A Novel NiFe2O4/Paper-Based Magnetoelastic Biosensor to Detect Human Serum Albumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Paper
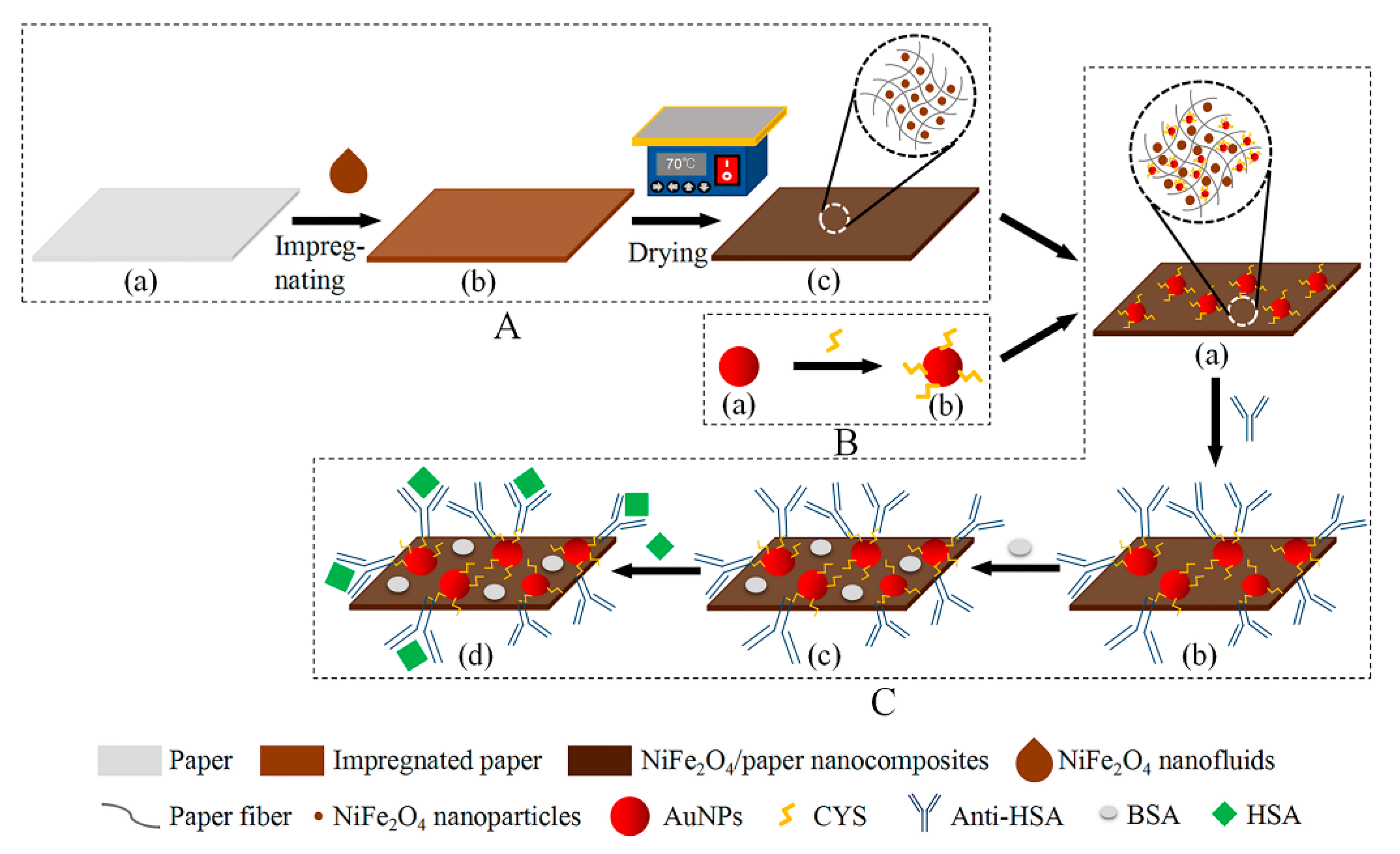
2.3. Fabrication of the NiFe2O4/Paper Nanocomposite
2.4. Fabrication of the Paper-Based ME Sensor
2.5. Antibody Immobilization
2.6. Signal Measurement
3. Results and Discussion
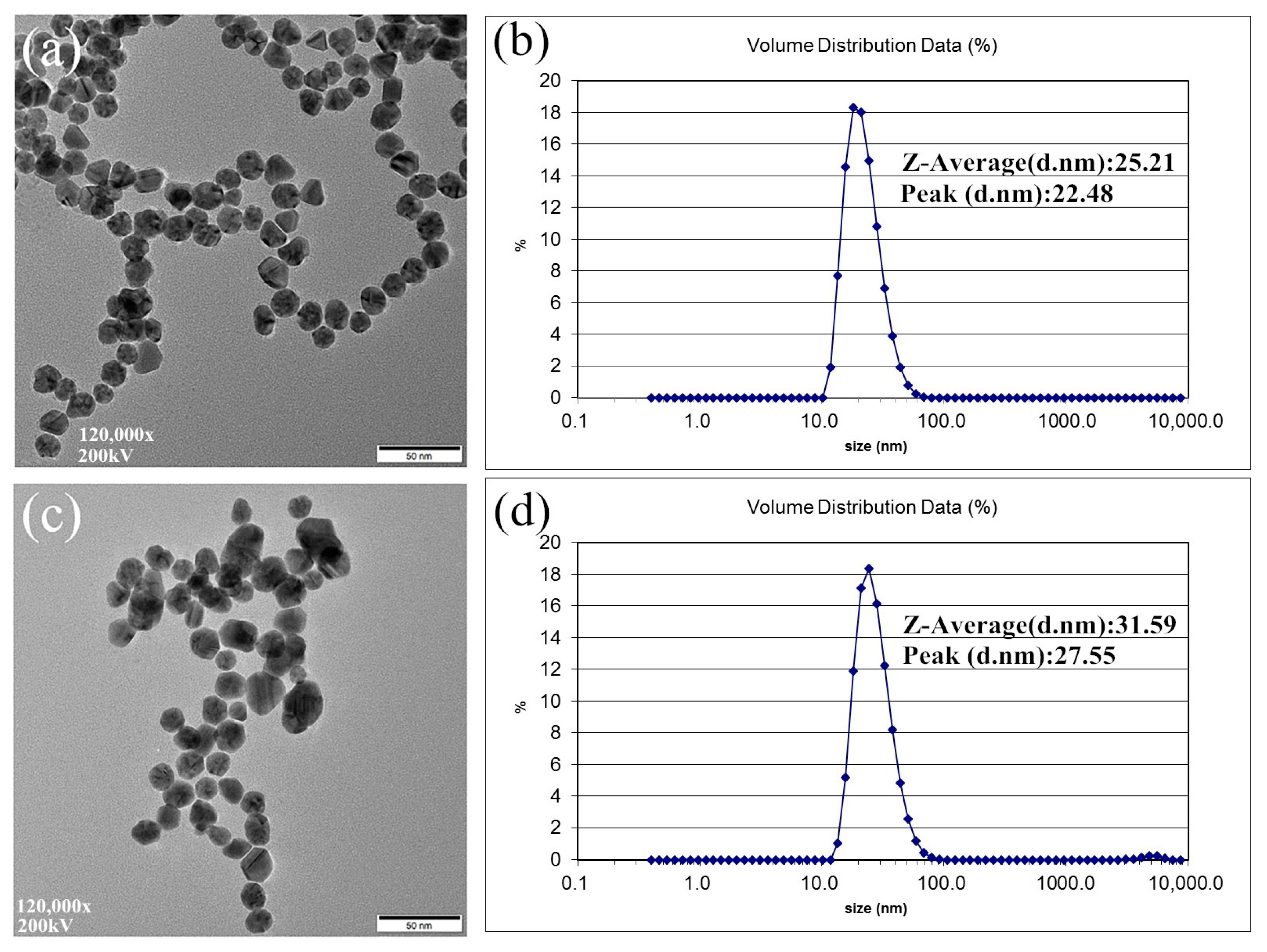
3.1. Characterization of NiFe2O4 Nanoparticles and AuNPs
3.2. Optimization of NiFe2O4 Nanofluid Concentration and the Number of Impregnation Times
3.3. Characterization of Paper and NiFe2O4/Paper Nanocomposite
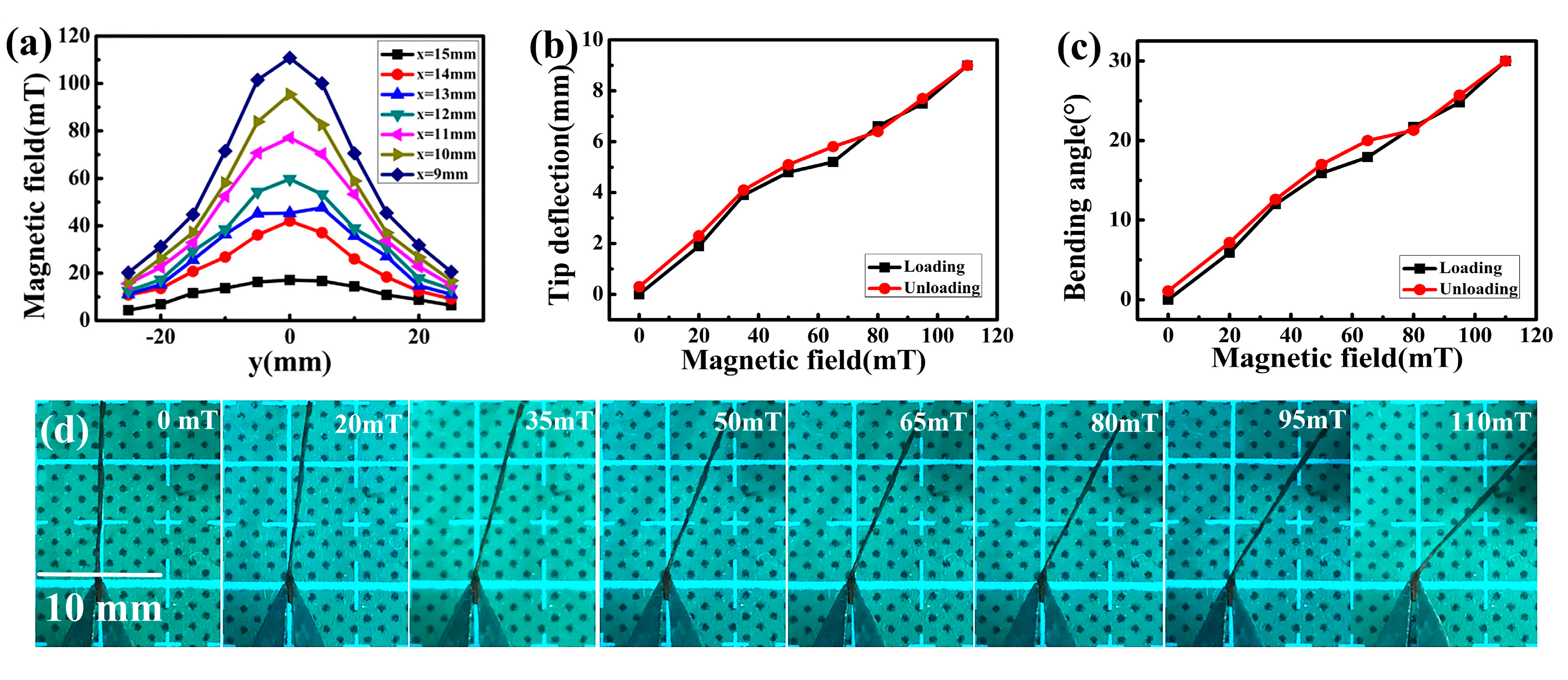
3.4. Magnet Field Strength Measurement of NiFe2O4/Paper Nanocomposite
3.5. Hysteresis Loop Measurement of the Biosensor Fabrication Process
3.6. HSA Detection
3.7. Characterization of the Preparing and Detecting Process
3.8. Specificity Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qaseem, A.; Wilt, T.; Denberg, T.D. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease. Ann. Intern. Med. 2014, 161, 82. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.M.; Nilsson, G.; Bruns, D.E.; Mcqueen, M.; Lieske, J.C.; Zakowski, J.J.; Miller, W.G. State of the art for measurement of urine albumin: Comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin. Chem. 2014, 60, 471. [Google Scholar] [CrossRef] [Green Version]
- Riella, M.C. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Friedman, A.N.; Fadem, S.Z. Reassessment of albumin as a nutritional marker in kidney disease. J. Am. Soc. Nephrol. 2010, 21, 223. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, Y.; Zhang, S.; Xu, L.; Wang, G.; Cui, J. An ultrasensitive rapid-response fluorescent probe for highly selective detection of HSA. Tetrahedron Lett. 2018, 59, 1390–1393. [Google Scholar] [CrossRef]
- Gama-Axelsson, T.; Heimbürger, O.; Stenvinkel, P.; Bárány, P.; Lindholm, B.; Qureshi, A.R. Serum albumin as predictor of nutritional status in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Guobing, X.; Lili, J.; Lihua, Z.; Tiean, X. Application of an improved biuret method to the determination of total protein in urine and cerebrospinal fluid without concentration step by use of Hitachi 7170 auto-analyzer. J. Clin. Lab. Anal. 2012, 15, 161–164. [Google Scholar] [CrossRef]
- Burenin, A.G.; Urusov, A.E.; Betin, A.V.; Orlov, A.V.; Nikitin, M.P.; Ksenevich, T.I.; Gorshkov, B.G.; Zherdev, A.V.; Dzantiev, B.B.; Nikitin, P.I. Direct immunosensing by spectral correlation interferometry: Assay characteristics versus antibody immobilization chemistry. Anal. Bioanal. Chem. 2015, 407, 3955–3964. [Google Scholar] [CrossRef]
- Lubran, M.M. The measurement of total serum proteins by the Biuret method. Ann. Clin. Lab. Sci. 1978, 8, 106. [Google Scholar]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2012, 82, 3–10. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Luckham, R.E.; Smith, A.M.; Lebert, J.M.; Davies, L.M.; Pelton, R.H.; Filipe, C.D.M.; Brennan, J.D. Development of a Bioactive Paper Sensor for Detection of Neurotoxins Using Piezoelectric Inkjet Printing of Sol-Gel-Derived Bioinks. Anal. Chem. 2011, 81, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Sadri, B.; Goswami, D.; Sala de Medeiros, M.; Pal, A.; Castro, B.; Kuang, S.; Martinez, R.V. Wearable and implantable epidermal paper-based electronics. ACS Appl. Mater. Interfaces 2018, 10, 31061–31068. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Dong, J.; Waterhouse, G.I.N.; Cheng, Z.; Ai, S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2011, 101, 110–115. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, N.; Luo, X.; Ding, S.N. Patchy gold coated Fe3O4 nanospheres with enhanced catalytic activity applied for paper-based bipolar electrode-electrochemiluminescence aptasensors. Biosens. Bioelectron. 2018, 114, 44–51. [Google Scholar] [CrossRef]
- Russell, S.M.; de la Rica, R. Paper transducers to detect plasmon variations in colorimetric nanoparticle biosensors. Sens. Actuators B Chem. 2018, 270, 327–332. [Google Scholar] [CrossRef]
- Chouler, J.; Cruz-Izquierdo, Á.; Rengaraj, S.; Scott, J.L.; Lorenzo, M.D. A screen-printed paper microbial fuel cell biosensor for detection of toxic compounds in water. Biosens. Bioelectron. 2018, 102, 49–56. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.P.; Nguyen, T.K.; Qamar, A.; Dao, D.V. Solvent-free fabrication of biodegradable hot-film flow sensor for noninvasive respiratory monitoring. J. Phys. D-Appl. Phys. 2017, 50, 215401. [Google Scholar] [CrossRef]
- Yan, W.; Wang, K.; Xu, H.; Huo, X.; Jin, Q.; Cui, D. Machine learning approach to enhance the performance of MNP-labeled lateral flow immunoassay. Nano-Micro Lett. 2019, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Haun, J.B.; Yoon, T.J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Grimes, C.A.; Roy, S.C.; Rani, S.; Cai, Q. Theory, Instrumentation and Applications of Magnetoelastic Resonance Sensors: A Review. Sensors 2011, 11, 2809–2844. [Google Scholar] [CrossRef]
- Saiz, P.G.; Gandia, D.; Lasheras, A.; Sagasti, A.; Quintana, I.; Fdez-Gubieda, M.L.; Gutiérrez, J.; Arriortua, M.I.; Lopes, A.C. Enhanced mass sensitivity in novel magnetoelastic resonators geometries for advanced detection systems. Sens. Actuators B Chem. 2019, 296, 126612. [Google Scholar] [CrossRef]
- Marín, P.; Marcos, M.; Hernando, A. High magnetomechanical coupling on magnetic microwire for sensors with biological applications. Appl. Phys. Lett. 2010, 96, 262512. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Li, Y.; Guo, X.; Zhang, B.; Xue, X.; Zhuo, K.; Zhang, C.; Zhang, W.; Yuan, Z. A Portable Device for Rapid Detection of Human Serum Albumin using an immunoglobulin-coating-based Magnetoelastic Biosensor. Biosens. Bioelectron. 2019, 141, 111399. [Google Scholar] [CrossRef] [PubMed]
- George, M.; John, A.M.; Nair, S.S.; Joy, P.A.; Anantharaman, M.R. Finite size effects on the structural and magnetic properties of sol–gel synthesized NiFe2O4 powders. J. Magn. Magn. Mater. 2006, 302, 190–195. [Google Scholar] [CrossRef]
- Chen, D.H.; He, X.R. Synthesis of nickel ferrite nanoparticles by sol-gel method. Mater. Res. Bull. 2001, 36, 1369–1377. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Zhang, W. Progress of new label-free techniques for biosensors: A review. Crit. Rev. Biotechnol. 2015, 36, 1–17. [Google Scholar] [CrossRef]
- Zhukova, V.; Zhukov, A.; Blanco, J.M.; Usov, N.; Gonzalez, J. Effect of applied stress on remagnetization and magnetization profile of Co–Si–B amorphous wire. J. Magn. Magn. Mater. 2003, 258, 189–191. [Google Scholar] [CrossRef]
- Apilux, A.; Dungchai, W.; Siangproh, W.; Praphairaksit, N.; Henry, C.S.; Chailapakul, O. Lab-on-Paper with Dual Electrochemical/Colorimetric Detection for Simultaneous Determination of Gold and Iron. Anal. Chem. 2010, 82, 1727–1732. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Garnier, G.; Shen, W. Fabrication of paper-based microfluidic sensors by printing. Colloid Surf. B-Biointerfaces 2010, 76, 564–570. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, K.; Li, S.; Wang, Y.; Huang, T.S.; Zhang, A.; Cheng, Z.Y. In situ real-time detection of E. coli in water using antibody-coated magnetostrictive microcantilever. Sens. Actuators B Chem. 2010, 150, 220–225. [Google Scholar] [CrossRef]
- Guo, X.; Gao, S.; Sang, S.; Jian, A.; Duan, Q.; Ji, J.; Zhang, W. Detection system based on magnetoelastic sensor for classical swine fever virus. Biosens. Bioelectron. 2016, 82, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Menti, C.; Henriques, J.A.; Missell, F.P.; Roeschely, M. Antibody-based magneto-elastic biosensors: Potential devices for detection of pathogens and associated toxins. Appl. Microbiol. Biotechnol. 2016, 100, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Seo, J. Nano zinc oxide-sodium alginate antibacterial cellulose fibres. Carbohydr. Polym. 2016, 135, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Nithyayini, K.N.; Harish, M.N.K.; Nagashree, K.L. Electrochemical detection of nitrite at NiFe2O4 nanoparticles synthesised by solvent deficient method. Electrochim. Acta 2019, 317, 701–710. [Google Scholar] [CrossRef]








| Wavenumber Range/cm−1 | Bonds | Labels |
|---|---|---|
| 3500–3200 | -OH stretching | 1 |
| 3000–2835 | C-H stretching | 2 |
| 1652–1623 | -OH bending of absorbed water | 3 |
| 1470–1420 | C-H bending | 4 |
| 1391–1361 | C-H and C-O vibrations in pyranose ring | 5 |
| 1260–1150 | C-O-C stretching | 6 |
| 1111–1056 | Out-of-phase bending | 7 |
| 1046–994 | C-C, C-OH, C-H ring and side group vibrations | 8 |
| 898–890 | COC, CCO and CCH deformation and stretching | 9 |
| 565–410 | Fe-O, Ni-O stretching | 10,11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Liu, R.; Li, H.; Wang, J.; Yuan, Z.; Zhang, W.; Sang, S. A Novel NiFe2O4/Paper-Based Magnetoelastic Biosensor to Detect Human Serum Albumin. Sensors 2020, 20, 5286. https://doi.org/10.3390/s20185286
Guo X, Liu R, Li H, Wang J, Yuan Z, Zhang W, Sang S. A Novel NiFe2O4/Paper-Based Magnetoelastic Biosensor to Detect Human Serum Albumin. Sensors. 2020; 20(18):5286. https://doi.org/10.3390/s20185286
Chicago/Turabian StyleGuo, Xing, Rong Liu, Hongmei Li, Jingzhe Wang, Zhongyun Yuan, Wendong Zhang, and Shengbo Sang. 2020. "A Novel NiFe2O4/Paper-Based Magnetoelastic Biosensor to Detect Human Serum Albumin" Sensors 20, no. 18: 5286. https://doi.org/10.3390/s20185286





