Colorimetric Paper-Based Device for Hazardous Compounds Detection in Air and Water: A Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Ammonia Detection
2.1.1. Reagents
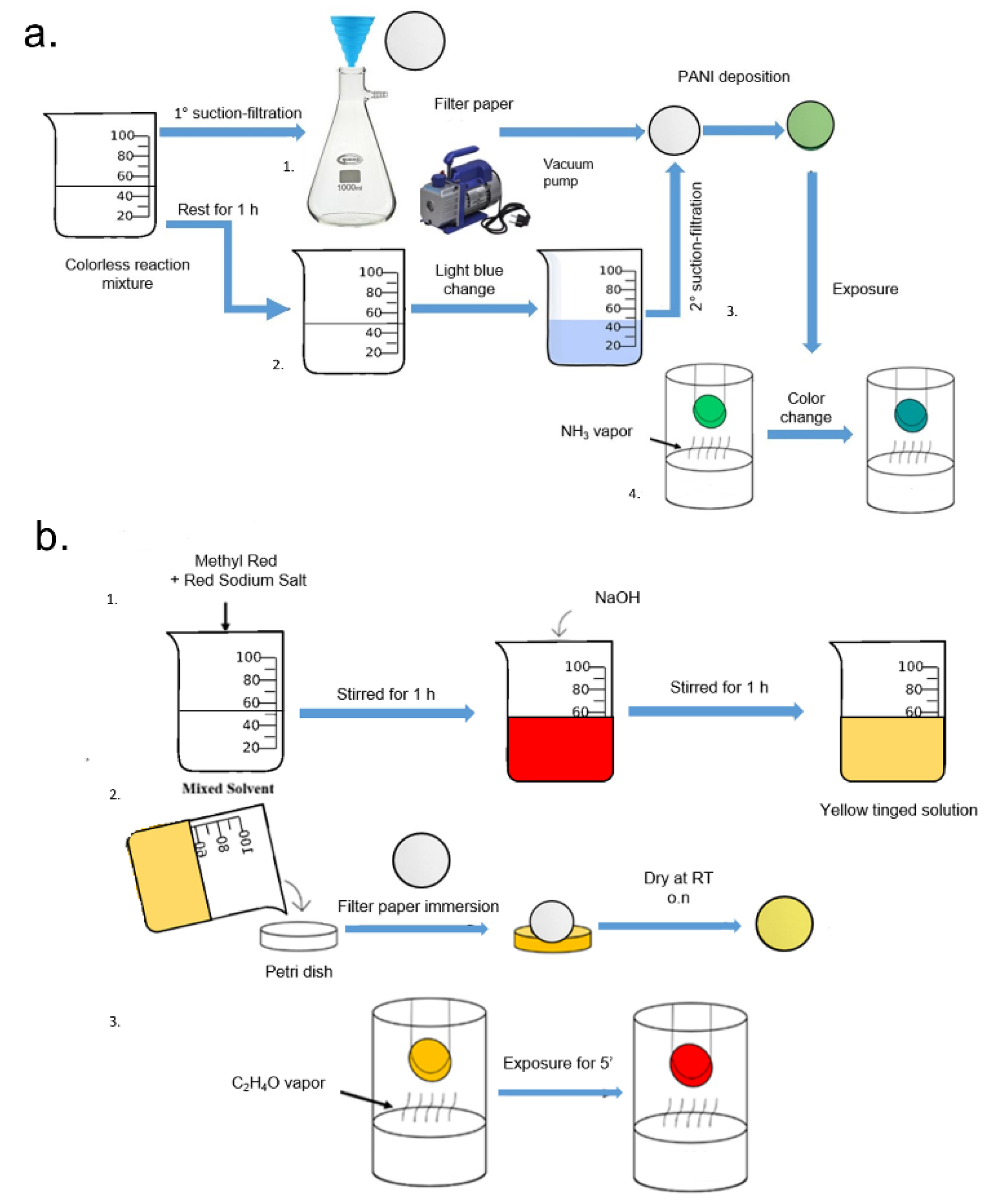
2.1.2. Functionalization of Whatman Paper for Reversible Ammonia Vapor Detection
2.1.3. Construction of Calibration Curve by Colorimetric Response to Ammonia Vapor
2.2. Acetaldehyde Detection
2.2.1. Reagents
2.2.2. Functionalization of Whatman Paper for Acetaldehyde Vapor Detection
2.2.3. Construction of Calibration Curve by Colorimetric Response to Acetaldehyde Vapor
2.3. Fabrication of Paper-Based Colorimetric Device for Fe2+ and Cu2+
2.3.1. Reagents
2.3.2. Iron and Copper Calibration Curve Standard Solutions Preparation
2.3.3. Fabrication of the Paper Analytical Device (PAD)
- The waxy channels on a piece of Whatman filter paper were obtained by using a wax pen. The shape of each channel was circular with a diameter of approximately 0.5 cm. Four spots were drawn on the filter paper, one for each standard.
- The PAD was heated on a hot plate at ~60 °C for 1 h to melt the wax. The liquid wax penetrated into the cellulose pores to achieve hydrophobic barriers.
- The PAD was dried at room temperature for approximately 30 min.
2.3.4. Assay Procedure
2.4. Quantitative Image Processing by ImageJ 1.47 Software
- The “Color Threshold” window was accessed through the ImageJ menu by selecting “Image”→ “Adjust”→“Color Threshold.”
- At the bottom of this window HSB was selected, which allowed the adjustment of hue, saturation, and brightness.
- The hue was adjusted by moving the sliders directly below the “Hue” spectrum until only the color of interest was visible. The hue threshold ranges set for each metal were fixed as follows: NH3 (244–255), C2H4O (38–240), Fe2+ (171–197), Cu2+ (37–255).
2.5. Interference Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 1–14. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Methods 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.-D. Physiology of iron metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 8–40. [Google Scholar] [CrossRef]
- International Maritime Organization. Marine Environmental Protection Committee. In Harmful Effects of the Use of Antifouling Paints for Ships; 40th Session, Agenda Item 11, Annex 2nd; United Nations Economic and Social Council: London, UK, 1997. [Google Scholar]
- Hall, L.W.; Anderson, R.D. A deterministic ecological risk assessment for copper in european saltwater environments. Mar. Pollut. Bull. 1999, 38, 207–218. [Google Scholar] [CrossRef]
- Hall, L.W., Jr.; Scott, M.C.; Killen, W.D. A Screening Level Probabilistic Ecological Risk Assessment of Copper and Cadmium in the Chesapeake Bay Watershed; US EPA, Chesapeake Bay Program Office: Annapolis, MD, USA, 1997. [Google Scholar]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2019, 65, 131–159. [Google Scholar] [CrossRef]
- Hébert, C.D. NTP Technical Report on Toxicity Studies of Cupric Sulphate (CAS N°7758-99-8) Administered in Drinking Water and Feed to F344/N Rats and B6C3F1 Mice; Technical Report No. 7758-99-8; United States Department of Health and Hum: Washington, DC, USA, 1993. [Google Scholar]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Phippen, B.; Horvath, C.; Nordin, R.N.N. Ambient Water Quality Guidelines for Iron: Overview; Water Stewardship Division, Ministry of Environment Province of British Columbia: Cranbrook, BC, Canada, 2008. [Google Scholar]
- Grazuleviciene, R.; Nadisauskiene, R.; Buinauskiene, J.G.T. Effects of elevated levels of manganese and iron in drinking water on birth outcomes. Pol. J. Environ. Stud. 2009, 18, 819–825. [Google Scholar]
- Sutton, M.A. Introduction. In Atmospheric Ammonia; Sutton, M.A., Reis, S., Howard, C., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Van Damme, M.; Clarisse, L.; Whitburn, S.; Hadji-Lazaro, J.; Hurtmans, D.; Clerbaux, C.; Coheur, P.-F. Industrial and agricultural ammonia point sources exposed. Nature 2018, 564, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Ammonia; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004. [Google Scholar]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Michaels, R.A. Emergency planning and the acute toxic potency of inhaled ammonia. Environ. Health Perspect. 1999. [Google Scholar] [CrossRef] [PubMed]
- Goldmacher, V.S.; Thilly, W.G. Formaldehyde is mutagenic for cultured human cells. Mutat. Res. Toxicol. 1983, 116, 417–422. [Google Scholar] [CrossRef]
- Fassett, D.W. Aldehydes and acetals. In Indus Trial Hygiene and Toxicology, 2nd ed.; Patty, F.A., Ed.; Interscience: New York, NY, USA, 1963; Volume 2, pp. 1959–1989. [Google Scholar]
- Woutersen, R.; Appelman, L.; Feron, V.J.; Van Der Heijden, C. Inhalation toxicity of acetaldehyde in rats II. Carcinogenicity study: Interim results after 15 months. Toxicology 1984, 31, 123–133. [Google Scholar] [CrossRef]
- Feron, V.J.; Kruysse, A.; Woutersen, R.A. Respiratory tract tumors in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo[a]pyrene or dimethylnitrosamine. Eur. J. Cancer Clin. Oncol. 1982, 18, 13–31. [Google Scholar] [CrossRef]
- Pandey, S.K.; Mohanta, G.C.; Kumar, P. Development of Disposable Sensor Strips for Point-of-Care Testing of Environmental Pollutants. Adv. Nanosens. Biol. Environ. Anal. 2019, 6, 95–118. [Google Scholar] [CrossRef]
- Cunningham, J.C.; DeGregory, P.R.; Crooks, R.M. New Functionalities for Paper-Based Sensors Lead to Simplified User Operation, Lower Limits of Detection, and New Applications. Annu. Rev. Anal. Chem. 2016, 9, 183–202. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; You, J.-Q.; Cheng, Y.; Sun, W.; Ding, L.; Feng, Y.-Q. Frontal elution paper chromatography for ambient ionization mass spectrometry: Analyzing powder samples. Anal. Methods 2013, 5, 4105. [Google Scholar] [CrossRef]
- Xiao-Wei, H.; Zou, X.; Ji-Yong, S.; Zhi-Hua, L.; Jie-Wen, Z. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple silver nanoparticle colorimetric sensing for copper by paper-based devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Noblitt, S.D.; Volckens, J.; Henry, C.S. Multiplexed paper analytical device for quantification of metals using distance-based detection. Lab Chip 2015, 15, 2808–2818. [Google Scholar] [CrossRef]
- Irvine, G.W.; Tan, S.N.; Stillman, M.J. A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury. Biosensors 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Suslick, K.S. Ultrasonic Preparation of Porous Silica-Dye Microspheres: Sensors for Quantification of Urinary Trimethylamine N-Oxide. ACS Appl. Mater. Interfaces 2018, 10, 15820–15828. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Dai, F.; Li, F.; Jin, X.; Wang, R.; Sun, D. A visual test paper based on Pb(ii) metal–organic nanotubes utilized as a H2S sensor with high selectivity and sensitivity. Anal. Methods 2017, 9, 3094–3098. [Google Scholar] [CrossRef]
- Francis, A.P.; Thiyagarajan, D. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Maity, A.; Ghosh, B. Fast response paper based visual color change gas sensor for efficient ammonia detection at room temperature. Sci. Rep. 2018, 8, 16851. [Google Scholar] [CrossRef]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, srep18721. [Google Scholar] [CrossRef]
- Khattab, T.A.; Dacrory, S.; Abou-Yousef, H.; Kamel, S. Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr. Polym. 2019, 210, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Qin, J.; Lin, B. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal. Chem. 2010, 82, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Khimji, I.; Akbas, R.; Qiu, W.; Edwards, D.; Cramer, D.W.; Ye, B.; Demirci, U. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab Chip 2011, 11, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Erenas, M.M.; Marinetto, E.; Abad, C.A.; De Orbe-Payá, I.; Palma, A.J.; Capitán-Vallvey, L. Mobile phone platform as portable chemical analyzer. Sens. Actuators B 2011, 156, 350–359. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Barik, S.; Adhikari, B. Polyaniline as a gas-sensor material. Mater. Manuf. Process. 2006, 21, 263–270. [Google Scholar] [CrossRef]
- ImageJ. Available online: http://imagej.nih.gov/ij/ (accessed on 24 September 2020).
- Yu, P.; Deng, M.; Yang, Y. New single-layered paper-based microfluidic devices for the analysis of nitrite and glucose built via deposition of adhesive tape. Sensors 2019, 19, 4082. [Google Scholar] [CrossRef]
- Joshi, B.P.; Park, J.; Lee, W.I.; Lee, K.H. Ratiometric and turn-on monitoring for heavy and transition metal ions in aqueous solution with a fluorescent peptide sensortle. Talanta 2009, 78, 903–909. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection a closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Vhahangwele, M.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Met. 2018. [Google Scholar] [CrossRef]
- Tuomisto, H.; Hodge, I.; Riordan, P.; Macdonald, D. Does organic farming reduce environmental impacts?—A meta-analysis of European research. J. Environ. Manag. 2012, 112, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2016, 7, 1043–1067. [Google Scholar] [CrossRef]
- WHO chronicle. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 978924154815 1. [Google Scholar]










| PADs | Concentrations Range | Limit of Detection (LOD) |
|---|---|---|
| NH3 | 100–1000 ppm | 7.64 ppm |
| C2H4O | 100–1000 ppm | 11.08 ppm |
| Fe2+ | 25–200 µg/mL | 3.8 µg/mL |
| Cu2+ | 25–200 µg/mL | 3.2 µg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matteis, V.; Cascione, M.; Fella, G.; Mazzotta, L.; Rinaldi, R. Colorimetric Paper-Based Device for Hazardous Compounds Detection in Air and Water: A Proof of Concept. Sensors 2020, 20, 5502. https://doi.org/10.3390/s20195502
De Matteis V, Cascione M, Fella G, Mazzotta L, Rinaldi R. Colorimetric Paper-Based Device for Hazardous Compounds Detection in Air and Water: A Proof of Concept. Sensors. 2020; 20(19):5502. https://doi.org/10.3390/s20195502
Chicago/Turabian StyleDe Matteis, Valeria, Mariafrancesca Cascione, Gabriele Fella, Laura Mazzotta, and Rosaria Rinaldi. 2020. "Colorimetric Paper-Based Device for Hazardous Compounds Detection in Air and Water: A Proof of Concept" Sensors 20, no. 19: 5502. https://doi.org/10.3390/s20195502
APA StyleDe Matteis, V., Cascione, M., Fella, G., Mazzotta, L., & Rinaldi, R. (2020). Colorimetric Paper-Based Device for Hazardous Compounds Detection in Air and Water: A Proof of Concept. Sensors, 20(19), 5502. https://doi.org/10.3390/s20195502








