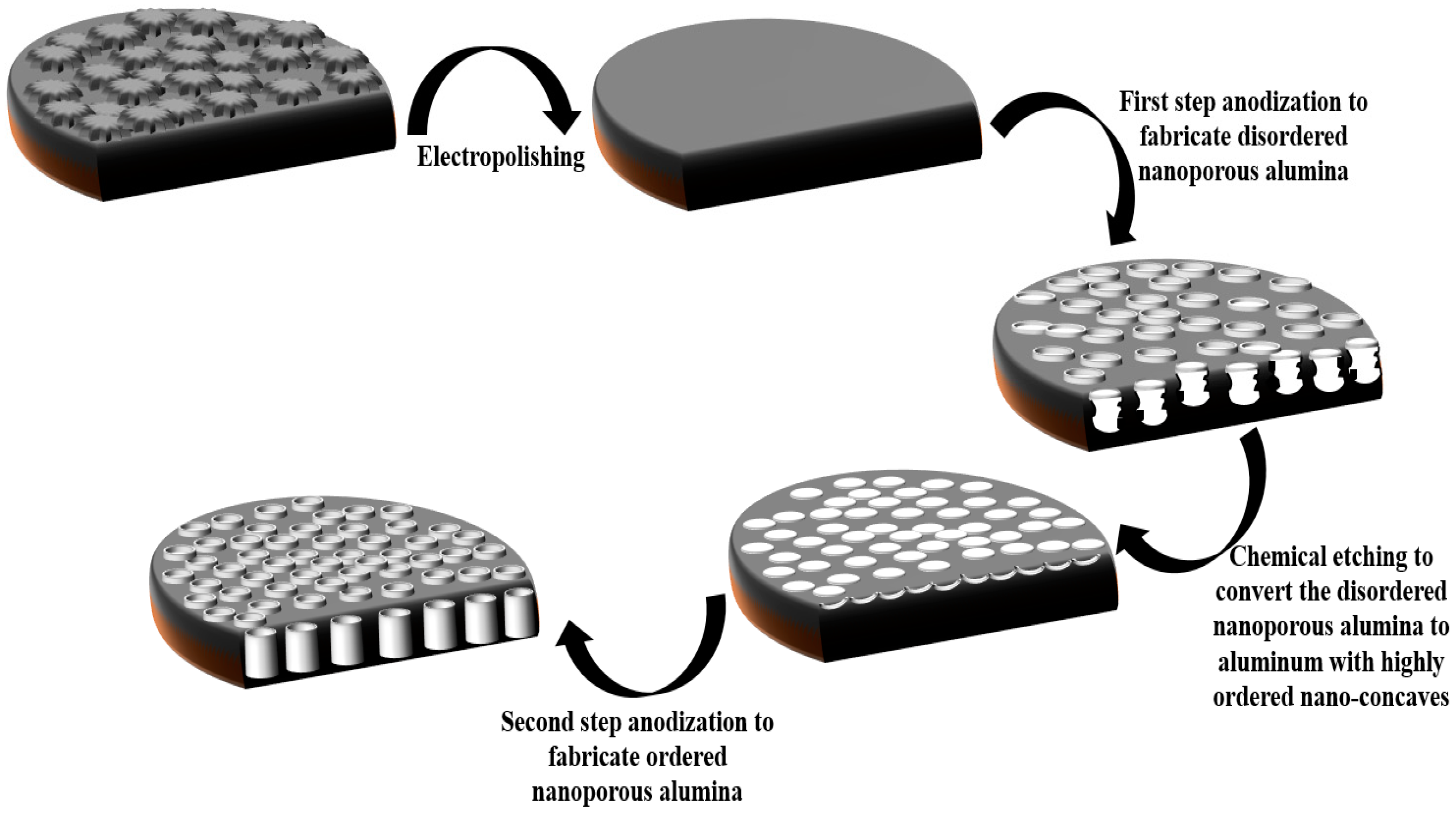
2. Fabrication and Functionalization of NAA
3. Biosensors (Biorecognizer-Based Sensors)
3.1. Immunosensor
3.2. Aptasensor
3.3. Genosensor
3.4. Peptide-Based Biosensor
3.5. Enzyme-Based Biosensor

4. Sensors
4.1. Gas Sensor
4.2. Non-Biorecognizer-Based Sensor
4.3. Ion Sensor
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Acronyms
| SOPMO | Self-ordered porous metal oxides |
| Al | Aluminum |
| NAA | Nanoporous anodic alumina |
| 3-APTS | 3-Aminopropyltriethoxysilane |
| 3-MPTES | 3-Mercaptopropyl-tirethoxysilane |
| 3-ISCN | 3-Isocyanatopropyl triethoxy |
| PLS | photoluminescence spectroscopy |
| IRS | Interferometric reflectance spectroscopy |
| SERS | Surface-enhanced Raman scattering |
| CCD | Charge-coupled device |
| α-TNF | Tumor necrosis factor alpha |
| anti-EpCAM | Anti- Epithelial cell adhesion molecule antibody |
| CTC | Circulating tumor cells |
| Glu | Glutaraldehyde |
| MB | Methylene blue |
| TB | Thrombin |
| EOT | Effective optical thickness |
| Aβ | Amyloid β |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| CFU | Colony-forming unit |
| Cat B | Cathepsin B |
| Cyt C | Cytochrome c |
| FLITC | Fluorescein 5(6)-isothiocyanate |
| Km | Michaelis-Menten constant |
| H2S | Hydrogen sulphide |
| H2 | Hydrogen |
| PEI | Polyethylenimine |
| Rh B | Rhodamine B |
| OWG | Optical waveguide |
References
- Wang, J.; Karnaushenko, D.; Medina-Sánchez, M.; Yin, Y.; Ma, L.; Schmidt, O.G. Three-Dimensional Microtubular Devices for Lab-on-a-Chip Sensing Applications. ACS Sens. 2019, 4, 1476–1496. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TRAC-Trend Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Losic, D. Nanoporous Anodic Alumina for Drug Delivery and Biomedical Applications. In Nanoporous Alumina: Fabrication, Structure, Properties and Applications; Losic, D., Santos, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 319–354. [Google Scholar]
- Sieber, I.; Hildebrand, H.; Friedrich, A.; Schmuki, P. Initiation of tantalum oxide pores grown on tantalum by potentiodynamic anodic oxidation. J. Electroceramics 2006, 16, 35–39. [Google Scholar] [CrossRef]
- Wang, G.; Lee, J.-H.; Yang, Y.; Ruan, G.; Kim, N.D.; Ji, Y.; Tour, J.M. Three-Dimensional Networked Nanoporous Ta2O5–x Memory System for Ultrahigh Density Storage. Nano Lett. 2015, 15, 6009–6014. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, S.; Yang, X.; Wang, X.; Sun, H.; Huo, M. Synthesis of Coral-Like Tantalum Oxide Films via Anodization in Mixed Organic-Inorganic Electrolytes. PLoS ONE 2013, 8, e66447. [Google Scholar] [CrossRef]
- Fialho, L.; Almeida Alves, C.F.; Marques, L.S.; Carvalho, S. Development of stacked porous tantalum oxide layers by anodization. Appl. Surf. Sci. 2020, 511, 145542. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.M.; Shrestha, N.K.; Schmuki, P. Thick Self-Ordered Nanoporous Ta2O5 Films with Long-Range Lateral Order. J. Electrochem. Soc. 2009, 156, K104–K111. [Google Scholar] [CrossRef]
- Barton, J.E.; Stender, C.L.; Li, P.; Odom, T.W. Structural control of anodized tantalum oxide nanotubes. J. Mater. Chem. 2009, 19, 4896–4898. [Google Scholar] [CrossRef]
- Anitha, V.C.; Goswami, A.; Sopha, H.; Nandan, D.; Gawande, M.B.; Cepe, K.; Ng, S.; Zboril, R.; Macak, J.M. Pt nanoparticles decorated TiO2 nanotubes for the reduction of olefins. Appl. Mater. Today 2018, 10, 86–92. [Google Scholar]
- Kim, C.; Kim, S.; Choi, J.; Lee, J.; Kang, J.S.; Sung, Y.-E.; Lee, J.; Choi, W.; Yoon, J. Blue TiO2 Nanotube Array as an Oxidant Generating Novel Anode Material Fabricated by Simple Cathodic Polarization. Electrochim. Acta 2014, 141, 113–119. [Google Scholar] [CrossRef]
- Zheng, Q.; Lee, H.-J.; Lee, J.; Choi, W.; Park, N.-B.; Lee, C. Electrochromic titania nanotube arrays for the enhanced photocatalytic degradation of phenol and pharmaceutical compounds. Chem. Eng. J. 2014, 249, 285–292. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Hwang, I.; Kondo, T.; Yanagishita, T.; Masuda, H.; Schmuki, P. Optimizing TiO2 nanotube morphology for enhanced photocatalytic H2 evolution using single-walled and highly ordered TiO2 nanotubes decorated with dewetted Au nanoparticles. Electrochem. Commun. 2017, 79, 46–50. [Google Scholar] [CrossRef]
- Altomare, M.; Cha, G.; Schmuki, P. Anodic nanoporous niobium oxide layers grown in pure molten ortho-phosphoric acid. Electrochim. Acta 2020, 344, 136158. [Google Scholar] [CrossRef]
- Agnieszka, S.; Tomasz, G.; Bożena, Ł. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Niobium (Review). Curr. Nanosci. 2019, 15, 42–48. [Google Scholar]
- Lu, Q.; Hashimoto, T.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Nanoporous Anodic Niobium Oxide Formed in Phosphate/Glycerol Electrolyte. Electrochem. Solid-State Lett. 2005, 8, B17–B20. [Google Scholar] [CrossRef]
- Sieber, I.; Hildebrand, H.; Friedrich, A.; Schmuki, P. Formation of self-organized niobium porous oxide on niobium. Electrochem. Commun. 2005, 7, 97–100. [Google Scholar] [CrossRef]
- Martín-González, M.; Martinez-Moro, R.; Aguirre, M.H.; Flores, E.; Caballero-Calero, O. Unravelling nanoporous anodic iron oxide formation. Electrochim. Acta 2020, 330, 135241. [Google Scholar] [CrossRef]
- Liang, F.-X.; Liang, L.; Zhao, X.-Y.; Tong, X.-W.; Hu, J.-G.; Lin, Y.; Luo, L.-B.; Wu, Y.-C. Mesoporous anodic α-Fe2O3 interferometer for organic vapor sensing application. RSC Adv. 2018, 8, 31121–31128. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Varghese, O.K.; Paulose, M.; Mor, G.K.; Grimes, C.A. Synthesis and photoelectrochemical properties of nanoporous iron (III) oxide by potentiostatic anodization. Nanotechnology 2006, 17, 4285–4291. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, H.; Chen, R.; Zhan, W.; Zhang, C.; Lei, R.; Zha, Y. A two-step anodic method to fabricate self-organised nanopore arrays on stainless steel. Appl. Surf. Sci. 2015, 351, 1161–1168. [Google Scholar] [CrossRef]
- Zhan, W.; Ni, H.; Chen, R.; Song, X.; Huo, K.; Fu, J. Formation of nanopore arrays on stainless steel surface by anodization for visible-light photocatalytic degradation of organic pollutants. J. Mater. Res. 2012, 27, 2417–2424. [Google Scholar] [CrossRef]
- Dhawan, U.; Pan, H.-A.; Shie, M.-J.; Chu, Y.H.; Huang, G.S.; Chen, P.-C.; Chen, W.L. The Spatiotemporal Control of Osteoblast Cell Growth, Behavior, and Function Dictated by Nanostructured Stainless Steel Artificial Microenvironments. Nanoscale Res. Lett. 2017, 12, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Formentín, P.; Catalán, Ú.; Fernández-Castillejo, S.; Alba, M.; Baranowska, M.; Solà, R.; Pallarès, J.; Marsal, L.F. Human aortic endothelial cell morphology influenced by topography of porous silicon substrates. J. Biomater. Appl. 2015, 30, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Molinero, D.; Valera, E.; Trifonov, T.; Marsal, L.F.; Pallarès, J.; Alcubilla, R. Fabrication of silicon oxide microneedles from macroporous silicon. Sens. Actuators B Chem. 2005, 109, 135–140. [Google Scholar] [CrossRef]
- Elia, P.; Nativ-Roth, E.; Zeiri, Y.; Porat, Z. Determination of the average pore-size and total porosity in porous silicon layers by image processing of SEM micrographs. Microporous Mater. 2016, 225, 465–471. [Google Scholar] [CrossRef]
- Xia, X.H.; Ashruf, C.M.A.; French, P.J.; Kelly, J.J. Galvanic Cell Formation in Silicon/Metal Contacts: The Effect on Silicon Surface Morphology. Chem. Mater. 2000, 12, 1671–1678. [Google Scholar] [CrossRef]
- Noh, K.; Brammer, K.S.; Kim, H.; Jung, S.-Y.; Seong, T.-Y.; Jin, S. Highly self-assembled nanotubular aluminum oxide by hard anodization. J. Mater. Res. 2011, 26, 186–193. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Balde, M.; Vena, A.; Sorli, B. Fabrication of porous anodic aluminium oxide layers on paper for humidity sensors. Sens. Actuators B Chem. 2015, 220, 829–839. [Google Scholar] [CrossRef]
- Zaraska, L.; Jaskuła, M.; Sulka, G.D. Porous anodic alumina layers with modulated pore diameters formed by sequential anodizing in different electrolytes. Mater. Lett. 2016, 171, 315–318. [Google Scholar] [CrossRef]
- Santos, A.; Balderrama, V.S.; Alba, M.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Nanoporous Anodic Alumina Barcodes: Toward Smart Optical Biosensors. Adv. Mater. 2012, 24, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Bertó-Roselló, F.; Xifré-Pérez, E.; Ferré-Borrull, J.; Marsal, L.F. 3D-FDTD modelling of optical biosensing based on gold-coated nanoporous anodic alumina. Results Phys. 2018, 11, 1008–1014. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Structural and Optical Nanoengineering of Nanoporous Anodic Alumina Rugate Filters for Real-Time and Label-Free Biosensing Applications. Anal. Chem. 2014, 86, 1837–1844. [Google Scholar] [CrossRef]
- Santos, A.; Balderrama, V.S.; Alba, M.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Tunable Fabry-Pérot interferometer based on nanoporous anodic alumina for optical biosensing purposes. Nanoscale Res. Lett. 2012, 7, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Singh, S.; Peltier-Pain, P.; Thorson, J.S.; Hinds, B.J. Functionalized Anodic Aluminum Oxide Membrane–Electrode System for Enzyme Immobilization. ACS Nano 2014, 8, 8104–8112. [Google Scholar] [CrossRef]
- Mey, I.; Steinem, C.; Janshoff, A. Biomimetic functionalization of porous substrates: Towards model systems for cellular membranes. J. Mater. Chem. 2012, 22, 19348–19356. [Google Scholar] [CrossRef]
- Aguilar-Sierra, S.M.; Ferré-Borrull, J.; Echeverría, F.E.; Marsal, L.F. Titanium dioxide-coated nanoporous anodic alumina optical properties. Appl. Surf. Sci. 2019, 489, 239–246. [Google Scholar] [CrossRef]
- Montero-Rama, M.P.; Viterisi, A.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. In-situ removal of thick barrier layer in nanoporous anodic alumina by constant current Re-anodization. Surf. Coat. Technol. 2019, 380, 125039. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Liu, L.; Abell, A.D.; Marsal, L.F.; Santos, A. Realization of high-quality optical nanoporous gradient-index filters by optimal combination of anodization conditions. Nanoscale 2020, 12, 9404–9415. [Google Scholar] [CrossRef]
- Matsumoto, F.; Harada, M.; Nishio, K.; Masuda, H. Nanometer-Scale Patterning of DNA in Controlled Intervals on a Gold-Disk Array Fabricated Using Ideally Ordered Anodic Porous Alumina. Adv. Mater. 2005, 17, 1609–1612. [Google Scholar] [CrossRef]
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Self-Ordering Behavior of Anodic Porous Alumina via Selenic Acid Anodizing. Electrochim. Acta 2014, 137, 728–735. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-Ordered Anodic Aluminum Oxide Formed by H2SO4 Hard Anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Self-Ordering Regimes of Porous Anodic Alumina Layers Formed in Highly Diluted Sulfuric Acid Electrolytes. J. Phys. Chem. C 2016, 120, 14590–14596. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Asadabadi, S.J.; Yousefi, M.H.; Ghasemzadeh, M. Photoluminescence emission of nanoporous anodic aluminum oxide films prepared in phosphoric acid. Nanoscale Res. Lett. 2012, 7, 689–696. [Google Scholar] [CrossRef]
- Choudhari, K.S.; Kulkarni, S.D.; Santhosh, C.; George, S.D. Photoluminescence enhancement and morphological properties of nanoporous anodic alumina prepared in oxalic acid with varying time and temperature. Microporous Mater. 2018, 271, 138–145. [Google Scholar] [CrossRef]
- Leontiev, A.P.; Roslyakov, I.V.; Napolskii, K.S. Complex influence of temperature on oxalic acid anodizing of aluminium. Electrochim. Acta 2019, 319, 88–94. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Forbot, D.; Norek, M.; Michalska-Domańska, M.; Król, A. The impact of viscosity of the electrolyte on the formation of nanoporous anodic aluminum oxide. Electrochim. Acta 2014, 133, 57–64. [Google Scholar] [CrossRef]
- Santos, A.; Alba, M.; Rahman, M.M.; Formentín, P.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Structural tuning of photoluminescence in nanoporous anodic alumina by hard anodization in oxalic and malonic acids. Nanoscale Res. Lett. 2012, 7, 228–239. [Google Scholar] [CrossRef]
- Lee, W.; Nielsch, K.; Gösele, U. In Self-ordering behavior of nanoporous anodic aluminum oxide (AAO) in malonic acid anodization. Nanotechnology 2007, 18, 475713–475721. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Li, Y.; Zhang, Z.; Feng, C.; Sun, R. Fabrication of Self-Ordered Alumina Films with Large Interpore Distance by Janus Anodization in Citric Acid. Sci. Rep. 2016, 6, 39165–39173. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Exploration for the Self-ordering of Porous Alumina Fabricated via Anodizing in Etidronic Acid. Electrochim. Acta 2016, 211, 515–523. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of Self-Ordered Porous Alumina via Etidronic Acid Anodizing and Structural Color Generation from Submicrometer-Scale Dimple Array. Electrochim. Acta 2015, 156, 235–243. [Google Scholar] [CrossRef]
- Vrublevsky, I.A.; Chernyakova, K.V.; Ispas, A.; Bund, A.; Zavadski, S. Optical properties of thin anodic alumina membranes formed in a solution of tartaric acid. Thin Solid Films 2014, 556, 230–235. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Marsal, L.F.; Santos, A. Structural tailoring of nanoporous anodic alumina optical microcavities for enhanced resonant recirculation of light. Nanoscale 2018, 10, 14139–14152. [Google Scholar] [CrossRef]
- Alvarez, S.D.; Li, C.-P.; Chiang, C.E.; Schuller, I.K.; Sailor, M.J. A Label-Free Porous Alumina Interferometric Immunosensor. ACS Nano 2009, 3, 3301–3307. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-Coated Ordered Nanoporous Anodic Alumina Bilayers for Future Label-Free Interferometric Biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef]
- Eckstein, C.; Acosta, L.K.; Pol, L.; Xifré-Pérez, E.; Pallares, J.; Ferré-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Surface Modification by Electrostatic, Covalent, and Immune Complexation Binding Investigated by Capillary Filling. ACS Appl. Mater. Interfaces 2018, 10, 10571–10579. [Google Scholar] [CrossRef]
- Rajeev, G.; Prieto Simon, B.; Marsal, L.F.; Voelcker, N.H. Advances in Nanoporous Anodic Alumina-Based Biosensors to Detect Biomarkers of Clinical Significance: A Review. Adv. Healthc. Mater. 2018, 7, 1700904–1700922. [Google Scholar] [CrossRef]
- Álvarez, J.; Sola, L.; Cretich, M.; Swann, M.J.; Gylfasson, K.B.; Volden, T.; Chiari, M.; Hill, D. A real time immunoassay in alumina membranes. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 1760–1763. [Google Scholar]
- Álvarez, J.; Sola, L.; Cretich, M.; Swann, M.J.; Gylfason, K.B.; Volden, T.; Chiari, M.; Hill, D. Real time optical immunosensing with flow-through porous alumina membranes. Sens. Actuators B Chem. 2014, 202, 834–839. [Google Scholar] [CrossRef]
- Tanvir, S.; Pantigny, J.; Boulnois, P.; Pulvin, S. Covalent immobilization of recombinant human cytochrome CYP2E1 and glucose-6-phosphate dehydrogenase in alumina membrane for drug screening applications. J. Membr. Sci. 2009, 329, 85–90. [Google Scholar] [CrossRef]
- Jia, R.-P.; Shen, Y.; Luo, H.-Q.; Chen, X.-G.; Hu, Z.-D.; Xue, D.-S. Photoluminescence spectra of human serum albumen and morin embedded in porous alumina membranes with ordered pore arrays. J. Phys. Condens. Matter. 2003, 15, 8271–8279. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, X.-Y.; Liang, Y.; Guo, H. The molecularly imprinted polymer supported by anodic alumina oxide nanotubes membrane for efficient recognition of chloropropanols in vegetable oils. Food Chem. 2018, 258, 295–300. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Machado, F.; Pereira, M.; Teixeira, V.; Costa, M.F.; Ribeiro, A.; Cavaco-Paulo, A.; Samantilleke, A.P. The influence of the morphological characteristics of nanoporous anodic aluminium oxide (AAO) structures on capacitive touch sensor performance: A biological application. RSC Adv. 2018, 8, 37254–37266. [Google Scholar] [CrossRef]
- Hong, C.; Chu, L.-A.; Lai, W.; Chiang, A.-S.; Fang, W. Implementation of a New Capacitive Touch Sensor Using the Nanoporous Anodic Aluminum Oxide (np-AAO) Structure. IEEE Sens. J. 2011, 11, 3409–3416. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Hong, C.; Hsu, F.-M.; Fang, W. Novel temperature sensor implemented on nanoporous Anodic Aluminum Oxide template. In Proceedings of the 2011 IEEE SENSORS, Limerick, Ireland, 28–31 October 2011; pp. 1253–1256. [Google Scholar]
- Salamat, A.; Islam, T. Fabrication of an anodized porous alumina relative humidity sensor with improved sensitivity. Instrum. Sci. Technol. 2020, 48, 128–145. [Google Scholar] [CrossRef]
- Lu, Z.; Ruan, W.; Yang, J.; Xu, W.; Zhao, C.; Zhao, B. Deposition of Ag nanoparticles on porous anodic alumina for surface enhanced Raman scattering substrate. J. Raman Spectrosc. 2009, 40, 112–116. [Google Scholar] [CrossRef]
- Sinn Aw, M.; Kurian, M.; Losic, D. Non-eroding drug-releasing implants with ordered nanoporous and nanotubular structures: Concepts for controlling drug release. Biomater. Sci. 2014, 2, 10–34. [Google Scholar] [CrossRef]
- Porta, I.B.M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. 3D Nanoporous Anodic Alumina Structures for Sustained Drug Release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef]
- Saji, V.S.; Kumeria, T.; Gulati, K.; Prideaux, M.; Rahman, S.; Alsawat, M.; Santos, A.; Atkins, G.J.; Losic, D. Localized drug delivery of selenium (Se) using nanoporous anodic aluminium oxide for bone implants. J. Mater. Chem. B 2015, 3, 7090–7098. [Google Scholar] [CrossRef][Green Version]
- Porta-i-Batalla, M.; Eckstein, C.; Xifré-Pérez, E.; Formentín, P.; Ferré-Borrull, J.; Marsal, L.F. Sustained, Controlled and Stimuli-Responsive Drug Release Systems Based on Nanoporous Anodic Alumina with Layer-by-Layer Polyelectrolyte. Nanoscale Res. Lett. 2016, 11, 372–381. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Kaur, G.; Evdokiou, A.; Losic, D. Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 2014, 35, 5517–5526. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Huang, G.; An, Z.; Chen, G.; Zhang, J.; Li, M.; Liu, R.; Mei, Y. Hierarchical nanoporous microtubes for high-speed catalytic microengines. NPG Asia Mater. 2014, 6, e94. [Google Scholar] [CrossRef]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro/nanomotors towards in vivo application: Cell, tissue and biofluid. Chem. Soc. Rev. 2017, 46, 5289–5310. [Google Scholar] [CrossRef]
- Mozalev, A.; Hubalek, J. On-substrate porous-anodic-alumina-assisted gold nanostructure arrays: Meeting the challenges of various sizes and interfaces. Electrochim. Acta 2019, 297, 988–999. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Santos, A.; Ferre-Borrull, J.; Marsal, L.F. Tailor-engineered structural and physico-chemical properties of anodic alumina nanotubes by pulse anodization: A step forward. Microporous Mater. 2020, 303, 110264. [Google Scholar] [CrossRef]
- Ferré-Borrull, J.; Xifré-Pérez, E.; Pallarès, J.; Marsal, L.F. Optical Properties of Nanoporous Anodic Alumina and Derived Applications. In Nanoporous Alumina: Fabrication, Structure, Properties and Applications; Losic, D., Santos, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 185–217. [Google Scholar]
- Ito, T.; Matsuda, Y.; Jinba, T.; Asai, N.; Shimizu, T.; Shingubara, S. Fabrication and characterization of nano porous lattice biosensor using anodic aluminum oxide substrate. Jpn. J. Appl. Phys. 2017, 56, 06GG02. [Google Scholar] [CrossRef]
- Law, C.S.; Marsal, L.F.; Santos, A. 9—Electrochemically engineered nanoporous photonic crystal structures for optical sensing and biosensing. In Handbook of Nanomaterials in Analytical Chemistry; Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 201–226. [Google Scholar]
- Fu, C.; Gu, Y.; Wu, Z.; Wang, Y.; Xu, S.; Xu, W. Surface-enhanced Raman scattering (SERS) biosensing based on nanoporous dielectric waveguide resonance. Sens. Actuators B Chem. 2014, 201, 173–176. [Google Scholar] [CrossRef]
- Takmakov, P.; Vlassiouk, I.; Smirnov, S. Hydrothermally shrunk alumina nanopores and their application to DNA sensing. Analyst 2006, 131, 1248–1253. [Google Scholar] [CrossRef]
- Hotta, K.; Yamaguchi, A.; Teramae, N. Nanoporous Waveguide Sensor with Optimized Nanoarchitectures for Highly Sensitive Label-Free Biosensing. ACS Nano 2012, 6, 1541–1547. [Google Scholar] [CrossRef]
- Koutsioubas, A.G.; Spiliopoulos, N.; Anastassopoulos, D.; Vradis, A.A.; Priftis, G.D. Nanoporous alumina enhanced surface plasmon resonance sensors. J. Appl. Phys. 2008, 103, 094521. [Google Scholar] [CrossRef]
- Dhathathreyan, A. Real-Time Monitoring of Invertase Activity Immobilized in Nanoporous Aluminum Oxide. J. Phys. Chem. B 2011, 115, 6678–6682. [Google Scholar] [CrossRef]
- Chen, R.; Du, X.; Cui, Y.; Zhang, X.; Ge, Q.; Dong, J.; Zhao, X. Vertical Flow Assay for Inflammatory Biomarkers Based on Nanofluidic Channel Array and SERS Nanotags. Small 2020, 1, 2002801. [Google Scholar] [CrossRef]
- Lazzara, T.D.; Mey, I.; Steinem, C.; Janshoff, A. Benefits and Limitations of Porous Substrates as Biosensors for Protein Adsorption. Anal. Chem. 2011, 83, 5624–5630. [Google Scholar] [CrossRef]
- Fan, Y.; Hotta, K.; Yamaguchi, A.; Ding, Y.; He, Y.; Teramae, N.; Sun, S.; Ma, H. Highly sensitive real-time detection of DNA hybridization by using nanoporous waveguide fluorescence spectroscopy. Appl. Phys. Lett. 2014, 105, 031103. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hotta, K.; Teramae, N. Optical Waveguide Sensor Based on a Porous Anodic Alumina/Aluminum Multilayer Film. Anal. Chem. 2009, 81, 105–111. [Google Scholar] [CrossRef]
- Hotta, K.; Yamaguchi, A.; Teramae, N. Deposition of Polyelectrolyte Multilayer Film on a Nanoporous Alumina Membrane for Stable Label-Free Optical Biosensing. J. Phys. Chem. C 2012, 116, 23533–23539. [Google Scholar] [CrossRef]
- Fan, Y.; Hotta, K.; Yamaguchi, A.; Teramae, N. Enhanced fluorescence in a nanoporous waveguide and its quantitative analysis. Opt. Express 2012, 20, 12850–12859. [Google Scholar] [CrossRef]
- Ji, N.; Ruan, W.; Wang, C.; Lu, Z.; Zhao, B. Fabrication of Silver Decorated Anodic Aluminum Oxide Substrate and Its Optical Properties on Surface-Enhanced Raman Scattering and Thin Film Interference. Langmuir 2009, 25, 11869–11873. [Google Scholar] [CrossRef]
- Hiep, H.M.; Yoshikawa, H.; Tamiya, E. Interference Localized Surface Plasmon Resonance Nanosensor Tailored for the Detection of Specific Biomolecular Interactions. Anal. Chem. 2010, 82, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Lee, J.-S.; Lee, S.-W.; Kang, B.-H.; Kwon, J.-B.; Kim, O.-S.; Kim, J.-S.; Kim, E.-S.; Kwon, D.-H.; Kang, S.-W. Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control. Sensors 2017, 17, 856. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, S.-W.; Jang, E.-Y.; Kang, B.-H.; Lee, S.-W.; Sai-Anand, G.; Lee, S.-H.; Kwon, D.-H.; Kang, S.-W. Rapid and Sensitive Detection of Lung Cancer Biomarker Using Nanoporous Biosensor Based on Localized Surface Plasmon Resonance Coupled with Interferometry. J. Nanomater. 2015, 2015, 183438–183440. [Google Scholar] [CrossRef]
- Yeom, S.H.; Kim, O.G.; Kang, B.H.; Kim, K.J.; Yuan, H.; Kwon, D.H.; Kim, H.R.; Kang, S.W. Highly sensitive nano-porous lattice biosensor based on localized surface plasmon resonance and interference. Opt. Express 2011, 19, 22882–22891. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Kerman, K.; Hiep, H.M.; Saito, M.; Yamamura, S.; Takamura, Y.; Kwon, Y.-S.; Tamiya, E. Label-free optical detection of aptamer–protein interactions using gold-capped oxide nanostructures. Anal. Biochem. 2008, 379, 1–7. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kerman, K.; Saito, M.; Sathuluri, R.R.; Endo, T.; Yamamura, S.; Kwon, Y.-S.; Tamiya, E. Label-Free DNA Biosensor Based on Localized Surface Plasmon Resonance Coupled with Interferometry. Anal. Chem. 2007, 79, 1855–1864. [Google Scholar] [CrossRef]
- Pla, L.; Xifré-Pérez, E.; Ribes, À.; Aznar, E.; Marcos, M.D.; Marsal, L.F.; Martínez-Máñez, R.; Sancenón, F. A Mycoplasma Genomic DNA Probe using Gated Nanoporous Anodic Alumina. ChemPlusChem 2017, 82, 337–341. [Google Scholar] [CrossRef]
- Yu, Z.; Lei, Y.; Yu, W.; Cheng, J.; Xing, J.; Zheng, X.; Zhan, Z.; Wang, B.; Guo, C. Fluorescence enhanced lab-on-a-chip patterned using a hybrid technique of femtosecond laser direct writing and anodized aluminum oxide porous nanostructuring. Nanoscale Adv. 2019, 1, 3474–3484. [Google Scholar] [CrossRef]
- Takmakov, P.; Vlassiouk, I.; Smirnov, S. Application of anodized aluminum in fluorescence detection of biological species. Anal. BioAnal. Chem. 2006, 385, 954–958. [Google Scholar] [CrossRef]
- Santos, A.; Macías, G.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Photoluminescent Enzymatic Sensor Based on Nanoporous Anodic Alumina. ACS Appl. Mater. Interfaces 2012, 4, 3584–3588. [Google Scholar]
- Jia, R.P.; Shen, Y.; Luo, H.Q.; Chen, X.G.; Hu, Z.D.; Xue, D.S. Enhanced photoluminescence properties of morin and trypsin absorbed on porous alumina films with ordered pores array. Solid State Commun. 2004, 130, 367–372. [Google Scholar] [CrossRef]
- Li, Y.B.; Zheng, M.J.; Ma, L. High-speed growth and photoluminescence of porous anodic alumina films with controllable interpore distances over a large range. Appl. Phys. Lett. 2007, 91, 073109–073113. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Q.; Chen, Z.; Zhao, C.; Chai, H.; Wu, Q.; Li, W.; Chen, X.; Deng, Y.; Song, Y. Arrayed nanopore silver thin films for surface-enhanced Raman scattering. RSC Adv. 2020, 10, 23908–23915. [Google Scholar] [CrossRef]
- Toccafondi, C.; Thorat, S.; La Rocca, R.; Scarpellini, A.; Salerno, M.; Dante, S.; Das, G. Multifunctional substrates of thin porous alumina for cell biosensors. J. Mater. Sci.: Mater. Med. 2014, 25, 2411–2420. [Google Scholar] [CrossRef]
- Velleman, L.; Bruneel, J.-L.; Guillaume, F.; Losic, D.; Shapter, J.G. Raman spectroscopy probing of self-assembled monolayers inside the pores of gold nanotube membranes. Phys. Chem. Chem. Phys. 2011, 13, 19587–19593. [Google Scholar] [CrossRef]
- Kodiyath, R.; Malak, S.T.; Combs, Z.A.; Koenig, T.; Mahmoud, M.A.; El-Sayed, M.A.; Tsukruk, V.V. Assemblies of silver nanocubes for highly sensitive SERS chemical vapor detection. J. Mater. Chem. A 2013, 1, 2777–2788. [Google Scholar] [CrossRef]
- Ko, H.; Tsukruk, V.V. Nanoparticle-Decorated Nanocanals for Surface-Enhanced Raman Scattering. Small 2008, 4, 1980–1984. [Google Scholar] [CrossRef]
- Lee, S.J.; Guan, Z.; Xu, H.; Moskovits, M. Surface-Enhanced Raman Spectroscopy and Nanogeometry: The Plasmonic Origin of SERS. J. Phys. Chem. C 2007, 111, 17985–17988. [Google Scholar] [CrossRef]
- Hao, Q.; Huang, H.; Fan, X.; Hou, X.; Yin, Y.; Li, W.; Si, L.; Nan, H.; Wang, H.; Mei, Y.; et al. Facile design of ultra-thin anodic aluminum oxide membranes for the fabrication of plasmonic nanoarrays. Nanotechnology 2017, 28, 105301–105311. [Google Scholar] [CrossRef]
- Kumeria, T.; Losic, D. Reflective interferometric gas sensing using nanoporous anodic aluminium oxide (AAO). Phys. Status Solidi-R 2011, 5, 406–408. [Google Scholar] [CrossRef]
- Pan, S.; Rothberg, L.J. Interferometric Sensing of Biomolecular Binding Using Nanoporous Aluminum Oxide Templates. Nano Lett. 2003, 3, 811–814. [Google Scholar] [CrossRef]
- Dronov, R.; Jane, A.; Shapter, J.G.; Hodges, A.; Voelcker, N.H. Nanoporous alumina-based interferometric transducers ennobled. Nanoscale 2011, 3, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Mutalib Md Jani, A.; Anglin, E.J.; McInnes, S.J.P.; Losic, D.; Shapter, J.G.; Voelcker, N.H. Nanoporous anodic aluminium oxide membranes with layered surface chemistry. ChemComm 2009, 3062–3064. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Losic, D. Controlling interferometric properties of nanoporous anodic aluminium oxide. Nanoscale Res. Lett. 2012, 7, 88–98. [Google Scholar] [CrossRef]
- Shi, W.; Shen, Y.; Ge, D.; Xue, M.; Cao, H.; Huang, S.; Wang, J.; Zhang, G.; Zhang, F. Functionalized anodic aluminum oxide (AAO) membranes for affinity protein separation. J. Membr. Sci. 2008, 325, 801–808. [Google Scholar] [CrossRef]
- Rajeev, G.; Xifre-Perez, E.; Prieto Simon, B.; Cowin, A.J.; Marsal, L.F.; Voelcker, N.H. A label-free optical biosensor based on nanoporous anodic alumina for tumour necrosis factor-alpha detection in chronic wounds. Sens. Actuators B Chem. 2018, 257, 116–123. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Highly sensitive aptasensor based on interferometric reflectance spectroscopy for the determination of amyloid β as an Alzheimer’s disease biomarkers using nanoporous anodic alumina. Biosens. Bioelectron. 2019, 137, 279–286. [Google Scholar] [CrossRef]
- Pol, L.; Acosta, L.K.; Ferré-Borrull, J.; Marsal, L.F. Aptamer-Based Nanoporous Anodic Alumina Interferometric Biosensor for Real-Time Thrombin Detection. Sensors 2019, 19, 4543. [Google Scholar] [CrossRef]
- Nemati, M.; Santos, A.; Kumeria, T.; Losic, D. Label-Free Real-Time Quantification of Enzyme Levels by Interferometric Spectroscopy Combined with Gelatin-Modified Nanoporous Anodic Alumina Photonic Films. Anal. Chem. 2015, 87, 9016–9024. [Google Scholar] [CrossRef]
- Lau, K.H.A.; Duran, H.; Knoll, W. In situ Characterization of N-Carboxy Anhydride Polymerization in Nanoporous Anodic Alumina. J. Phys. Chem. B 2009, 113, 3179–3189. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Kurkuri, M.D.; Diener, K.R.; Parkinson, L.; Losic, D. Label-free reflectometric interference microchip biosensor based on nanoporous alumina for detection of circulating tumour cells. Biosens. Bioelectron. 2012, 35, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.-H.; Han, M.-E.; Kang, B.-H.; Kim, K.-J.; Yuan, H.; Eum, N.-S.; Kang, S.-W. Enhancement of the sensitivity of LSPR-based CRP immunosensors by Au nanoparticle antibody conjugation. Sens. Actuators B Chem. 2013, 177, 376–383. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef]
- Thiviyanathan, V.; Gorenstein, D.G. Aptamers and the next generation of diagnostic reagents. Proteom. Clin. Appl. 2012, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Ribes, À.; Aznar, E.; Bernardos, A.; Marcos, M.D.; Amorós, P.; Martínez-Máñez, R.; Sancenón, F. Fluorogenic Sensing of Carcinogenic Bisphenol A using Aptamer-Capped Mesoporous Silica Nanoparticles. Chemistry 2017, 23, 8581–8584. [Google Scholar] [CrossRef]
- Liang, G.; Man, Y.; Li, A.; Jin, X.; Liu, X.; Pan, L. DNAzyme-based biosensor for detection of lead ion: A review. Microchem. J. 2017, 131, 145–153. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Highly sensitive remote biosensor for the determination of lead (II) ions by using nanoporous anodic alumina modified with DNAzyme. Sens. Actuators B Chem. 2020, 321, 128314. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Liu, Q.; Lin, Z.; Qiu, B.; Chen, G. Pb2+-introduced activation of horseradish peroxidase (HRP)-mimicking DNAzyme. ChemComm 2011, 47, 7437–7439. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Nanoporous Anodic Alumina Rugate Filters for Sensing of Ionic Mercury: Toward Environmental Point-of-Analysis Systems. ACS Appl. Mater. Interfaces 2014, 6, 12971–12978. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Law, C.S.; Williamson, N.H.; Kempson, I.; Popat, A.; Kumeria, T.; Santos, A. Environmental Copper Sensor Based on Polyethylenimine-Functionalized Nanoporous Anodic Alumina Interferometers. Anal. Chem. 2019, 91, 5011–5020. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Santos, A.; Losic, D. Ultrasensitive Nanoporous Interferometric Sensor for Label-Free Detection of Gold(III) Ions. ACS Appl. Mater. Interfaces 2013, 5, 11783–11790. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Santos, A. Real-Time Binding Monitoring between Human Blood Proteins and Heavy Metal Ions in Nanoporous Anodic Alumina Photonic Crystals. Anal. Chem. 2018, 90, 10039–10048. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, C.; Law, C.S.; Lim, S.Y.; Kaur, S.; Kumeria, T.; Ferré-Borrull, J.; Abell, A.D.; Marsal, L.F.; Santos, A. Nanoporous photonic crystals with tailored surface chemistry for ionic copper sensing. J. Mater. Chem. C 2019, 7, 12278–12289. [Google Scholar] [CrossRef]
- Chen, Y.; Santos, A.; Wang, Y.; Kumeria, T.; Wang, C.; Li, J.; Losic, D. Interferometric nanoporous anodic alumina photonic coatings for optical sensing. Nanoscale 2015, 7, 7770–7779. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Shamsipur, M. A label-free electrochemical DNA biosensor based on covalent immobilization of salmonella DNA sequences on the nanoporous glassy carbon electrode. Biosens. Bioelectron. 2015, 69, 100–105. [Google Scholar] [CrossRef]
- Gong, Q.; Han, H.; Yang, H.; Zhang, M.; Sun, X.; Liang, Y.; Liu, Z.; Zhang, W.; Qiao, J. Sensitive electrochemical DNA sensor for the detection of HIV based on a polyaniline/graphene nanocomposite. J. Mater. 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.Á.; Pemán, J.; Marsal, L.F.; Martínez-Máñez, R. Selective and Sensitive Probe Based in Oligonucleotide-Capped Nanoporous Alumina for the Rapid Screening of Infection Produced by Candida albicans. ACS Sensors 2019, 4, 1291–1298. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Remote sensing of Salmonella-specific DNA fragment by using nanoporous alumina modified with the single-strand DNA probe. Sens. Actuators B Chem. 2020, 304, 127302. [Google Scholar] [CrossRef]
- Rohs, R.; Sklenar, H.; Lavery, R.; Röder, B. Methylene Blue Binding to DNA with Alternating GC Base Sequence: A Modeling Study. J. Am. Chem. Soc. 2000, 122, 2860–2866. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. An electrochemical DNA-sensor developed with the use of methylene blue as a redox indicator for the detection of DNA damage induced by endocrine-disrupting compounds. Anal. Chim. Acta 2015, 867, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Boyd, B.J. Peptide-based biosensors. Talanta 2015, 136, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. An optical biosensor for the determination of cathepsin B as a cancer-associated enzyme using nanoporous anodic alumina modified with human serum albumin-thionine. Mikrochim. Acta 2020, 187, 230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Highly sensitive IRS based biosensor for the determination of cytochrome c as a cancer marker by using nanoporous anodic alumina modified with trypsin. Biosens. Bioelectron. 2020, 149, 111828. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, H.; Cui, W.; Zhou, Y.; Du, J. Label–free, turn–on fluorescent sensor for trypsin activity assay and inhibitor screening. Talanta 2016, 161, 535–540. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Cheng, M.; Guo, Y.; Šponer, J.; Monchaud, D.; Mergny, J.-L.; Ju, H.; Zhou, J. How Proximal Nucleobases Regulate the Catalytic Activity of G-Quadruplex/Hemin DNAzymes. ACS Catal. 2018, 8, 11352–11361. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina As a Three-dimensional Nanostructured material for the Remote Optical Sensing of Urea. ECS Meet. Abstr. 2020, MA2020-01, 1424. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Gonzalez Vara, A.; Ciurli, S. The structure of urease inactivated by Ag(i): A new paradigm for enzyme inhibition by heavy metals. Dalton Trans. 2018, 47, 8240–8247. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferré-Borrull, J.; Marsal, L.F. Remote biosensor for the determination of trypsin by using nanoporous anodic alumina as a three-dimensional nanostructured material. Sci. Rep. 2020, 10, 2356. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Santos, A.; Wang, Y.; Kumeria, T.; Li, J.; Wang, C.; Losic, D. Biomimetic Nanoporous Anodic Alumina Distributed Bragg Reflectors in the Form of Films and Microsized Particles for Sensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 19816–19824. [Google Scholar] [CrossRef] [PubMed]
- Macias, G.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Effect of pore diameter in nanoporous anodic alumina optical biosensors. Analyst 2015, 140, 4848–4854. [Google Scholar] [CrossRef] [PubMed]
- Pol, L.; Eckstein, C.; Acosta, L.K.; Xifré-Pérez, E.; Ferré-Borrull, J.; Marsal, L.F. Real-Time Monitoring of Biotinylated Molecules Detection Dynamics in Nanoporous Anodic Alumina for Bio-Sensing. Nanomaterials 2019, 9, 478. [Google Scholar] [CrossRef]
- Acosta, L.K.; Bertó-Roselló, F.; Xifre-Perez, E.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Stacked Nanoporous Anodic Alumina Gradient-Index Filters with Tunable Multispectral Photonic Stopbands as Sensing Platforms. ACS Appl. Mater. Interfaces 2019, 11, 3360–3371. [Google Scholar] [CrossRef]
- Acosta, L.K.; Bertó-Roselló, F.; Xifre-Perez, E.; Law, C.S.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Tunable Nanoporous Anodic Alumina Photonic Crystals by Gaussian Pulse Anodization. ACS Appl. Mater. Interfaces 2020, 12, 19778–19787. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Optically Optimized Photoluminescent and Interferometric Biosensors Based on Nanoporous Anodic Alumina: A Comparison. Anal. Chem. 2013, 85, 7904–7911. [Google Scholar] [CrossRef]
- Ferro, L.M.M.; Lemos, S.G.; Ferreira, M.; Trivinho-Strixino, F. Use of multivariate analysis on Fabry-Pérot interference spectra of nanoporous anodic alumina (NAA) for optical sensors purposes. Sens. Actuators B Chem. 2017, 248, 718–723. [Google Scholar] [CrossRef]
- Nemati, M.; Santos, A.; Losic, D. Fabrication and Optimization of Bilayered Nanoporous Anodic Alumina Structures as Multi-Point Interferometric Sensing Platform. Sensors 2018, 18, 470. [Google Scholar] [CrossRef]
- Dai, J.; Baker, G.L.; Bruening, M.L. Use of Porous Membranes Modified with Polyelectrolyte Multilayers as Substrates for Protein Arrays with Low Nonspecific Adsorption. Anal. Chem. 2006, 78, 135–140. [Google Scholar] [CrossRef]
- Ribes, À.; Xifré -Pérez, E.; Aznar, E.; Sancenón, F.; Pardo, T.; Marsal, L.F.; Martínez-Máñez, R. Molecular gated nanoporous anodic alumina for the detection of cocaine. Sci. Rep. 2016, 6, 38649–38658. [Google Scholar] [CrossRef] [PubMed]
- Pla, L.; Santiago-Felipe, S.; Tormo-Mas, M.Á.; Pemán, J.; Sancenón, F.; Aznar, E.; Martínez-Máñez, R. Aptamer-Capped nanoporous anodic alumina for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128281. [Google Scholar] [CrossRef]















| Sensor | Type of Recognizers | Analyte | Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| Nanoporous anodic alumina - Human serum albumin-Thionine | Labeled Peptide (Human serum albumin-Thionine) | Cathepsin B | Interferometric reflectance spectroscopy | 0.5–64.0 nM | 0.08 nM | [137] |
| Nanoporous anodic alumina -Urease- Fluorescein 5(6)-isothiocyanate | Labeled Enzyme (Urease- Fluorescein 5(6)-isothiocyanate) | Trypsin | Interferometric reflectance spectroscopy | 0.25–20 μg/mL | 0.06 μg/mL | [156] |
| Nanoporous anodic alumina-Gelatin | Peptide (Gelatin) | Trypsin | Interferometric reflectance spectroscopy | 1–7 mg/mL | 1 mg/mL | [124] |
| Nanoporous anodic alumina-Trypsin | Enzyme (Trypsin) | Cytochrome c | Interferometric reflectance spectroscopy | 1–100 nM | 0.5 nM | [151] |
| Nanoporous anodic alumina -ssDNAsal | Short chains of nucleotides (ssDNAsal) | Salmonella-specific DNA fragment | Interferometric reflectance spectroscopy | 0.25–50.0 nM | 0.01 nM | [145] |
| Nanoporous anodic alumina -AptamerTB | Short chains of nucleotides (AptamerTB) | Thrombin | Interferometric reflectance spectroscopy | 0.54–2.70 nM | 7.2 nM | [123] |
| Nanoporous anodic alumina -AptamerAβ | Short chains of nucleotides (AptamerAβ) | Amyloid β oligomers | Interferometric reflectance spectroscopy | 0.5–50.0 μg/mL | 0.02 μg/mL | [122] |
| Nanoporous anodic alumina -Anti- Tumor necrosis factor alpha | Antibody (Anti- Tumor necrosis factor alpha) | Tumour necrosis factor-alpha | Interferometric reflectance spectroscopy | 100–1500 ng/mL | 100 ng/mL | [121] |
| Nanoporous anodic alumina- Anti- Epithelial cell adhesion molecule antibody | Antibody (Anti- Epithelial cell adhesion molecule antibody) | Circulating tumor cells | Interferometric reflectance spectroscopy | 103–105 | >1000 | [128] |
| Nanoporous anodic alumina-Anti- human immunoglobulin G | Antibody (Anti- human immunoglobulin G) | Human immunoglobulin G | Interferometric reflectance spectroscopy | 10–100 μg/mL | 1 μg/mL | [158] |
| Nanoporous anodic alumina -Streptavidin | Peptide (Streptavidin) | Biotinylated thrombin | Interferometric reflectance spectroscopy | 10–100 μg/mL | 10 μg/mL | [159] |
| Nanoporous anodic alumina gradient-index | - | Glucose | Interferometric reflectance spectroscopy | 0.025–1 M | 0.025 M | [160] |
| Nanoporous anodic alumina -3-Aminopropyltriethoxysilane -Glutaraldehyde | Small molecule (Glutaraldehyde) | Vitamin C | Interferometric reflectance spectroscopy | 0.125–0.5 µM | 20 nM | [157] |
| Nanoporous anodic alumina | - | Glucose | Interferometric reflectance spectroscopy | 0.0125–1 M | 0.0125 | [161] |
| Nanoporous anodic alumina | - | Glucose | Interferometric reflectance spectroscopy | 0.01–1.2 M | 100 mM | [162] |
| Photoluminescence spectroscopy | 0.01–1.2 M | 10 mM | ||||
| Nanoporous anodic alumina | - | L-cysteine | Interferometric reflectance spectroscopy | 0.005–0.1 M | 5 mM | [162] |
| Photoluminescence spectroscopy | 0.005–0.1 M | 5 mM | ||||
| Nanoporous anodic alumina | - | Glucose | Photoluminescence spectroscopy | 0.01–1.1 mM | 0.01 mM | [163] |
| Nanoporous anodic alumina -3-Mercaptopropyl-tirethoxysilane | Small molecule (3-Mercaptopropyl-tirethoxysilane) | Mercury(II) ion | Interferometric reflectance spectroscopy | 1–100 μM | 1 μM | [136] |
| Nanoporous anodic alumina - Polyethylenimine- Glutaraldehyde-Polyethylenimine | Polymer (Polyethylenimine - Glutaraldehyde - Polyethylenimine) | Copper (II) ion | Interferometric reflectance spectroscopy | 1–100 mg/L | 0.007 mg/L (7 ppb) | [137] |
| Nanoporous anodic alumina -3-Mercaptopropyl-tirethoxysilane | Small molecule 3-Mercaptopropyl-tirethoxysilane | Glod (III) ion | Interferometric reflectance spectroscopy | 0.1–80 µM | 0.1 µM | [138] |
| Nanoporous anodic alumina -DNAzyme | Short chains of nucleotides and heme group (DNAzyme) | Lead ion (II) | Interferometric reflectance spectroscopy | 50–3200 nM | 12 nM | [134] |
| Nanoporous anodic alumina-Bovine serum albumin-5- Fluorouracil | Labeled Protein (Bovine serum albumin-5- Fluorouracil) | Fluorouracil antibody | Interference localized surface plasmon resonance | 10–104 ng/mL | 10 ng/mL | [96] |
| Nanoporous anodic alumina-Human serum albumin | Protein (Human serum albumin) | Quercetin | Interferometric reflectance spectroscopy | 0.25–0.5 mg/mL | 0.14 mg/mL | [164] |
| Nanoporous anodic alumina- poly(acrylic acid) [poly(acrylic acid)/protonated poly(allylamine)]3 | poly(acrylic acid)/protonated poly(allylamine) | Cy5-labeled human immunoglobulin G | Photoluminescence spectroscopy | 0.02–1 ng/mL | 0.02 ng/mL | [165] |
| Nanoporous anodic alumina | - | Bovine serum albumin | Nanoporous optical waveguide | 60 nM–6 µM | 5.7 pg/mm2 | [86] |
| Nanoporous anodic alumina-short aptamer/Rhodamine B sequence/AptamerCocaine probe | Short chains of nucleotides (aptamerCocaine probe) | Cocaine | photoluminescence spectroscopy | 0.5–10 µM | 0.5 µM | [166] |
| Nanoporous anodic alumina-short aptamer/Rhodamine B sequence/Aptamer probe | Short chains of nucleotides | Mycoplasma species genome | Photoluminescence spectroscopy | 20–80 copies/mL | 20 copies/mL | [102] |
| Nanoporous anodic alumina-short aptamer/Rhodamine B sequence/AptamerCandida albicans species probe | Short chains of nucleotides (AptamerCandida albicans speciesprobe) | Candida albicans species genome | Photoluminescence spectroscopy | 7−2 × 102 CFU/mL | 8 CFU/mL | [144] |
| Nanoporous anodic alumina-short aptamer/Rhodamine B sequence/Aptamer Staphylococcus aureus species genome probe | Short chains of nucleotides (Aptamer Staphylococcus aureus species) | Staphylococcus aureus species genome | Photoluminescence spectroscopy | 2–100 CFU/mL | 2 CFU/mL | [167] |
| Nanoporous anodic alumina -biotin-Stripavidin/Aptamer probe | Short chains of adenines | Timine rich oligumer | Optical waveguide | 50 pM–1 nM | 20 pM | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amouzadeh Tabrizi, M.; Ferre-Borrull, J.; Marsal, L.F. Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina. Sensors 2020, 20, 5068. https://doi.org/10.3390/s20185068
Amouzadeh Tabrizi M, Ferre-Borrull J, Marsal LF. Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina. Sensors. 2020; 20(18):5068. https://doi.org/10.3390/s20185068
Chicago/Turabian StyleAmouzadeh Tabrizi, Mahmoud, Josep Ferre-Borrull, and Lluis F. Marsal. 2020. "Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina" Sensors 20, no. 18: 5068. https://doi.org/10.3390/s20185068
APA StyleAmouzadeh Tabrizi, M., Ferre-Borrull, J., & Marsal, L. F. (2020). Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina. Sensors, 20(18), 5068. https://doi.org/10.3390/s20185068