Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Treatments and Wine Samples
2.2. Electronic Nose
2.3. Chemical Analysis of Glycoconjugates and Volatile Phenols
2.4. Sensory Evaluation-Consumer Test
2.5. Statistical Analysis and Machine Learning Modeling
3. Results
3.1. Electronic Nose Results
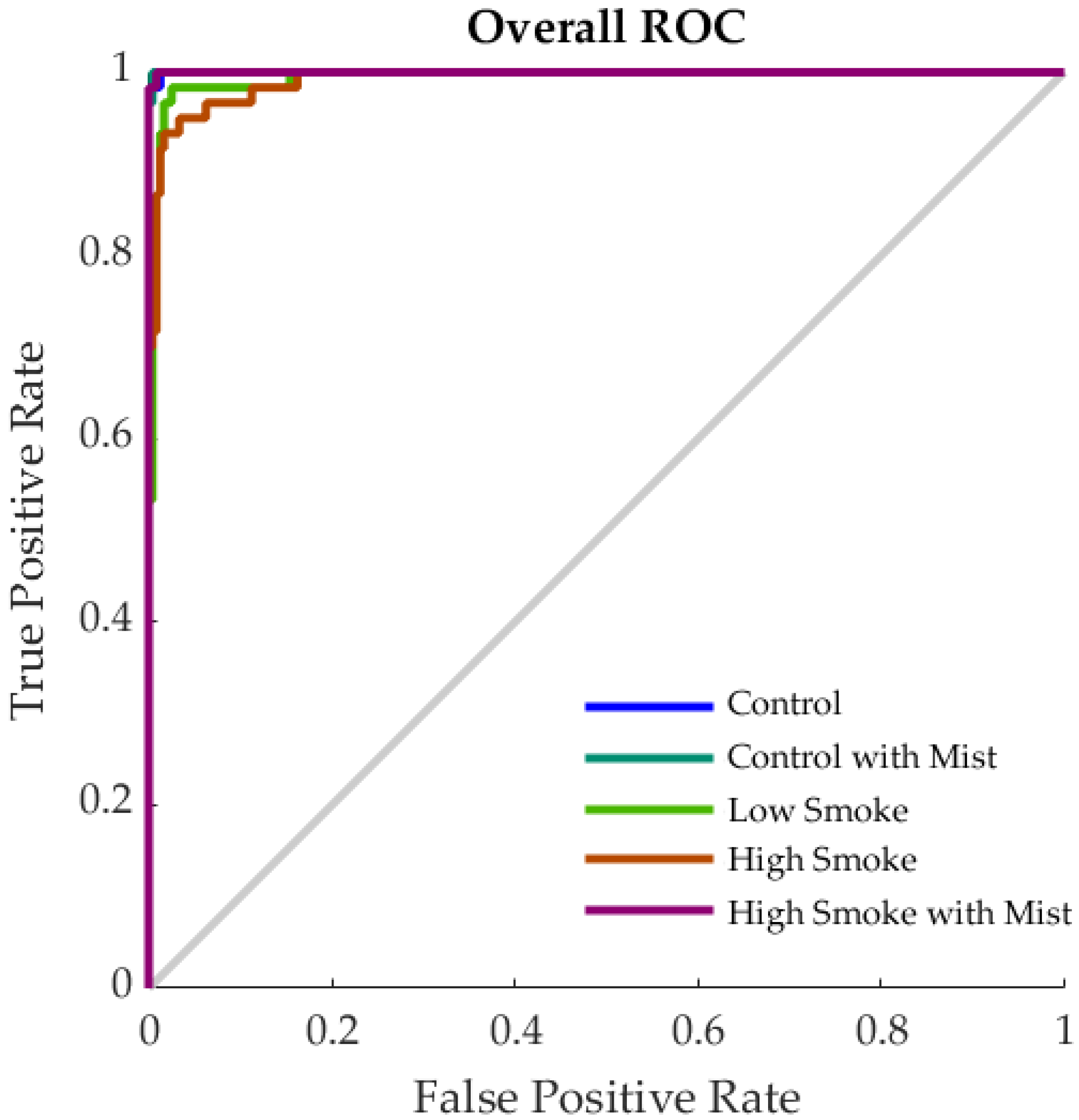
3.2. Machine Learning Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennison, K.; Wilkinson, K.L.; Pollnitz, A.; Williams, H.; Gibberd, M.R. Effect of smoke application to field–grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Aust. J. Grape Wine Res. 2011, 17, S5–S12. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.D.; Wilkinson, K.L. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Physiol. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and Glycosylation of Smoke-Derived Volatile Phenols by Cabernet Sauvignon Grapes and Their Subsequent Fate during Winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, C.; Holland, G.; Prein, A.; Done, J.; Buckley, B.; Chan, P.; Leplastrier, M.; Dyer, A. Severe Weather in a Changing Climate; Insurance Australia Group and National Center for Atmospheric Research, November. Insurance Australia Group Limited, 2019. Available online: https://www.iag.com.au/sites/default/files/documents/Severe-weather-in-a-changing-climate-report-011119.pdf (accessed on 3 August 2020).
- Fuentes, S.; Tongson, E.J.; De Bei, R.; Gonzalez Viejo, C.; Ristic, R.; Tyerman, S.; Wilkinson, K. Non-Invasive Tools to Detect Smoke Contamination in Grapevine Canopies, Berries and Wine: A Remote Sensing and Machine Learning Modeling Approach. Sensors 2019, 19, 3335. [Google Scholar] [CrossRef] [Green Version]
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography–tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of a HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2012, 61, 25–33. [Google Scholar] [CrossRef]
- Cipriano, D.; Capelli, L. Evolution of Electronic Noses from Research Objects to Engineered Environmental Odour Monitoring Systems: A Review of Standardization Approaches. Biosensors 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; DeWeerth, S.P. Odor discrimination using steady-state and transient characteristics of tin-oxide sensors. Sens. Actuators B Chem. 1995, 28, 123–128. [Google Scholar] [CrossRef]
- Roussel, S.; Forsberg, G.; Steinmetz, V.; Grenier, P.; Bellon-Maurel, V. Optimisation of electronic nose measurements. Part I: Methodology of output feature selection. J. Food Eng. 1998, 37, 207–222. [Google Scholar] [CrossRef]
- Carmel, L.; Levy, S.; Lancet, D.; Harel, D. A feature extraction method for chemical sensors in electronic noses. Sens. Actuators B Chem. 2003, 93, 67–76. [Google Scholar] [CrossRef]
- Ayhan, B.; Kwan, C.; Zhou, J.; Kish, L.B.; Benkstein, K.D.; Rogers, P.H.; Semancik, S. Fluctuation enhanced sensing (FES) with a nanostructured, semiconducting metal oxide film for gas detection and classification. Sens. Actuators B Chem. 2013, 188, 651–660. [Google Scholar] [CrossRef]
- Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in medical diagnostics. Curr. Med. Chem. 2019, 26, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Buttner, W.J.; Linnell, B.R.; Ramesham, R. Electronic nose for space program applications. Sens. Actuators B Chem. 2003, 93, 7–16. [Google Scholar] [CrossRef]
- Ryan, M.A.; Zhou, H.; Buehler, M.G.; Manatt, K.S.; Mowrey, V.S.; Jackson, S.P.; Kisor, A.K.; Shevade, A.V.; Homer, M.L. Monitoring space shuttle air quality using the jet propulsion laboratory electronic nose. IEEE Sens. J. 2004, 4, 337–347. [Google Scholar] [CrossRef]
- Li, W.; Leung, H.; Kwan, C.; Linnell, B.R. E-nose vapor identification based on Dempster–Shafer fusion of multiple classifiers. IEEE Trans. Instrum. Meas. 2008, 57, 2273–2282. [Google Scholar] [CrossRef]
- Peveler, W.J.; Parkin, I.P. Electronic Noses: The Chemistry of Smell and Security; Wortley, R., Sidebottom, A., Tilley, N., Laycock, Eds.; Routledge: Abingdon, Oxon, UK; New York, NY, USA, 2019; pp. 384–392. ISBN 9780415826266. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Tech. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Yan, B.; Zhang, L.; Gu, Y. Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection. Sensors 2019, 19, 45. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Viejo, C.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Turner, A.P.; Magan, N. Electronic noses and disease diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. Lebensm-Wiss Technol 1998, 31, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses: Powerful tools in meat quality assessment. Meat. Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic noses and tongues in wine industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, J.; Santos, J.P.; Horrillo, M.C. Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Elsevier: Cambridge, MA, USA, 2016; pp. 137–148, 301–307. ISBN 9780128002438. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; de Andrade Lima, L.L.; Ferreira, T.A. Wine quality rapid detection using a compact electronic nose system: Application focused on spoilage thresholds by acetic acid. LWT 2019, 108, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.M.; Duncan, S.E.; Zoecklein, B.W. Aroma characterization of Petit Manseng wines using sensory consensus training, SPME GC-MS, and electronic nose analysis. Am. J. Enol. Vitic. 2017, 68, 112–119. [Google Scholar] [CrossRef]
- Han, F.; Zhang, D.; Aheto, J.H.; Feng, F.; Duan, T. Integration of a low-cost electronic nose and a voltammetric electronic tongue for red wines identification. J. Food Sci. 2020, 8, 4330–4339. [Google Scholar] [CrossRef]
- Fan, H.; Hernandez Bennetts, V.; Schaffernicht, E.; Lilienthal, A.J. Towards gas discrimination and mapping in emergency response scenarios using a mobile robot with an electronic nose. Sensors 2019, 19, 685. [Google Scholar] [CrossRef] [Green Version]
- Valente, J.; Almeida, R.; Kooistra, L. A Comprehensive Study of the Potential Application of Flying Ethylene-Sensitive Sensors for Ripeness Detection in Apple Orchards. Sensors 2019, 19, 372. [Google Scholar] [CrossRef] [Green Version]
- Van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef]
- Fudge, A.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Fudge, A.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.; Hayasaka, Y.; Fudge, A.; Wilkinson, K.L. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Prieto, N.; Gay, M.; Vidal, S.; Aagaard, O.; De Saja, J.; Rodriguez-Mendez, M. Analysis of the influence of the type of closure in the organoleptic characteristics of a red wine by using an electronic panel. Food Chem. 2011, 129, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Rodrigues, C.M.; Schäfer, T.; Crespo, J.G. Monitoring the aroma production during wine–must fermentation with an electronic nose. Biotechnol Bioeng 2002, 77, 632–640. [Google Scholar] [CrossRef]
- Wei, Y.J.; Yang, L.L.; Liang, Y.P.; Li, J.M. Application of electronic nose for detection of wine-aging methods. Adv. Mater. Res. 2014, 875–877, 2206–2213. [Google Scholar] [CrossRef]
- Apetrei, I.; Rodríguez-Méndez, M.; Apetrei, C.; Nevares, I.; Del Alamo, M.; De Saja, J. Monitoring of evolution during red wine aging in oak barrels and alternative method by means of an electronic panel test. Food Res. Int. 2012, 45, 244–249. [Google Scholar] [CrossRef]
- Lozano, J.; Arroyo, T.; Santos, J.; Cabellos, J.; Horrillo, M. Electronic nose for wine ageing detection. Sens. Actuators B Chem. 2008, 133, 180–186. [Google Scholar] [CrossRef]
- Cynkar, W.; Dambergs, R.; Smith, P.; Cozzolino, D. Classification of Tempranillo wines according to geographic origin: Combination of mass spectrometry based electronic nose and chemometrics. Anal. Chim. Acta 2010, 660, 227–231. [Google Scholar] [CrossRef]
- Macías, M.M.; Manso, A.G.; Orellana, C.J.G.; Velasco, H.M.G.; Caballero, R.G.; Chamizo, J.C.P. Acetic acid detection threshold in synthetic wine samples of a portable electronic nose. Sensors 2013, 13, 208–220. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Long, F.; Guo, C.; Yuan, Y.; Yue, T. Early detection of Zygosaccharomyces Rouxii—spawned spoilage in apple juice by electronic nose combined with chemometrics. Int. J. Food Microbiol. 2016, 217, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, M.; Cabellos, J.M.; Arroyo, T.; Horrillo, M. Quantification of Wine Mixtures with an electronic nose and a human Panel. Front. Bioeng. Biotechnol. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, S.; Tongson, E.; Chen, J.; Gonzalez Viejo, C. A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages 2020, 6, 39. [Google Scholar] [CrossRef]
- Valente, J.; Munniks, S.; de Man, I.; Kooistra, L. Validation of a small flying e-nose system for air pollutants control: A plume detection case study from an agricultural machine. In Proceedings of the 2018 IEEE International Conference on Robotics and Biomimetics (ROBIO), Kuala Lumpur, Malaysia, 12–15 December 2018; pp. 1993–1998. [Google Scholar]
- Muralidhara, B.; Geethanjali, B. Review on different technologies used in Agriculture. Int. J. Pure Appl. Math. 2018, 119, 4117–4134. [Google Scholar]



| Sensor Name | Gases | Manufacturer |
|---|---|---|
| MQ3 | Ethanol | Henan Hanwei Electronics Co., Ltd., Henan, China |
| MQ4 | Methane | |
| MQ7 | Carbon monoxide (CO) | |
| MQ8 | Hydrogen | |
| MQ135 | Ammonia, alcohol, and benzene | |
| MQ136 | Hydrogen sulfide | |
| MQ137 | Ammonia | |
| MQ138 | Benzene, alcohol, and ammonia | |
| MG811 | Carbon dioxide (CO2) |
| Compound | Abbreviation/Label | Sample |
|---|---|---|
| Glycoconjugates | ||
| Syringol gentiobiosides | SyGG | Berries/Wine |
| Syringol glucosides | SyMG | Berries/Wine |
| Syringol pentosylglucosides | SyPG | Berries/Wine |
| Cresol glucosylpentosides | CrPG | Berries/Wine |
| Cresol gentiobioside | CrGG | Berries |
| Cresol glucosides | CrMG | Berries |
| Cresol rutinosides | CrRG | Berries/Wine |
| Guaiacol pentosylglucosides | GuPG | Berries/Wine |
| Guaiacol gentiobiosides | GuGG | Berries/Wine |
| Guaiacol rutinosides | GuRG | Berries/Wine |
| Guaiacol glucosides | GuMG | Berries/Wine |
| Methylguaiacol pentosylglucosides | MGuPG | Berries/Wine |
| Methylguaiacol rutinosides | MGuRG | Berries/Wine |
| Methylguaiacol glucosides | MGuMG | Berries |
| Methylsyringol gentiobiosides | MSyGG | Berries/Wine |
| Methylsyringol pentosylglucosides | MSyPG | Berries/Wine |
| Phenol rutinosides | PhRG | Berries/Wine |
| Phenol gentiobiosides | PhGG | Berries/Wine |
| Phenol pentosylglucosides | PhPG | Berries/Wine |
| Phenol glucosides | PhMG | Berries/Wine |
| Volatile Phenols | ||
| Guaiacol | Guaiacol | Berries/Wine |
| 4-Methylguaiacol | 4-Methylguaiacol | Berries/Wine |
| Phenol | Phenol | Berries |
| o-Cresol | o-Cresol | Berries/Wine |
| Total m/p-cresols | Total m/p-cresol | Berries |
| m-Cresol | m-Cresol | Berries/Wine |
| p-Cresol | p-Cresol | Berries/Wine |
| Syringol | Syringol | Berries/Wine |
| 4-Methylsyringol | 4-Methylsyringol | Berries/Wine |
| Total cresols | Cresols | Berries |
| Compound | Berries 1 h After Smoking | Berries at Harvest | Wine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| Syringol gentiobioside | 2.37 | 56.93 | 15.42 | 6.30 | 772.81 | 186.55 | 10.43 | 582.11 | 152.58 |
| Syringol monoglucoside | 0.14 | 26.97 | 6.38 | 2.65 | 68.34 | 19.22 | 0.36 | 14.54 | 4.26 |
| Syringol pentosylglucosides | 0.76 | 4.52 | 1.79 | 6.41 | 369.14 | 88.76 | 1.70 | 103.37 | 27.73 |
| Cresol glucosylpentosides | 8.07 | 47.12 | 18.13 | 41.69 | 1395.52 | 382.63 | 0.40 | 17.67 | 5.28 |
| Cresol gentiobioside | 0.18 | 0.71 | 0.45 | 1.94 | 6.46 | 3.55 | NA | NA | NA |
| Cresol monoglucoside | 0.24 | 61.87 | 16.36 | 0 | 35.47 | 8.70 | NA | NA | NA |
| Cresol rutinoside | 1.62 | 13.34 | 4.90 | 3.11 | 122.07 | 38.35 | 2.91 | 133.85 | 40.55 |
| Guaiacol pentosylglucosides | 2.29 | 25.61 | 7.57 | 15.76 | 1233.46 | 268.39 | 5.30 | 330.36 | 80.47 |
| Guaiacol gentiobioside | 0.05 | 1.38 | 0.40 | 0.54 | 67.44 | 16.33 | 0.30 | 2.81 | 0.99 |
| Guaiacol rutinoside | 0 | 1.35 | 0.48 | 1.13 | 32.03 | 9.97 | 0 | 48.60 | 15.24 |
| Guaiacol monoglucoside | 0.03 | 30.04 | 7.07 | 1.22 | 30.25 | 7.15 | 0.12 | 12.60 | 3.46 |
| Methylguaiacol pentosylglucosides | 0.55 | 11.51 | 3.29 | 6.79 | 266.50 | 57.32 | 1.43 | 51.79 | 12.72 |
| Methylguaiacol rutinoside | 0.60 | 5.58 | 1.89 | 6.45 | 153.06 | 44.36 | 0.79 | 40.92 | 11.97 |
| Methylguaiacol monoglucoside | 0 | 0 | 0 | 0.94 | 11.52 | 3.89 | NA | NA | NA |
| Methylsyringol gentiobioside | 0.33 | 13.34 | 3.49 | 2.53 | 302.51 | 72.52 | 0.15 | 30.69 | 7.41 |
| Methylsyringol pentosylglucosides | 0.07 | 0.39 | 0.17 | 1.57 | 34.84 | 10.36 | 0.20 | 8.35 | 2.46 |
| Phenol rutinoside | 0.31 | 3.78 | 1.26 | 3.75 | 175.57 | 53.28 | 1.42 | 77.58 | 23.40 |
| Phenol gentiobioside | 0.01 | 0.61 | 0.15 | 0 | 28.54 | 6.57 | 0.08 | 6.22 | 1.70 |
| Phenol pentosylglucosides | 1.44 | 24.97 | 7.02 | 16.21 | 812.10 | 215.13 | 0.53 | 22.59 | 6.31 |
| Phenol monoglucoside | 0.04 | 2.55 | 0.63 | 0.99 | 21.52 | 5.65 | 0.74 | 43.48 | 11.86 |
| Guaiacol | 2.39 | 139.72 | 41.57 | 2.06 | 12.97 | 5.08 | 0 | 39.00 | 11.73 |
| 4-Methylguaiacol | 3.54 | 27.72 | 9.50 | 3.52 | 4.45 | 3.80 | 0 | 5.00 | 1.40 |
| Phenol | 1.40 | 85.68 | 21.12 | 1.26 | 26.38 | 9.61 | NA | NA | NA |
| o-Cresol | 1.65 | 54.02 | 16.31 | 1.74 | 8.08 | 4.02 | 0 | 14.00 | 4.87 |
| Total m/p-cresol | 0.56 | 63.07 | 16.01 | 0.52 | 7.71 | 2.99 | NA | NA | NA |
| m-Cresol | 1.90 | 45.07 | 12.08 | 1.84 | 5.89 | 3.24 | 0 | 14.00 | 4.53 |
| p-Cresol | 0 | 18.00 | 4.38 | 0 | 2.04 | 0.44 | 0 | 9.00 | 2.60 |
| Syringol | 5.17 | 180.31 | 47.67 | 9.32 | 13.77 | 11.73 | 1.00 | 6.00 | 3.13 |
| 4-Methylsyringol | 1.83 | 24.36 | 6.62 | 1.75 | 2.11 | 1.83 | 0 | 0 | 0 |
| Total cresols | 2.22 | 117.08 | 32.32 | 2.26 | 15.79 | 7.01 | NA | NA | NA |
| Data/Sensory Attribute | Min | Max | Mean |
|---|---|---|---|
| Appearance liking | 0.45 | 15.00 | 7.19 |
| Overall aroma liking | 0.30 | 14.85 | 6.21 |
| Smoke aroma intensity | 0 | 15.00 | 4.98 |
| Smoke aroma liking | 0 | 15.00 | 4.72 |
| Bitter liking | 0.30 | 15.00 | 5.98 |
| Sweet liking | 0 | 14.70 | 6.16 |
| Acidity liking | 0 | 14.70 | 6.23 |
| Astringency liking | 0.30 | 15.00 | 6.27 |
| Warming liking | 0.30 | 15.00 | 6.20 |
| Overall liking | 0.30 | 14.85 | 6.07 |
| Perceived quality | 0 | 14.85 | 5.66 |
| FaceScale | 0 | 99.00 | 42.15 |
| Stage Model 1 | Samples | Accuracy | Error | Performance (Cross-Entropy) |
|---|---|---|---|---|
| Training | 180 | 99% | 1% | 0.01 |
| Validation | 60 | 93% | 7% | 0.04 |
| Testing | 60 | 92% | 8% | 0.05 |
| Overall | 300 | 97% | 3% | - |
| Stage/ Model 2 (Berries 1 h Smoke) | Samples | Observations | R | R2 | b | Performance (MSE) |
|---|---|---|---|---|---|---|
| Training | 180 | 5400 | 0.98 | 0.96 | 0.96 | 0.01 |
| Validation | 60 | 1800 | 0.96 | 0.92 | 0.97 | 0.03 |
| Testing | 60 | 1800 | 0.97 | 0.95 | 0.97 | 0.02 |
| Overall | 300 | 9000 | 0.98 | 0.95 | 0.97 | - |
| Stage/ Model 3 (Berries at Harvest) | Samples | Observations | R | R2 | b | Performance (MSE) |
| Training | 180 | 5400 | 0.99 | 0.98 | 0.97 | 0.01 |
| Validation | 60 | 1800 | 0.98 | 0.95 | 0.96 | 0.02 |
| Testing | 60 | 1800 | 0.98 | 0.97 | 0.95 | 0.01 |
| Overall | 300 | 9000 | 0.99 | 0.97 | 0.96 | - |
| Stage/ Model 4 (Wine) | Samples | Observations | R | R2 | b | Performance (MSE) |
| Training | 180 | 4320 | 0.99 | 0.99 | 0.99 | <0.01 |
| Validation | 60 | 1440 | 0.98 | 0.95 | 0.96 | 0.02 |
| Testing | 60 | 1440 | 0.98 | 0.96 | 0.95 | 0.01 |
| Overall | 300 | 7200 | 0.99 | 0.98 | 0.98 | - |
| Stage/ Model 5 (Wine Sensory) | Samples | Observations | R | R2 | b | Performance (MSE) |
| Training | 180 | 2160 | 0.98 | 0.97 | 0.97 | 0.02 |
| Validation | 60 | 720 | 0.97 | 0.94 | 0.97 | 0.04 |
| Testing | 60 | 720 | 0.97 | 0.94 | 0.97 | 0.04 |
| Overall | 300 | 3600 | 0.98 | 0.96 | 0.97 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, S.; Summerson, V.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach. Sensors 2020, 20, 5108. https://doi.org/10.3390/s20185108
Fuentes S, Summerson V, Gonzalez Viejo C, Tongson E, Lipovetzky N, Wilkinson KL, Szeto C, Unnithan RR. Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach. Sensors. 2020; 20(18):5108. https://doi.org/10.3390/s20185108
Chicago/Turabian StyleFuentes, Sigfredo, Vasiliki Summerson, Claudia Gonzalez Viejo, Eden Tongson, Nir Lipovetzky, Kerry L. Wilkinson, Colleen Szeto, and Ranjith R. Unnithan. 2020. "Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach" Sensors 20, no. 18: 5108. https://doi.org/10.3390/s20185108
APA StyleFuentes, S., Summerson, V., Gonzalez Viejo, C., Tongson, E., Lipovetzky, N., Wilkinson, K. L., Szeto, C., & Unnithan, R. R. (2020). Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach. Sensors, 20(18), 5108. https://doi.org/10.3390/s20185108











