Modelling of Chest Wall Motion for Cardiorespiratory Activity for Radar-Based NCVS Systems
Abstract
1. Introduction
2. Proposed Model
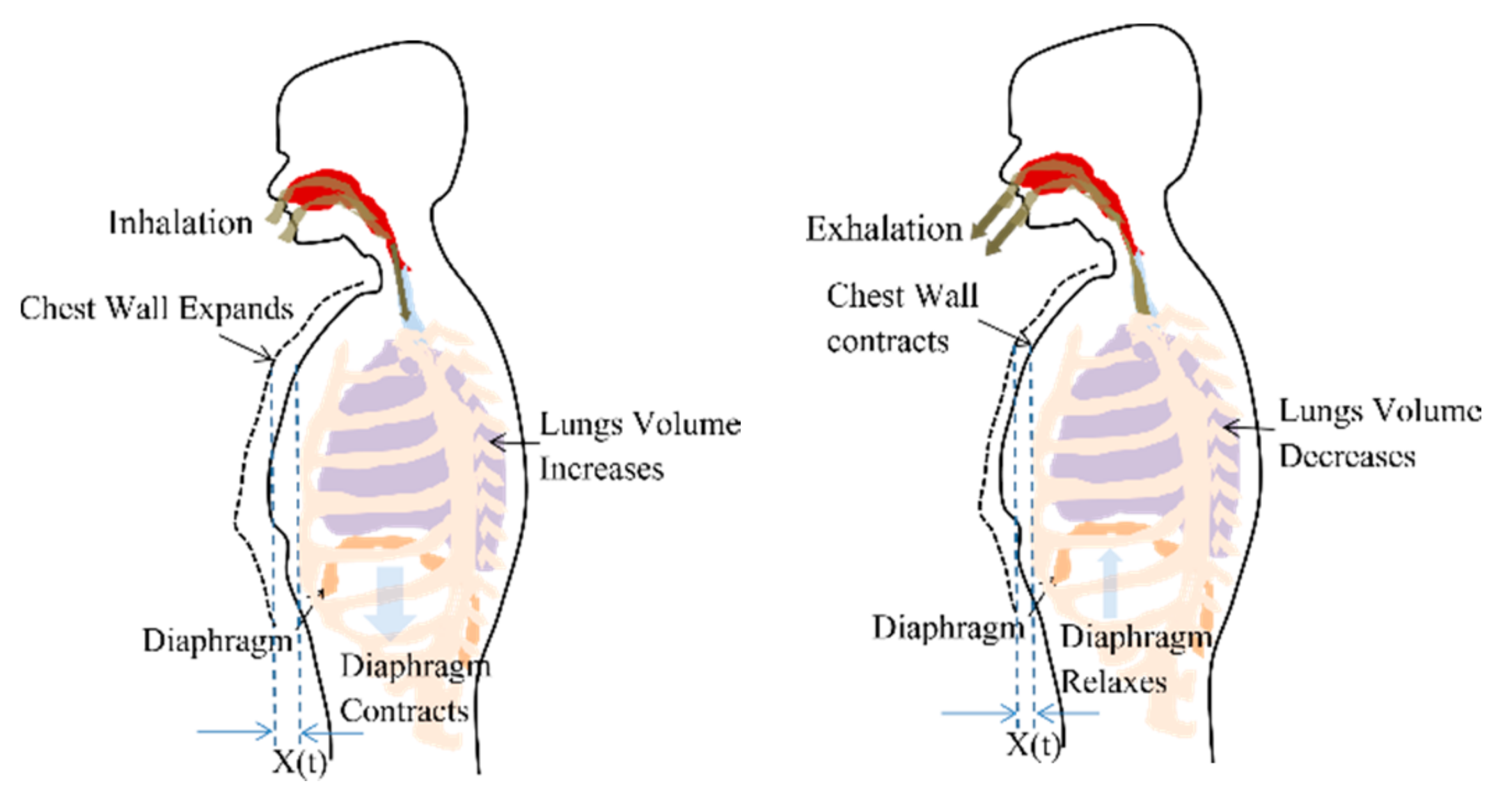
2.1. Respiration Signal
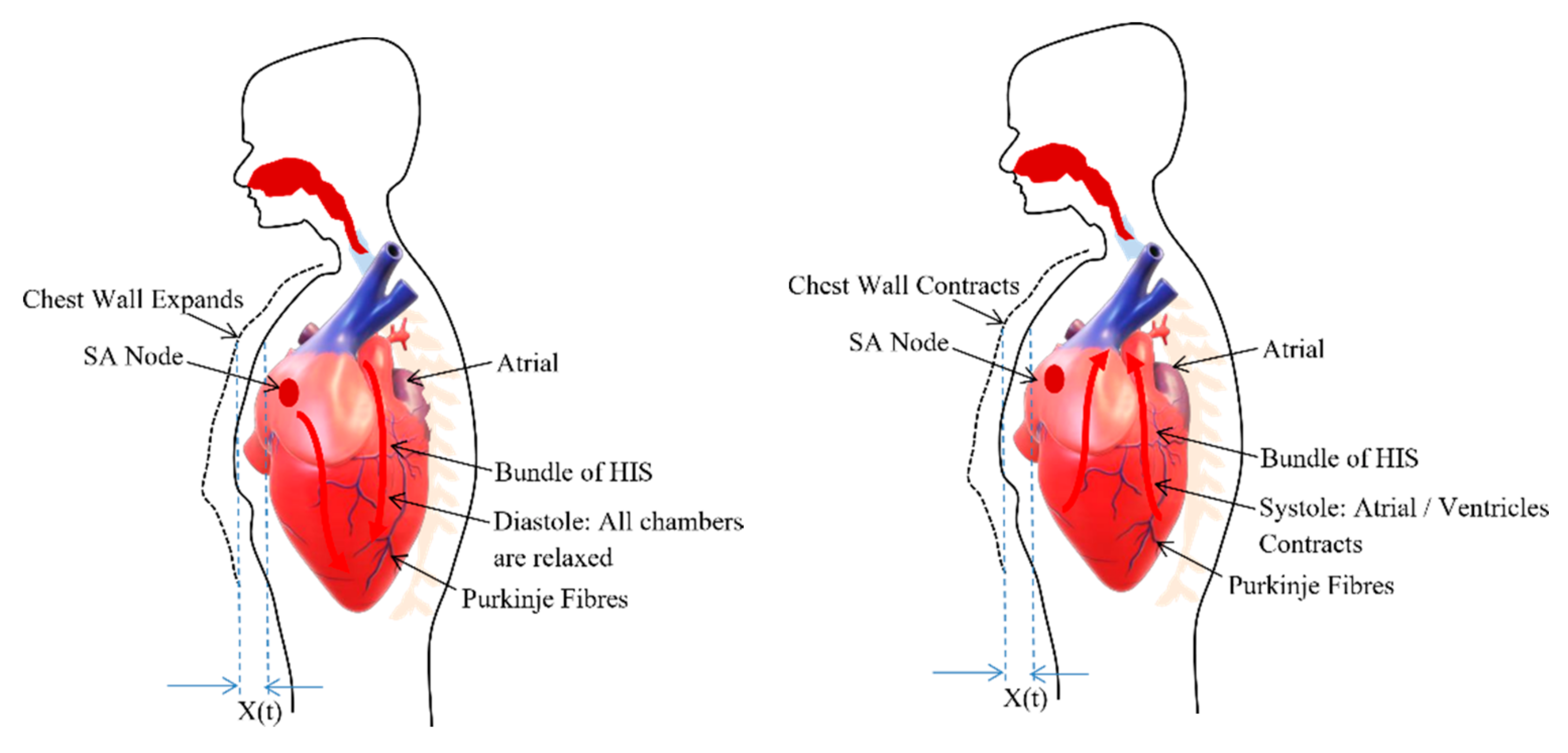
2.2. Cardiac Signal
3. Experimental Results and Discussion
3.1. Simulation Result
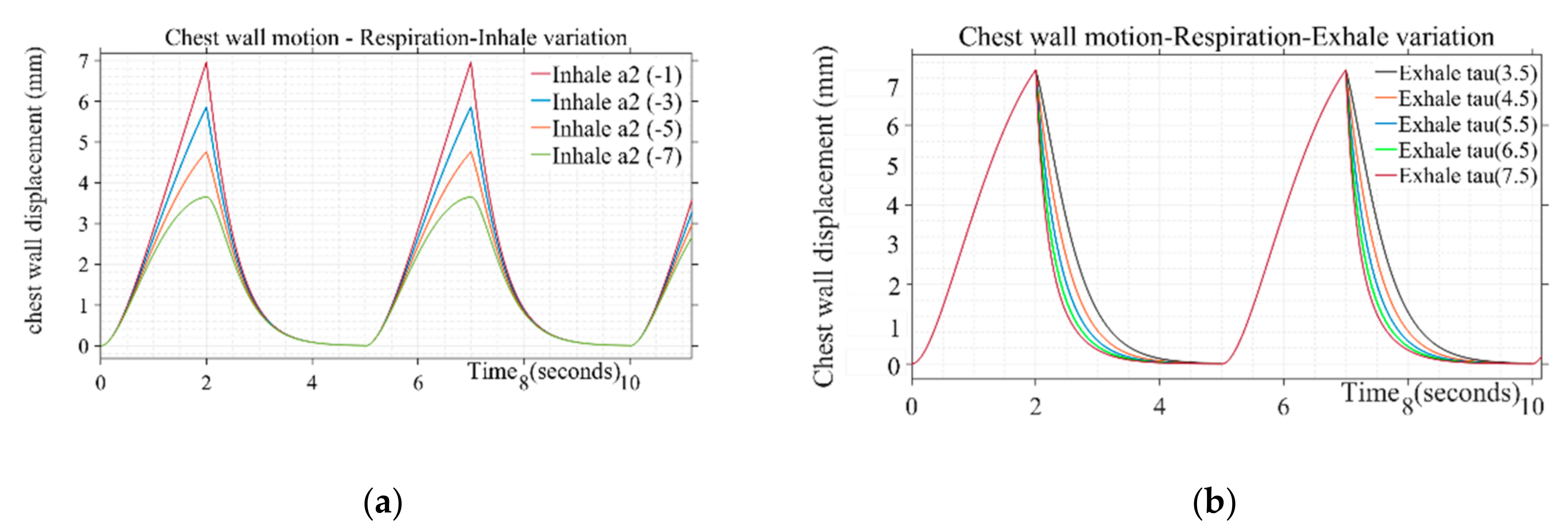
3.1.1. Chest Wall Motion—Respiration
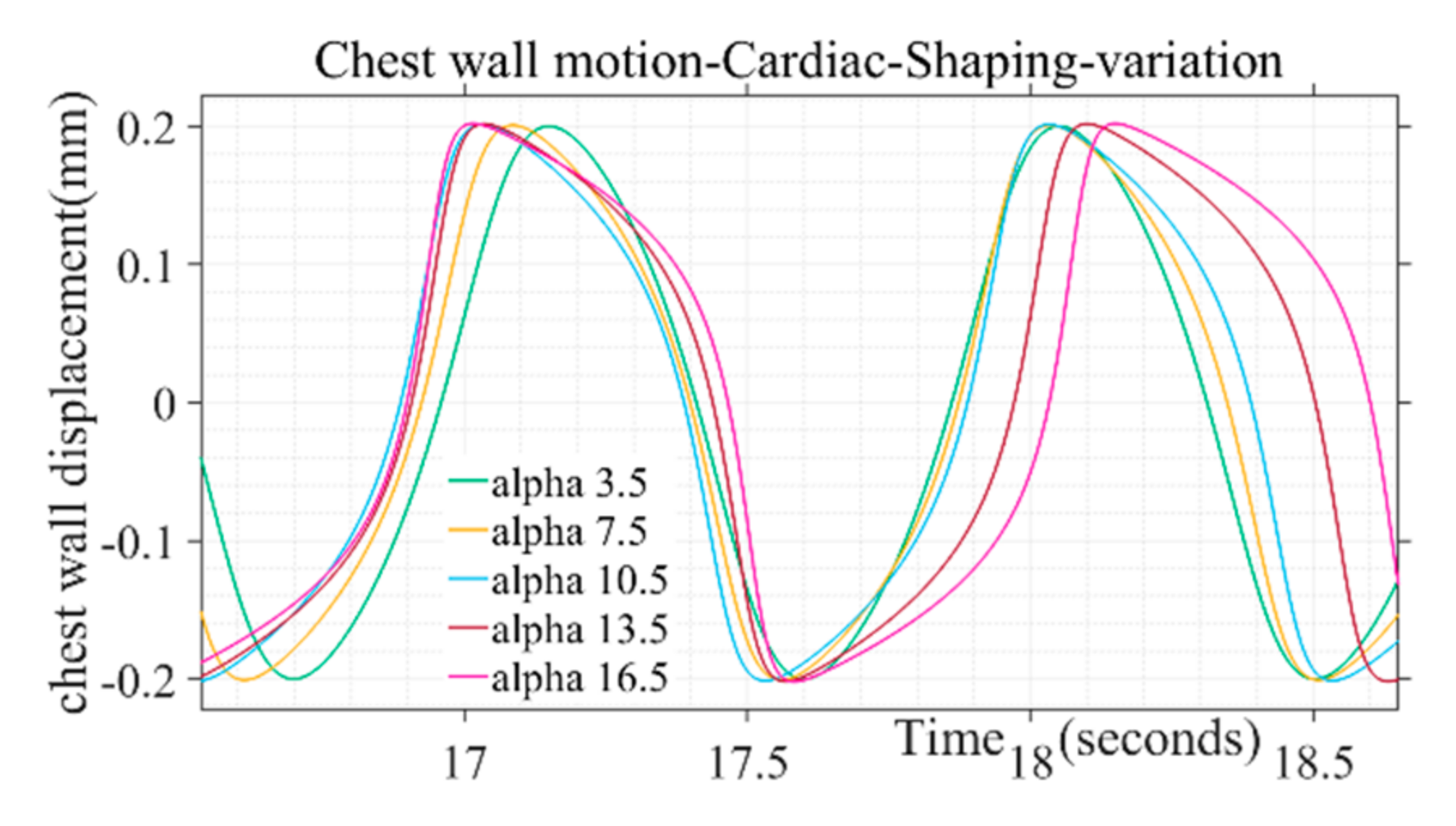
3.1.2. Chest Wall Motion—Cardiac
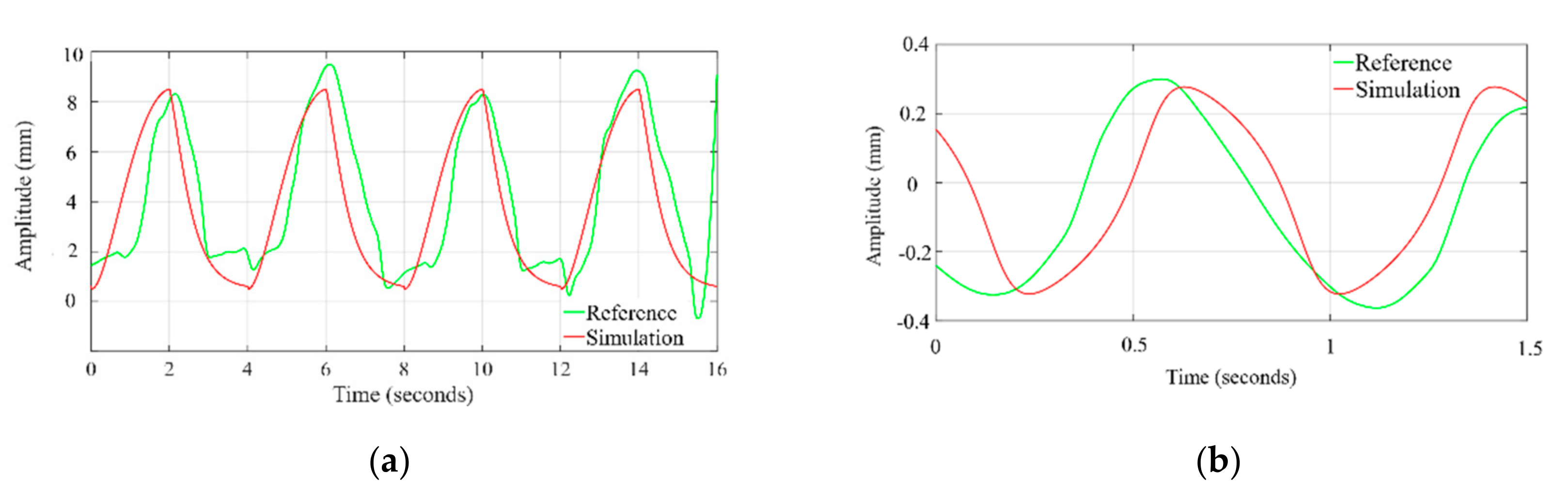
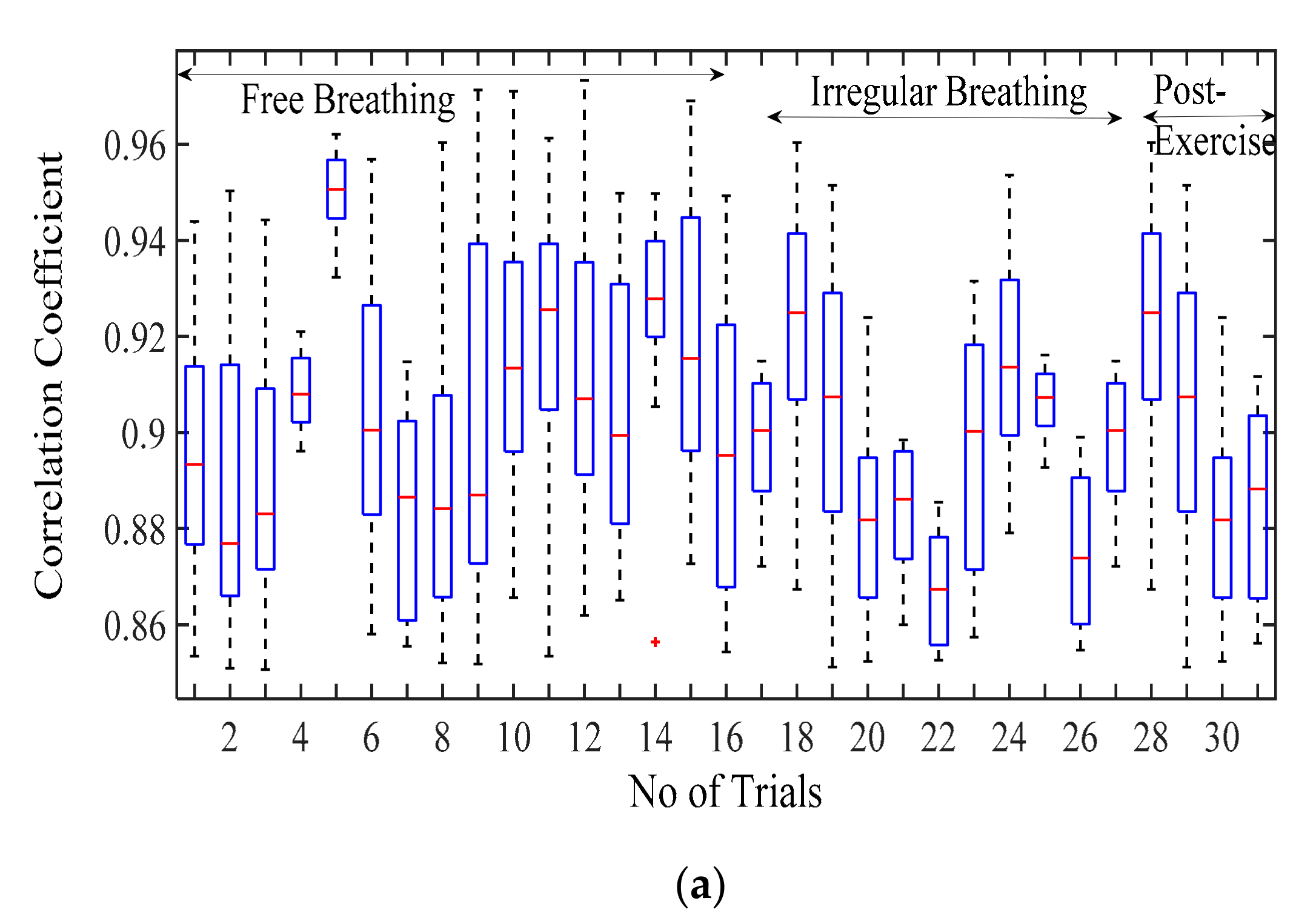
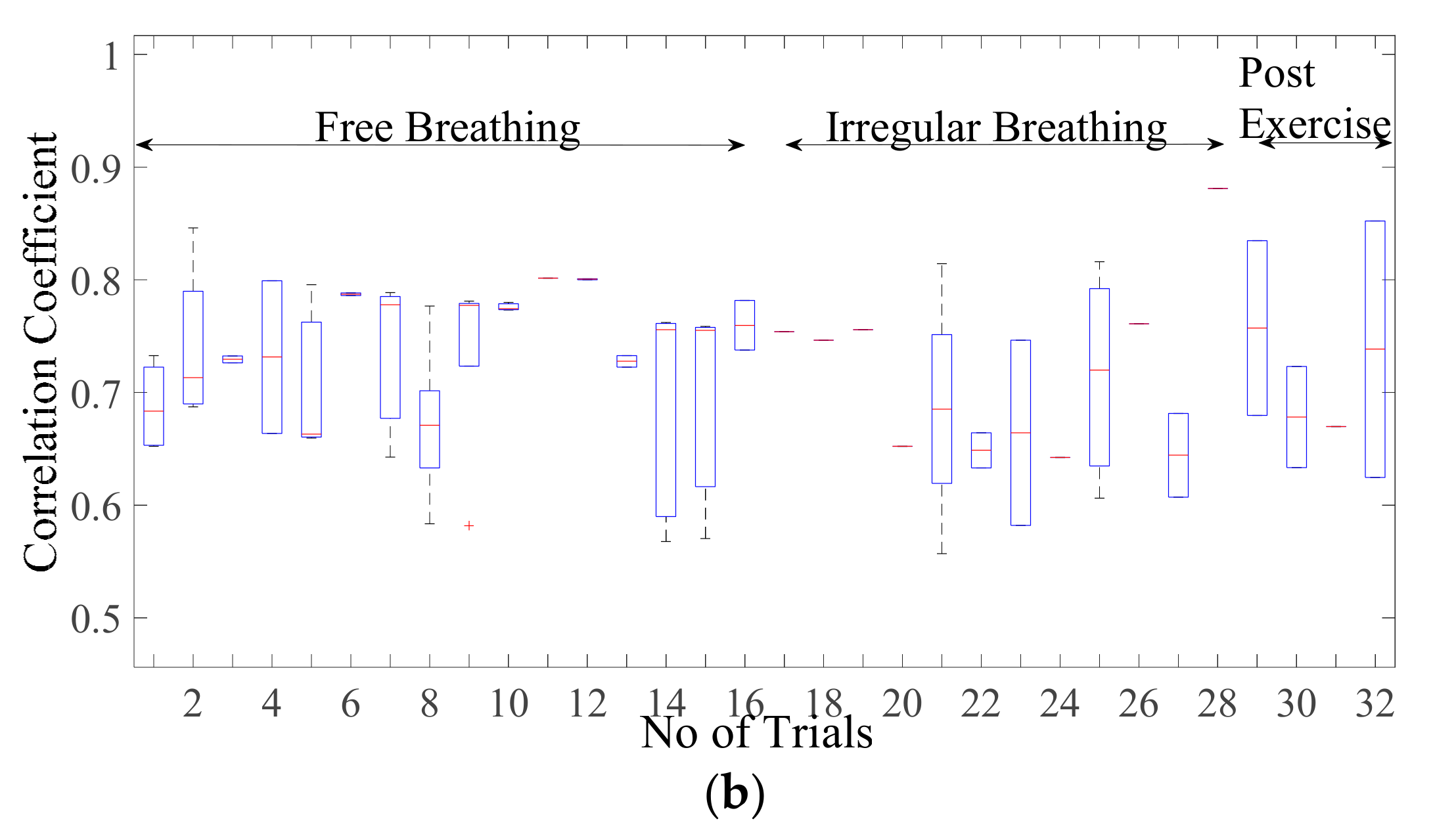
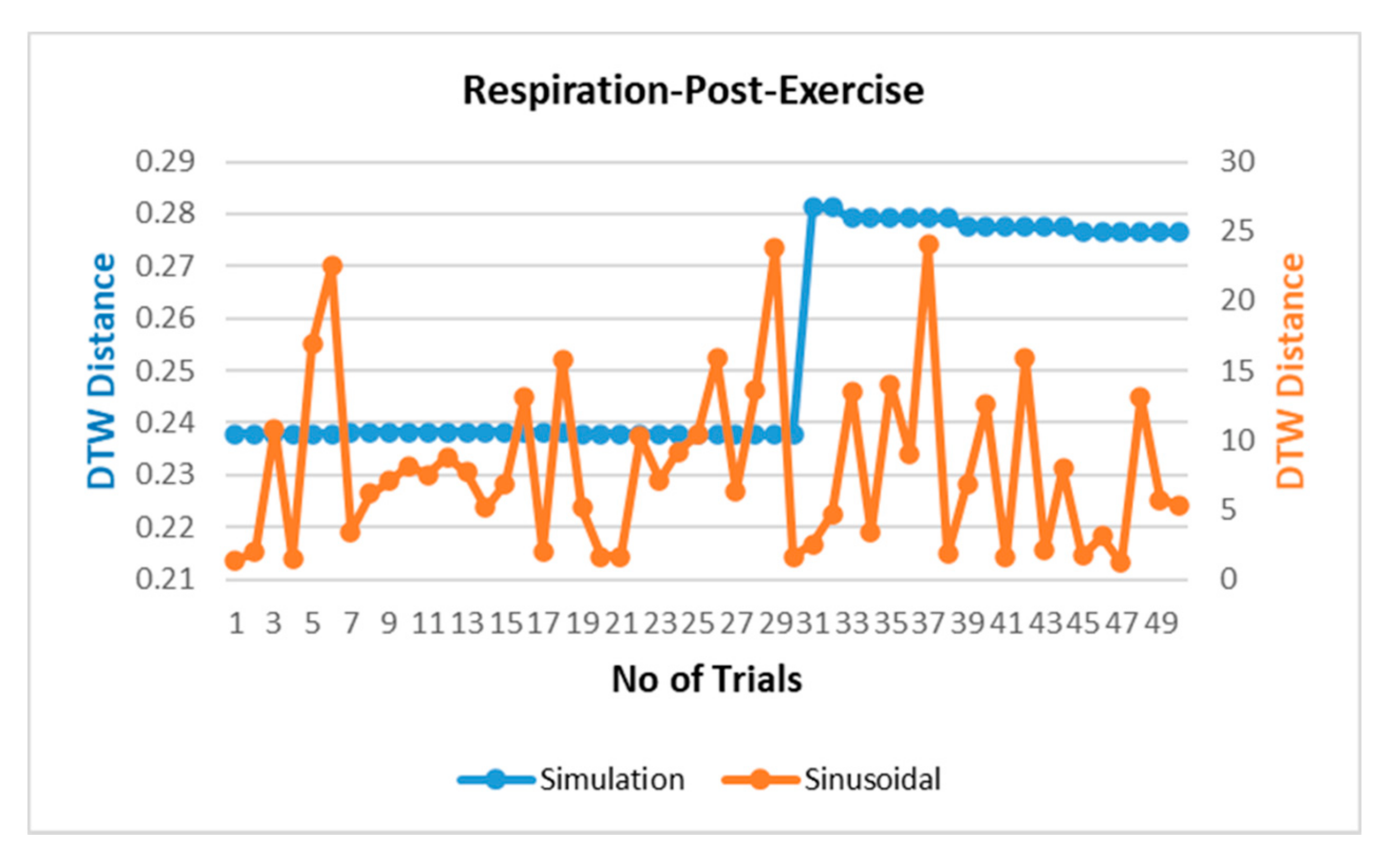
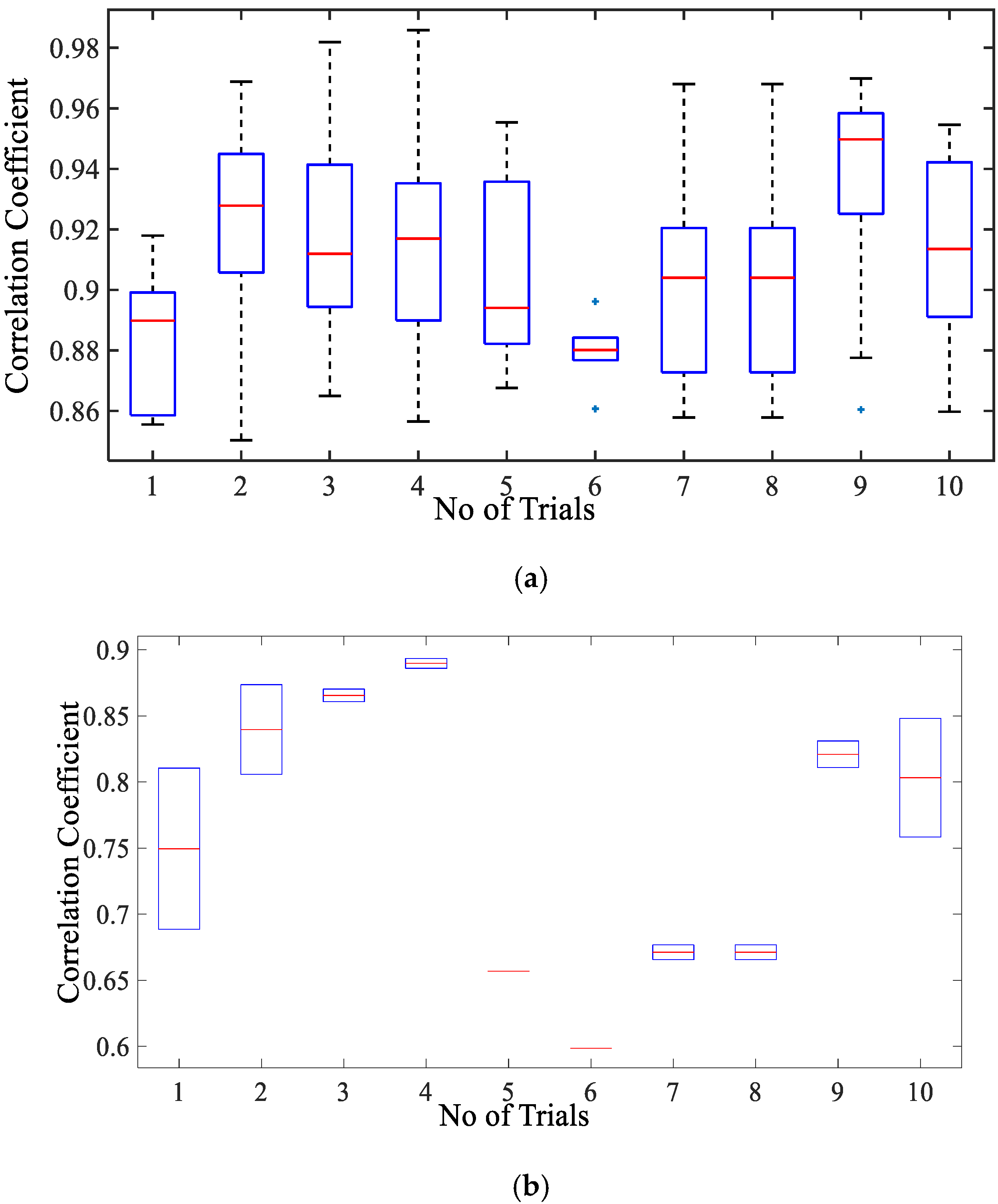
3.2. Validation and Comparision Analysis
3.2.1. Comparison of Respiration Simulation Signal
3.2.2. Comparison of Cardiac Simulation Signal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ridley, S. Cardiac scoring systems—What is their value? Anaesthesia 2003, 58, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Adib, F.; Kabelac, Z.; Katabi, D.; Miller, R. Smart Homes that Monitor Breathing and Heart Rate. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems, Seoul, Korea, 18–23 April 2015; pp. 837–846. [Google Scholar]
- Wang, Y. Assessment of respiration-induced motion and its impact on treatment outcome for lung cancer. BioMed. Res. Int. 2013, 2013, 872739. [Google Scholar] [CrossRef] [PubMed]
- Scalise, L. Non Contact Heart Monitoring. In Advances in Electrocardiograms—Methods and Analysis; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Kranjec, J. Non-contact heart rate and heart rate variability measurements: A review. Biomed. Signal Process. Control 2014, 13, 102–112. [Google Scholar] [CrossRef]
- Fouladi, R.F.; Oncu, A. Vital signs modeling for Doppler radar cardiorespiratory monitoring. In Proceedings of the 2013 36th International Conference on Telecommunications and Signal Processing (TSP), Rome, Italy, 2–4 July 2013; pp. 363–366. [Google Scholar]
- Tehrani, F.T. Mathematical analysis and computer simulation of the respiratory system in the new born. IEEE Trans. Biomed. Eng. 1993, 40, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiao, D.; Li, Y. An analytical model for regular respiratory signal. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 102–105. [Google Scholar]
- Xu, Y.Y.; Wu, S.Y.; Chen, C.; Chen, J.; Fang, G.Y. A Novel Method for Automatic Detection of Trapped Victims by Ultrawideband Radar. IEEE Trans. Geosci. Remote Sens. 2012, 50, 3132–3142. [Google Scholar] [CrossRef]
- Vedam, S.S.; Keall, P.; Docef, A.; Todor, D.A.; Kini, V.R.; Mohan, R. Predicting Respiratory Motion for Four-Dimensional Radiotherapy. Med. Phys. 2004, 31, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Nezirovic, A.; Tesfay, S.; Ezhil Valavan, S.; Yarovoy, A. Experimental Study on Human Being Detection using UWB Radar. In Proceedings of the International Radar Symposium, Krakow, Poland, 24–26 May 2006. [Google Scholar]
- Li, X.; Qiao, D.; Li, Y.; Dai, H. A Novel Through-wall Respiration Detection Algorithm Using UWB Radar. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Lujan, A.E.; Larsen, E.W.; Balter, J.M.; Ten Haken, R.K. A Method for Incorporating Organ Motion due to Breathing into 3D Dose Calculations. Med. Phys. 1999, 26, 715–720. [Google Scholar] [CrossRef]
- Seppenwoolde, Y.; Shirato, H.; Kitamura, K.; Shimizu, S.; Herk, M.V.; Lebesque, J.V. Precise and Real-Time Measurement of 3D Tumor Motion in Lung due to Breathing and Heartbeat, Measured during Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 822–834. [Google Scholar] [CrossRef]
- Kranjec, J.; Begus, S.; Gersak, G.; Drnovsek, J. Design and Clinical Evaluation of a Non-Contact Heart Rate Variability Measuring Device. Sensors 2017, 17, 2637. [Google Scholar] [CrossRef]
- Mercuri, M.; Lorato, I.R.; Liu, Y.H.; Wieringa, F.; Hoof, C.V.; Torfs, T. A Direct Phase-Tracking Doppler Radar Using Wavelet Independent Component Analysis for Non-Contact Respiratory and Heart Rate Monitoring. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 632–643. [Google Scholar] [CrossRef]
- Nahar, S.; Phan, T.; Quiyum, F.; Ren, L.; Fathy, A.E.; Kilic, O. An Electromagnetic Model of Human Vital Signs Detection and Its Experimental Validation. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 338–349. [Google Scholar] [CrossRef]
- Sekine, M.; Maeno, K. Non-contact heart rate detection using periodic variation in Doppler frequency. In Proceedings of the IEEE Sensors Applications Symposium, San Antonio, TX, USA, 22–24 February 2011. [Google Scholar]
- Morgan, D.R.; Zierdt, M.G. Novel signal processing techniques for Doppler radar cardiopulmonary sensing. Signal Process. 2009, 89, 45–66. [Google Scholar] [CrossRef]
- Ko, S.M. Assessment of left ventricular ejection fraction and regional wall motion with 64-slice multidetector CT: A comparison with two-dimensional transthoracic echocardiography. Br. J. Radiol. 2010, 83, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.S.V.; Saksena, F.B.; Ranganathan, N.; Sivaciyan, V. Precordial Pulsations. In The Art and Science of Cardiac Physical Examination. Contemporary Cardiology; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar]
- Weishaupt, F. Vital Sign Localization and Measurement Using an LFMCW MIMO Radar. In Proceedings of the 19th International Radar Symposium (IRS), Bonn, Germany, 20–22 June 2018; pp. 1–8. [Google Scholar]
- Mikhelson, I. Remote Sensing of Heart Rate and Patterns of Respiration on a Stationary Subject Using 94-GHz Millimeter-Wave Interferometry. IEEE Trans. Biomed. Eng. 2011, 58, 1671–1677. [Google Scholar] [CrossRef]
- Obeid, D.; Issa, G.; Sadek, S.; Zaharia, G.; Zein, G.E. Position-Free Vital Sign Monitoring: Measurements and Processing. In Advanced Biosignal Processing and Diagnostic Methods; Hintermüller, C., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Kaneko, H.; Horie, J. Breathing Movements of the Chest and Abdominal Wall in Healthy Subjects. Respir. Care 2012, 57, 1442. [Google Scholar] [CrossRef]
- Tukanova, K.; Papi, E.; Jamel, S.; Hanna, G.B.; McGregor, A.H.; Markar, S.R. Assessment of chest wall movement following thoracotomy: A systematic review. J. Thorac. Dis. 2020, 12, 1031–1040. [Google Scholar] [CrossRef]
- Diaz, C.E.; Deoras, K.S.; Allen, J.L. Chest wall motion before and during mechanical ventilation in children with neuromuscular disease. Pediatr. Pulmonol. 1993, 16, 89–95. [Google Scholar] [CrossRef]
- Kumar, N.; Akangire, G.; Sullivan, B.; Fairchild, K.; Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: Big data to the forefront. Pediatr. Res. 2020, 87, 210–220. [Google Scholar] [CrossRef]
- Das, V. Antenna evaluation of a non-contact vital signs sensor for continuous heart and respiration rate monitoring. In Proceedings of the 2012 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), Santa Clara, CA, USA, 15–18 January 2012. [Google Scholar]
- Lin, S.; Guo, N.R. Modeling and Simulation of Respiratory Control with LabVIEW. J. Med. Biol. Eng. 2012, 32, 51–60. [Google Scholar] [CrossRef]
- Riddle, W. A model for the relation between respiratory neural and mechanical outputs. II. Methods. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 979–989. [Google Scholar] [CrossRef]
- Feher, J. Quantitative Human Physiology, 2nd ed.; Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2016; pp. 446–454. [Google Scholar]
- Carroll, R. Elsevier’s Integrated Physiology, 1st ed.; Mosby Elsevier: Philadelphia, PA, USA, 2006; pp. 65–75. [Google Scholar]
- Van der Pol, B.; Van der Mark, J. The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1928, 6, 763–775. [Google Scholar] [CrossRef]
- Gois, S.; Savi, M. An analysis of heart rhythm dynamics using a three-coupled oscillator model. Chaos Solitons Fractals 2009, 41, 2553–2565. [Google Scholar] [CrossRef]
- Shafiq, G.; Veluvolu, K.C. Multimodal chest surface motion data for respiratory and cardiovascular monitoring applications. Sci. Data 2017, 4, 170052. [Google Scholar] [CrossRef] [PubMed]
- Groote, A. Chest wall motion during tidal breathing. J. Appl. Phys. 1997, 83, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.; Singh, M. Three-dimensional reconstruction of cardiac displacement patterns on the chest wall during the P, QRS and T-segment of the ECG by laser speckle interferometry. Med. Biol. Eng. Comput. 1989, 27, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, L.; Gupta, S. Image recognition using coefficient of correlation and structural similarity index in uncontrolled environment. Int. J. Comput. Appl. 2012, 59, 32–39. [Google Scholar] [CrossRef]
- Starovoitov, V.; Eldarova, E.E.; Iskakov, K.T. Comparative analysis of the SSIM index and the pearson coefficient as a criterion for image similarity. Eurasian J. Math. Comput. Appl. 2020, 8, 76–90. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Wu, Z. Dynamic Time Warping Distance Method for Similarity Test of Multipoint Ground Motion Field. Math. Probl. Eng. 2010, 2010, 749517. [Google Scholar] [CrossRef]


| Pulse Duration ( | 4, 5 |
| Inhale shaping () | (−1), (−3), (−5), (−7) |
| Exhale shaping (τ) | 3.5, 4.5, 5.5, 6.5, 7.5 |
| Peak amplitude | 0.035, 0.045, 0.05, 0.06, 0.07 |
| Peak amplitude | 0.2, 0.5 |
| Pulse Duration | 50, 60, 70, 80, 90, 110 |
| Pulse Shaping (α) | 3.5, 7.5, 10.5, 13.5, 16.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Rehman, S.U.; Yongchareon, S.; Chong, P.H.J. Modelling of Chest Wall Motion for Cardiorespiratory Activity for Radar-Based NCVS Systems. Sensors 2020, 20, 5094. https://doi.org/10.3390/s20185094
Singh A, Rehman SU, Yongchareon S, Chong PHJ. Modelling of Chest Wall Motion for Cardiorespiratory Activity for Radar-Based NCVS Systems. Sensors. 2020; 20(18):5094. https://doi.org/10.3390/s20185094
Chicago/Turabian StyleSingh, Anuradha, Saeed Ur Rehman, Sira Yongchareon, and Peter Han Joo Chong. 2020. "Modelling of Chest Wall Motion for Cardiorespiratory Activity for Radar-Based NCVS Systems" Sensors 20, no. 18: 5094. https://doi.org/10.3390/s20185094
APA StyleSingh, A., Rehman, S. U., Yongchareon, S., & Chong, P. H. J. (2020). Modelling of Chest Wall Motion for Cardiorespiratory Activity for Radar-Based NCVS Systems. Sensors, 20(18), 5094. https://doi.org/10.3390/s20185094







