Feasibility Study of Precise Balloon Catheter Tracking and Visualization with Fast Photoacoustic Microscopy
Abstract
:1. Introduction
2. Materials and Methods
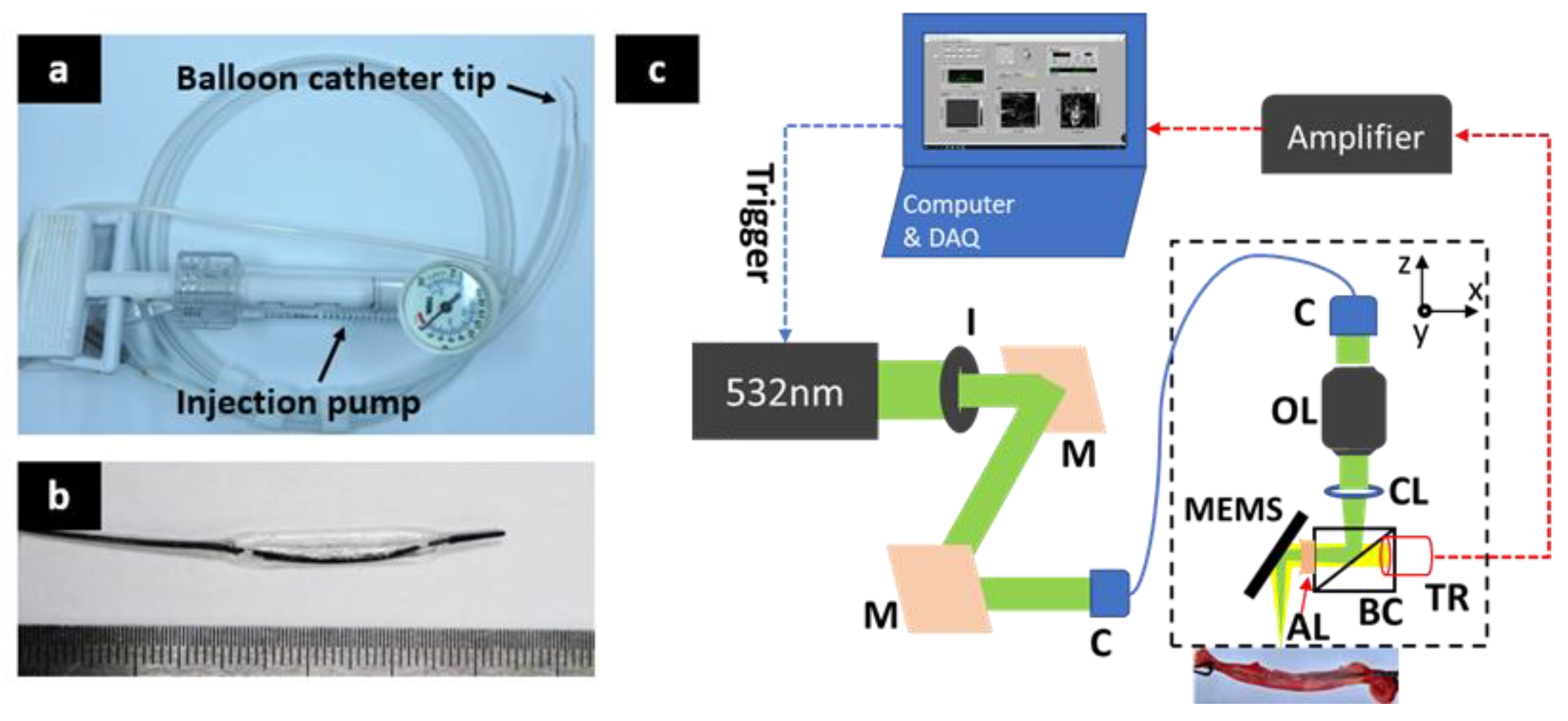
2.1. Balloon Catheter
2.2. Fast Photoacoustic Microscopic Imaging
2.3. Automatic Surface Removing Algorithm
2.4. Animal Preparing
3. Results
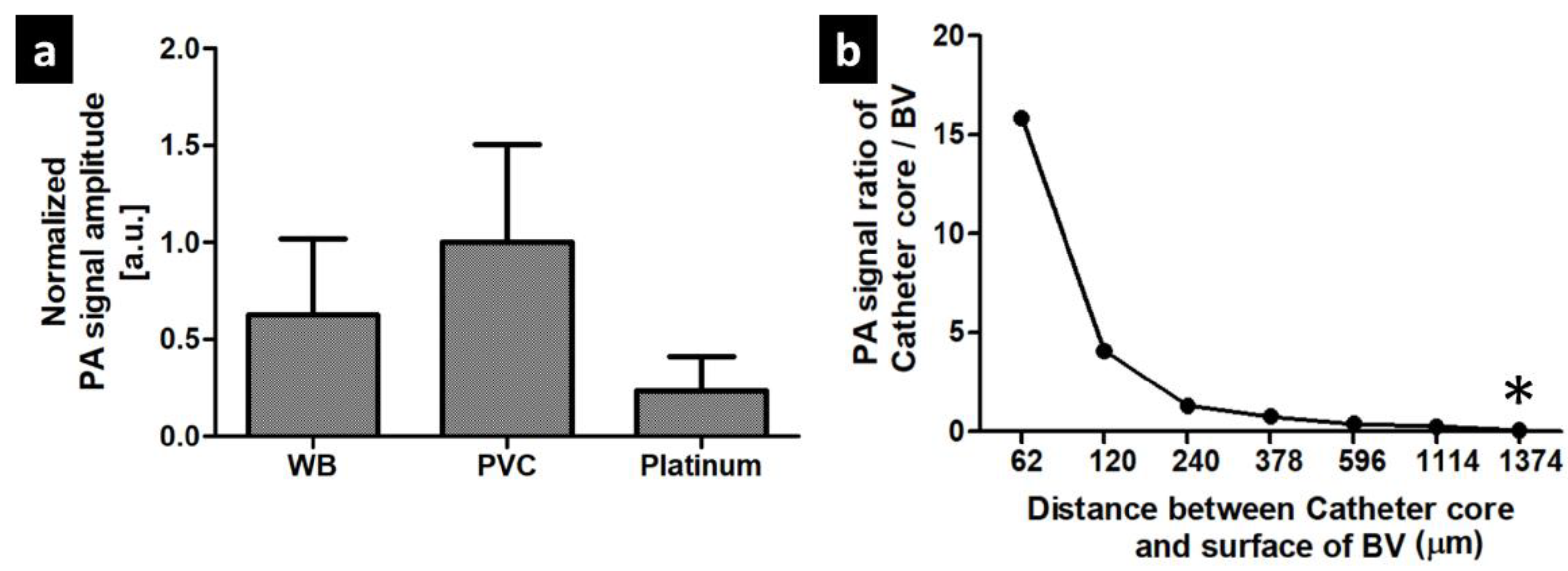
3.1. PA signal Characteristics of The Balloon Catheter
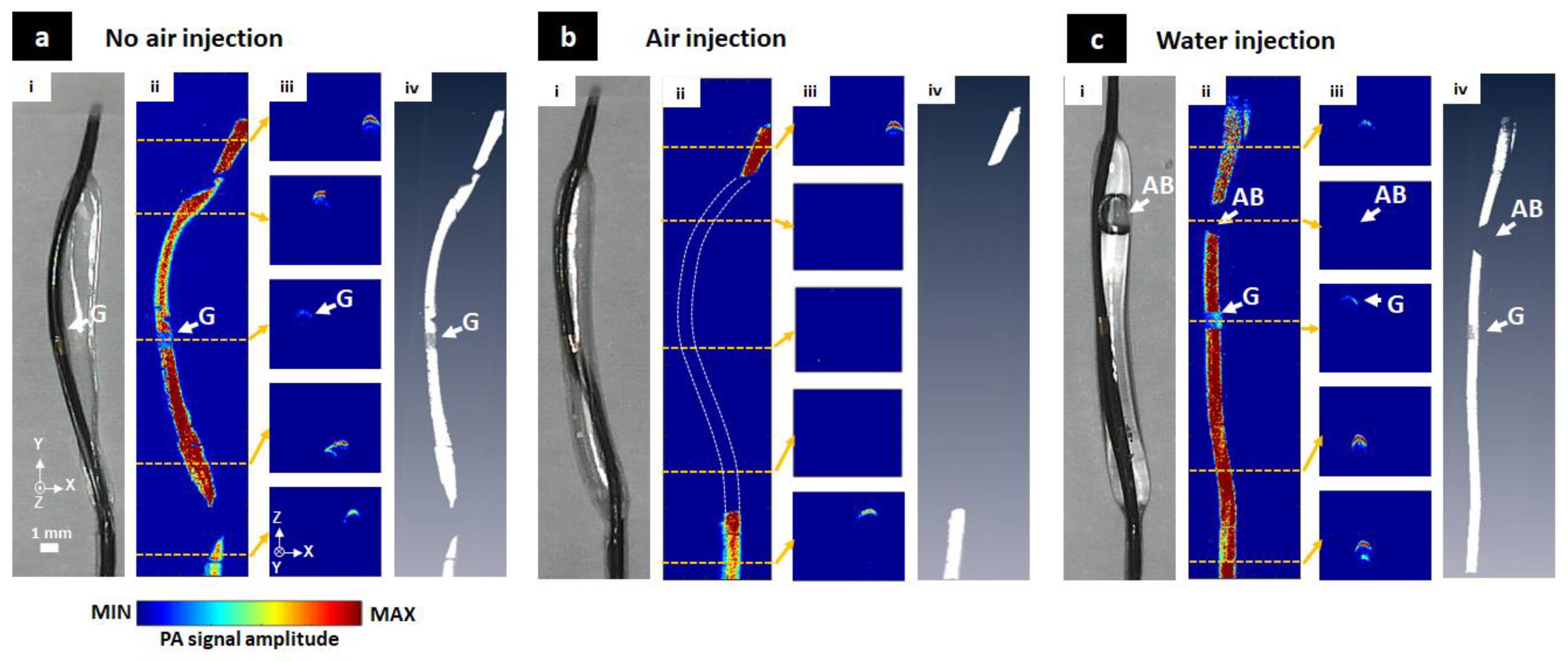
3.2. The Balloon Catheter Visualization
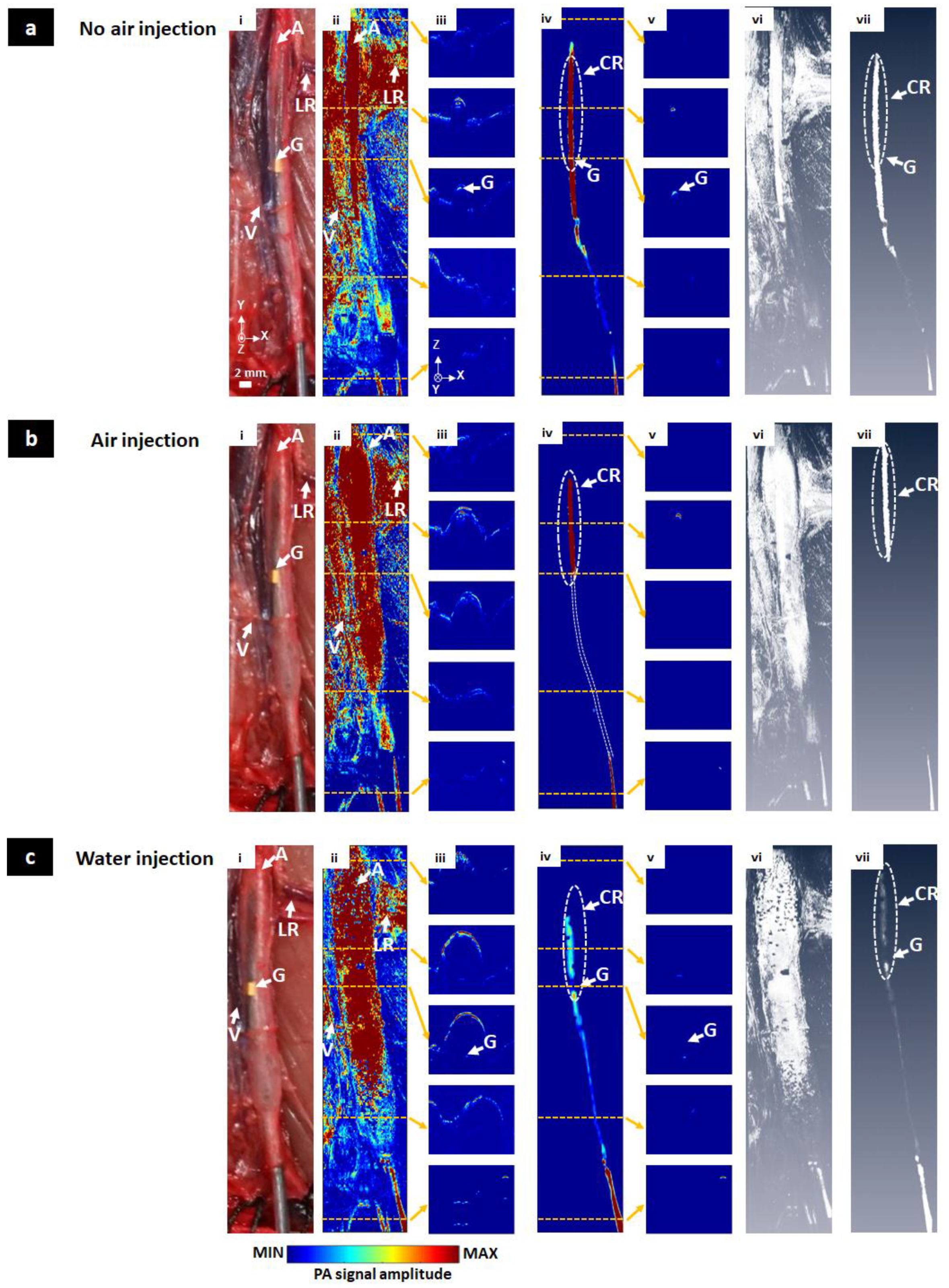
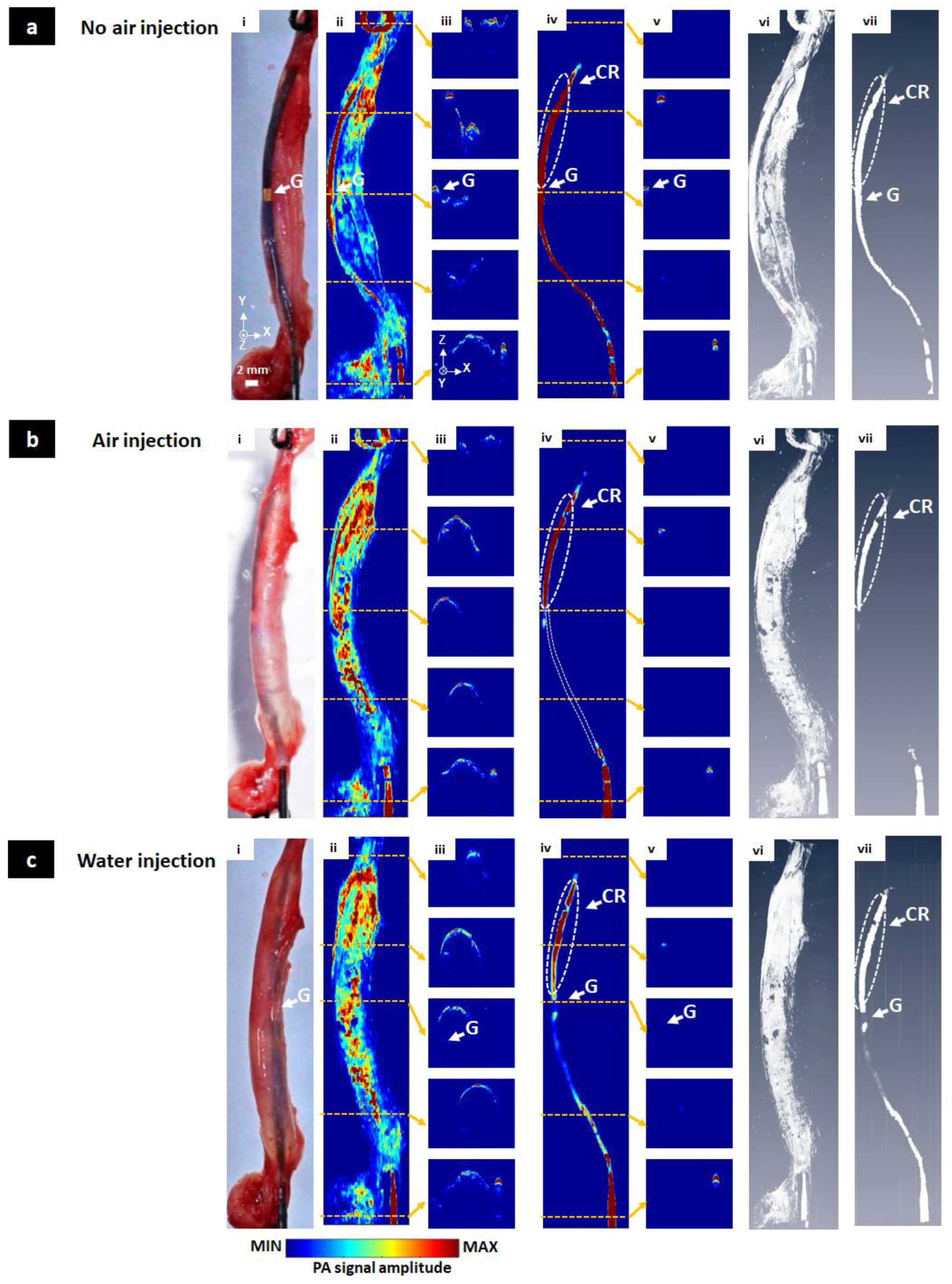
3.3. In Vivo Visualization of the Balloon Catheter
3.4. Ex Vivo Visualization of the Balloon Catheter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiemeneij, F.; Laarman, G.J.; de Melker, E. Transradial artery coronary angioplasty. Am. Heart J. 1995, 129, 1–7. [Google Scholar] [CrossRef]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef]
- Higashida, R.T.; Tsai, F.Y.; Halbach, V.V.; Barnwell, S.L.; Dowd, C.F.; Hieshima, G.B. Interventional neurovascular techniques in the treatment of stroke—State-of-the-art therapy. J. Intern. Med. 1995, 237, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, H.-Y.; Ko, H.K.; Park, J.-H.; Na, H.K.; Kim, Y.H.; Jung, H.-Y. Fluoroscopically guided balloon dilation or temporary stent placement for patients with gastric conduit strictures after esophagectomy with esophagogastrostomy. Am. J. Roentgenol. 2013, 201, 202–207. [Google Scholar] [CrossRef]
- Kim, K.Y.; Tsauo, J.; Song, H.-Y.; Park, H.J.; Kang, W.S.; Park, J.-H.; Wang, Z. Fluoroscopy-guided balloon dilation in patients with Eustachian tube dysfunction. Eur. Radiol. 2018, 28, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Elabd, S.A.; Elbahnasy, A.M.; Farahat, Y.A.; Soliman, M.G.; Taha, M.R.; Elgarabawy, M.A.; Figenshau, R. Minimally-invasive correction of ureteropelvic junction obstruction: Do retrograde endo-incision techniques still have a role in the era of laparoscopic pyeloplasty? Ther. Adv. Urol. 2009, 1, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stannard, A.; Eliason, J.L.; Rasmussen, T.E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J. Trauma Acute Care Surg. 2011, 71, 1869–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Chang, S.W.; Matsumoto, J. The utilization of resuscitative endovascular balloon occlusion of the aorta: Preparation, technique, and the implementation of a novel approach to stabilizing hemorrhage. J. Thorac. Dis. 2018, 10, 5550. [Google Scholar] [CrossRef]
- Angelini, G.; Wilde, P.; Salerno, T.; Bosco, G.; Calafiore, A. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 1996, 347, 757–758. [Google Scholar] [CrossRef]
- Frevert, S.; Dahl, B.; Lönn, L. Update on the roles of angiography and embolisation in pelvic fracture. Injury 2008, 39, 1290–1294. [Google Scholar] [CrossRef]
- Karkos, C.D.; Mair, R.; Markose, G.; Fishwick, G.; London, N.J.; Naylor, A.R. Hybrid procedures combining open and endovascular surgical techniques for the management of subclavian artery injuries. J. Trauma Acute Care Surg. 2007, 63, E107–E110. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, L.-Y.; Guo, Q.-S.; Yao, Y.-Z.; Sun, S.-J.; Wang, T.; Li, Y.-C.; Xiong, K.-L. “One-Stop Hybrid Procedure” in the Treatment of Vascular Injury of Lower Extremity. Indian J. Surg. 2015, 77, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Rehani, M.; Ciraj-Bjelac, O.; Vañó, E.; Miller, D.; Walsh, S.; Giordano, B.; Persliden, J. Radiological protection in fluoroscopically guided procedures performed outside the imaging department. Ann. ICRP 2010, 40, 1–102. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.R.; Katzen, B.T.; Scheinert, D.; Lammer, J.; Carpenter, J.; Buchbinder, M.; Dave, R.; Ansel, G.; Lansky, A.; Cristea, E. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the RESILIENT randomized trial. Circ. Cardiovasc. Interv. 2010, 3, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, M.; Meier, P.; Tamhane, U.U.; Welch, K.B.; Moscucci, M.; Gurm, H.S. The relative renal safety of iodixanol compared with low-osmolar contrast media: A meta-analysis of randomized controlled trials. Jacc Cardiovasc. Interv. 2009, 2, 645–654. [Google Scholar] [PubMed] [Green Version]
- Mogabgab, O.; Patel, V.G.; Michael, T.T.; Kotsia, A.; Christopoulos, G.; Banerjee, S.; Brilakis, E.S. Impact of contrast agent viscosity on coronary balloon deflation times: Bench testing results. J. Interv. Cardiol. 2014, 27, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Krueger, J.J.; Ewert, P.; Yilmaz, S.; Gelernter, D.; Peters, B.; Pietzner, K.; Bornstedt, A.; Schnackenburg, B.; Abdul-Khaliq, H.; Fleck, E. Magnetic resonance imaging–guided balloon angioplasty of coarctation of the aorta. Circulation 2006, 113, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Kusuyama, T.; Iida, H.; Mitsui, H. Intravascular ultrasound complements the diagnostic capability of carbon dioxide digital subtraction angiography for patients with allergies to iodinated contrast medium. Catheter. Cardiovasc. Interv. 2012, 80, E82–E86. [Google Scholar] [CrossRef]
- Ewert, P.; Berger, F.; Daehnert, I.; Krings, G.; Dittrich, S.; Lange, P.E. Diagnostic Catheterization and Balloon Sizing of Atrial Septal Defects by Echocardiography Guidance Without Fluoroscopy. Echocardiography 2000, 17, 159–163. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Han, S.; Kim, C. High-speed and high-SNR photoacoustic microscopy based on a galvanometer mirror in non-conducting liquid. Sci. Rep. 2016, 6, 34803. [Google Scholar] [CrossRef]
- Lee, C.; Han, S.; Kim, S.; Jeon, M.; Jeon, M.Y.; Kim, C.; Kim, J. Combined photoacoustic and optical coherence tomography using a single near-infrared supercontinuum laser source. Appl. Opt. 2013, 52, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yao, J. Photoacoustic microscopy: Principles and biomedical applications. Biomed. Eng. Lett. 2018, 8, 203–213. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, J.W.; Kim, J.Y.; Cho, S.; Choi, S.; Kim, J.; Kim, C. Super Wide-field Photoacoustic Microscopy of Animals and Humans In Vivo. IEEE Trans. Med. Imaging 2019, 39, 975–984. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.; Kim, J.; Kim, C. Acoustic resolution photoacoustic microscopy. Biomed. Eng. Lett. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848. [Google Scholar] [CrossRef]
- Liu, C.; Liao, J.; Chen, L.; Chen, J.; Ding, R.; Gong, X.; Cui, C.; Pang, Z.; Zheng, W.; Song, L. The integrated high-resolution reflection-mode photoacoustic and fluorescence confocal microscopy. Photoacoustics 2019, 14, 12–18. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical photoacoustic imaging platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In vitro photoacoustic measurement of hemoglobin oxygen saturation using a single pulsed broadband supercontinuum laser source. Appl. Opt. 2014, 53, 3884–3889. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, J.; Ning, B.; Sun, N.; Wang, T.; Zuo, Z.; Hu, S. Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. Neuroimage 2017, 150, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.; Jiang, M.; Hu, J.; Fawzi, A.; Zhou, Q.; Shung, K.K.; Puliafito, C.A.; Zhang, H.F. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt. Express 2010, 18, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.V. Neurovascular photoacoustic tomography. Front. Neuroenerg. 2010, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, L.V.; Cheng, Y.-J.; Chen, J.; Wickline, S.A. Label-free photoacoustic microscopy of myocardial sheet architecture. J. Biomed. Opt. 2012, 17, 060506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tavakoli, B.; Kang, H.-J.; Kang, J.U.; Etienne-Cummings, R.; Boctor, E.M. Photoacoustic active ultrasound element for catheter tracking. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing, San Francisco, CA, USA, 3 March 2014; p. 89435M. [Google Scholar]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.T.V.; Bui, N.Q.; Cho, S.-W.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Kim, C.-S.; Oh, J. Photoacoustic Imaging-Guided Photothermal Therapy with Tumor-Targeting HA-FeOOH@ PPy Nanorods. Sci. Rep. 2018, 8, 8809. [Google Scholar] [CrossRef] [Green Version]
- Francis, K.J.; Manohar, S. Photoacoustic imaging in percutaneous radiofrequency ablation: Device guidance and ablation visualization. Phys. Med. Biol. 2019, 64, 184001. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; Kruizinga, P.; Beurskens, R.; Springeling, G.; Mastik, F.; de Groot, N.M.; Knops, P.; van der Steen, A.F.; van Soest, G. Real-time photoacoustic assessment of radiofrequency ablation lesion formation in the left atrium. Photoacoustics 2019, 16, 100150. [Google Scholar] [CrossRef]
- Lee, D.; Lee, C.; Kim, S.; Zhou, Q.; Kim, J.; Kim, C. In vivo near infrared virtual intraoperative surgical photoacoustic optical coherence tomography. Sci. Rep. 2016, 6, 35176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shubert, J.; Lediju Bell, M.A. Photoacoustic imaging of a human vertebra: Implications for guiding spinal fusion surgeries. Phys. Med. Biol. 2018, 63, 144001. [Google Scholar] [CrossRef] [PubMed]
- Lediju Bell, M.A. Photoacoustic imaging for surgical guidance: Principles, applications, and outlook. J. Appl. Phys. 2020, 128, 060904. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Jokerst, J.V. Strategies for image-guided therapy, surgery, and drug delivery using photoacoustic imaging. Theranostics 2019, 9, 1550. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef]
- Song, W.; Wei, Q.; Liu, T.; Kuai, D.; Zhang, H.F.; Burke, J.M.; Jiao, S. Integrating photoacoustic ophthalmoscopy with scanning laser ophthalmoscopy, optical coherence tomography, and fluorescein angiography for a multimodal retinal imaging platform. J. Biomed. Opt. 2012, 17, 061206. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, H.; Song, W.; Jiao, S.; Zhang, H.F. Fundus camera guided photoacoustic ophthalmoscopy. Curr. Eye Res. 2013, 38, 1229–1234. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.P.; Li, Y.; Zhang, W.; Wang, X.; Paulus, Y.M. High-resolution multimodal photoacoustic microscopy and optical coherence tomography image-guided laser induced branch retinal vein occlusion in living rabbits. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Faber, D.J.; Aalders, M.C.G.; Mik, E.G.; Hooper, B.A.; van Gemert, M.J.C.; van Leeuwen, T.G. Oxygen Saturation-Dependent Absorption and Scattering of Blood. Phys. Rev. Lett. 2004, 93, 028102. [Google Scholar] [CrossRef] [Green Version]
- Rajian, J.R.; Fabiilli, M.L.; Fowlkes, J.B.; Carson, P.L.; Wang, X. Drug delivery monitoring by photoacoustic tomography with an ICG encapsulated double emulsion. Opt. Express 2011, 19, 14335–14347. [Google Scholar] [CrossRef]
- Ashkenazi, S. Photoacoustic lifetime imaging of dissolved oxygen using methylene blue. J. Biomed. Opt. 2010, 15, 040501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H.; et al. Bright Aggregation-Induced-Emission Dots for Targeted Synergetic NIR-II Fluorescence and NIR-I Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Mater. 2018, 30, 1800766. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, C.; Law, W.-C.; Zhu, D.; Liu, M.; Jeon, M.; Kim, J.; Prasad, P.N.; Kim, C.; Swihart, M.T. Au–Cu2–xSe Heterodimer Nanoparticles with Broad Localized Surface Plasmon Resonance as Contrast Agents for Deep Tissue Imaging. Nano Lett. 2013, 13, 4333–4339. [Google Scholar] [CrossRef]
- Zhou, Y.; Xing, W.; Maslov, K.I.; Cornelius, L.A.; Wang, L.V. Handheld photoacoustic microscopy to detect melanoma depth in vivo. Opt. Lett. 2014, 39, 4731–4734. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Mai, T.T.; Kim, J.Y.; Min, J.-J.; Kim, C.; Lee, C. Feasibility Study of Precise Balloon Catheter Tracking and Visualization with Fast Photoacoustic Microscopy. Sensors 2020, 20, 5585. https://doi.org/10.3390/s20195585
Kim J, Mai TT, Kim JY, Min J-J, Kim C, Lee C. Feasibility Study of Precise Balloon Catheter Tracking and Visualization with Fast Photoacoustic Microscopy. Sensors. 2020; 20(19):5585. https://doi.org/10.3390/s20195585
Chicago/Turabian StyleKim, Jahae, Thi Thao Mai, Jin Young Kim, Jung-Joon Min, Chulhong Kim, and Changho Lee. 2020. "Feasibility Study of Precise Balloon Catheter Tracking and Visualization with Fast Photoacoustic Microscopy" Sensors 20, no. 19: 5585. https://doi.org/10.3390/s20195585





