Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors
Abstract
:1. Introduction
2. Material and Methods
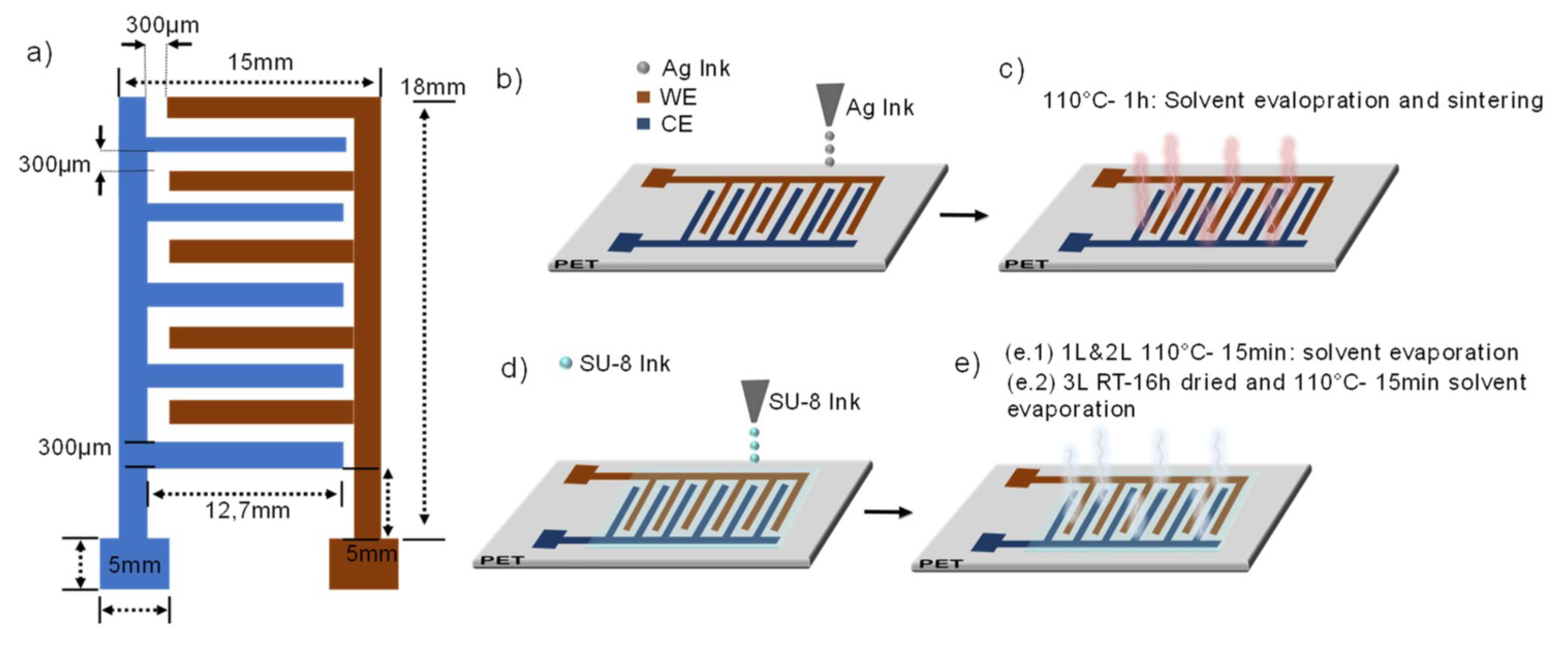
2.1. Fabrication of Interdigitated Sensors
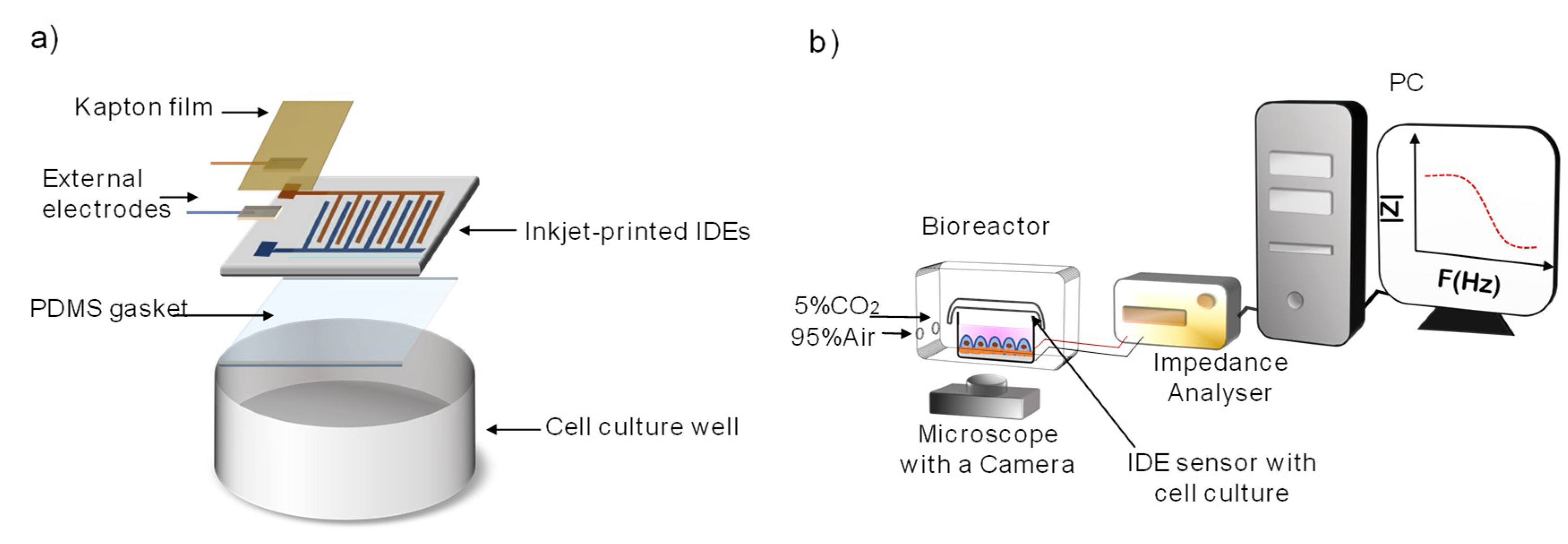
2.2. Assembly of IDE-Based Devices and Measuring System
2.3. Characterization of the Inkjet-Printed Sensors
2.4. Cell Culture
2.5. Preparation of the Inkjet-Printed IDEs-Based Device for Cellular Impedance Monitoring
2.6. Impedance Monitoring Protocol
2.7. Proliferation Assay
2.8. Migration Assay
2.9. Image Processing
2.10. Triton X-100
2.11. Statistical Methods
3. Results
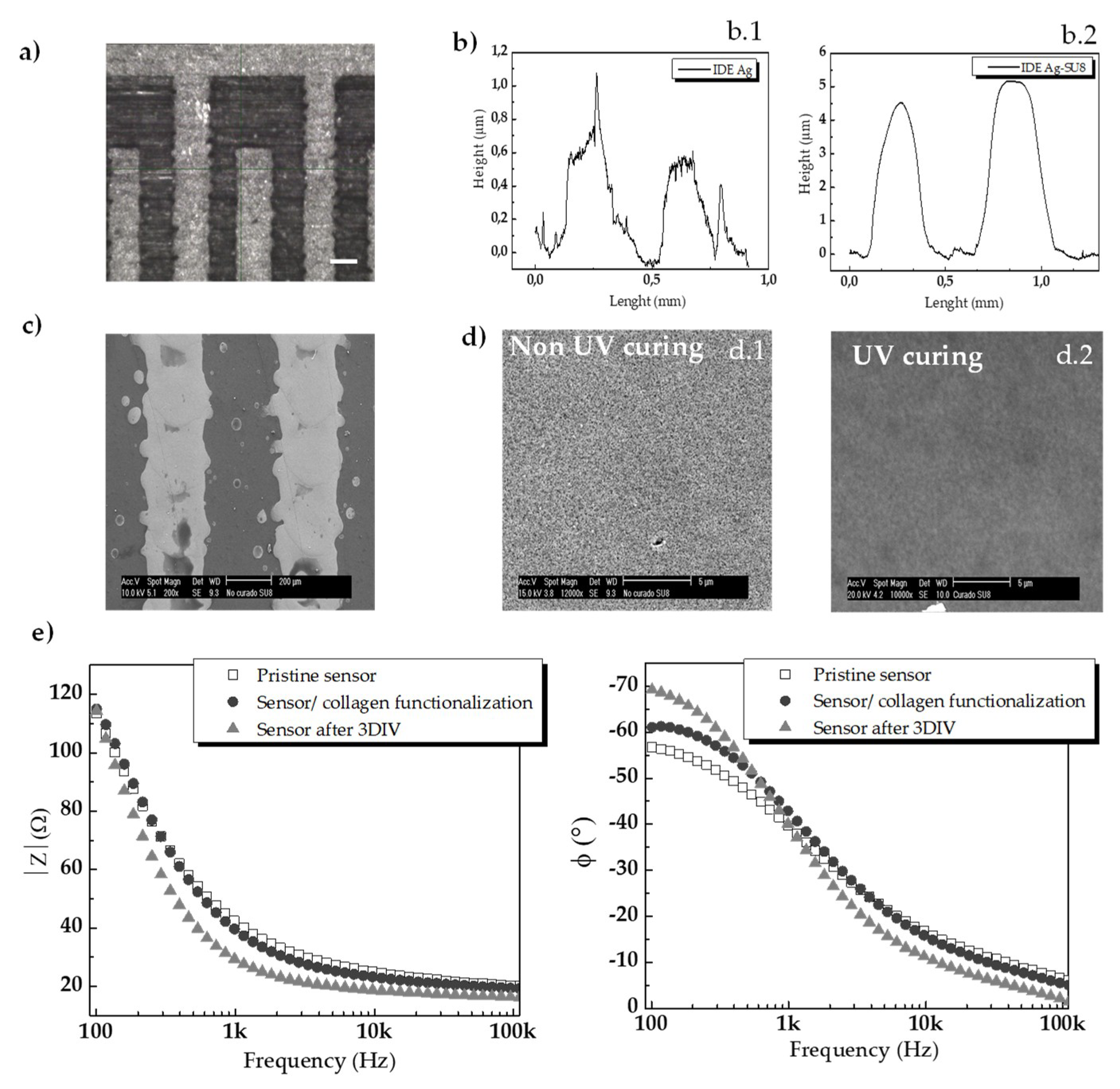
3.1. Characterization of the Inkjet-Printed Sensors
3.1.1. Morphological and Electrical Characterization of the Inkjet-Printed Sensors
3.1.2. Cytocompatibility Evaluation of the Inkjet-Printed-Based Device
3.2. Impedance Monitoring of 2D Epithelial Cultures
3.2.1. Demonstration of the Use of Inkjet-Printed Sensors for Impedance-Based Monitoring of Cell Proliferation
Study of the Contribution of the Cell Membranes Electrical Characteristics in the Impedance at the Endpoint of the Proliferation Assay
3.2.2. Demonstration of the Use of Inkjet-Printed Sensors for Impedance-Based Monitoring of Cell Detachment or Death
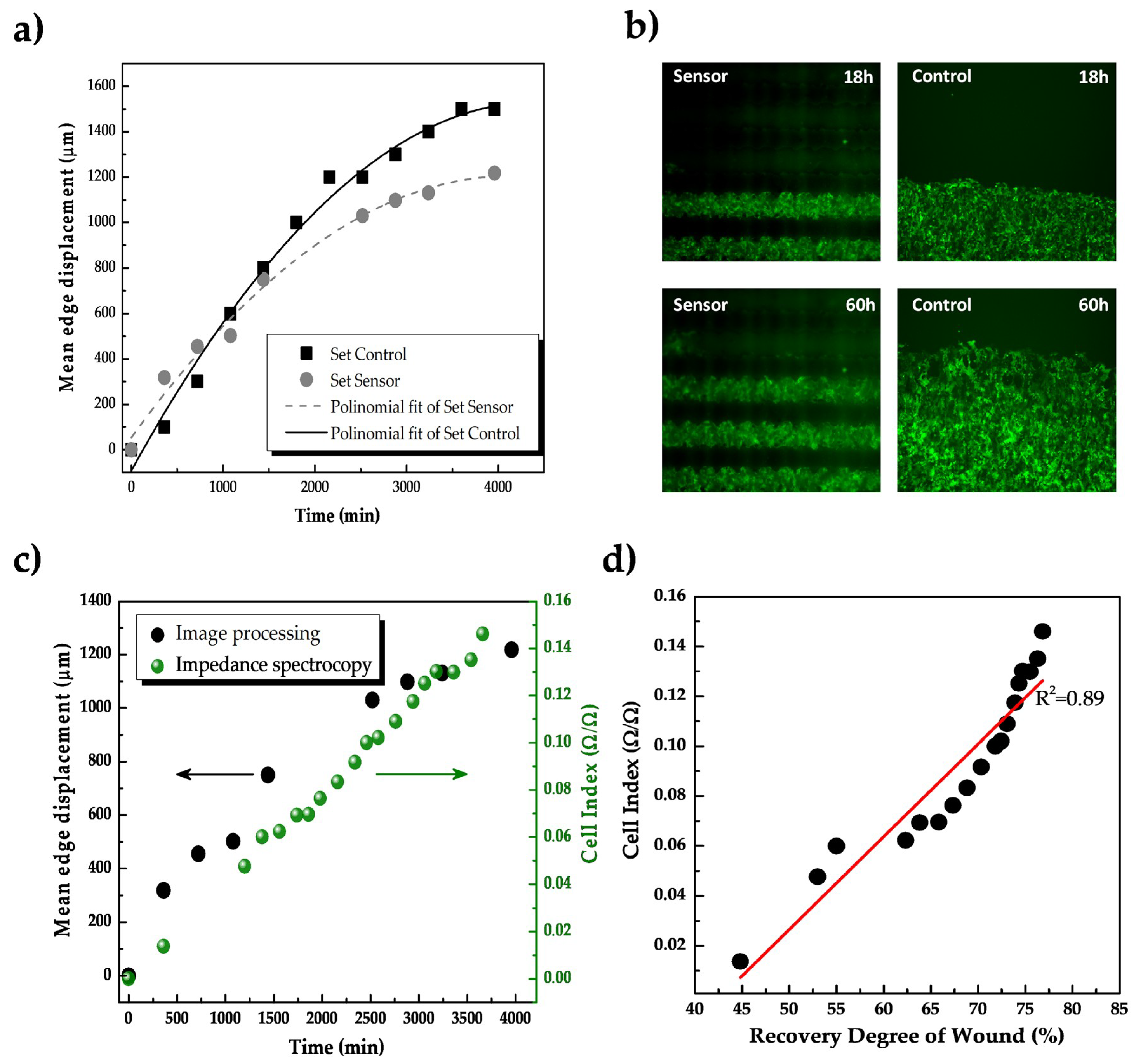
3.2.3. Preliminary Use of Inkjet-Printed Sensors for Impedance-Based Monitoring of Cell Migration
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Baeuscher, M.; Hu, X.; Woehrmann, M.; Becker, K.; Juergensen, N.; Hubl, M.; Mackowiak, P.; Schneider-Ramelow, M.; Lang, K.D.; et al. Development and Characterization of a Novel Low-Cost Water-Level and Water Quality Monitoring Sensor by Using Enhanced Screen Printing Technology with PEDOT: PSS. Micromachines 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Mojena-Medina, D.; Martínez-Hernández, M.; de la Fuente, M.; García-Isla, G.; Posada, J.; Jorcano, J.L.; Acedo, P. Design, Implementation, and Validation of a Piezoelectric Device to Study the Effects of Dynamic Mechanical Stimulation on Cell Proliferation, Migration and Morphology. Sensors 2020, 20, 2155. [Google Scholar] [CrossRef] [Green Version]
- Feiner, R.; Dvir, T. Engineering smart hybrid tissues with built-in electronics. Iscience 2020, 23, 100833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, C.; Galán, J.M.; Torrero, V.; Ferreiro, I.; Pérez, D.; Palao, R.; Martínez, E.; Llames, S.; Meana, A.; Holguín, P. Use of an autologous bioengineered composite skin in extensive burns: Clinical and functional outcomes. A multicentric study. Burns 2011, 37, 580–589. [Google Scholar] [CrossRef]
- Mohammed, A.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Grimenes, S.; Martinsen, Ø.G. Bioimpedance and Bioelectricty Basics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.M.; Keese, C.R.; Giaever, I. Impedance analysis of MDCK cells measured by electric cell-substrate impedance sensing. Biophys. J. 1995, 69, 2800–2807. [Google Scholar] [CrossRef] [Green Version]
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Stegbauer, K.; Wolf, B. Monitoring of cellular behaviour by impedance measurements on interdigitated electrode structures. Biosens. Bioelectron. 1997, 12, 29–41. [Google Scholar] [CrossRef]
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Wolf, B. On-line control of cellular adhesion with impedance measurements using interdigitated electrode structures. Med. Biol. Eng. Comput. 1998, 36, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonello, S.; Bianchetti, A.; Braga, S.; Almici, C.; Marini, M.; Piovani, G.; Guindani, M.; Dey, K.; Sartore, L.; Re, F.; et al. Impedance-Based Monitoring of Mesenchymal Stromal Cell Three-Dimensional Proliferation Using Aerosol Jet Printed Sensors: A Tissue Engineering Application. Materials 2020, 13, 2231. [Google Scholar] [CrossRef]
- Marziano, M.; Tonello, S.; Cantù, E.; Abate, G.; Vezzoli, M.; Rungratanawanich, W.; Serpelloni, M.; Lopomo, N.F.; Memo, M.; Sardini, E. Monitoring Caco-2 to enterocyte-like cells differentiation by means of electric impedance analysis on printed sensors. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 893–902. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.W.; Kim, J.; Noh, M. A novel fabrication method of Parylene-based microelectrodes utilizing inkjet printing. Sens. Actuators B Chem. 2017, 238, 862–870. [Google Scholar] [CrossRef]
- Blaschke, B.M.; Lottner, M.; Drieschner, S.; Calia, A.B.; Stoiber, K.; Rousseau, L.; Lissourges, G.; Garrido, J.A. Flexible graphene transistors for recording cell action potentials. 2D Mater. 2016, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Brischwein, M.; Herrmann, S.; Vonau, W.; Berthold, F.; Grothe, H.; Motrescu, E.R.; Wolf, B. Electric cell-substrate impedance sensing with screen printed electrode structures. Lab Chip 2006, 6, 819–822. [Google Scholar] [CrossRef]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet printing of proteins. Soft Matter 2009, 5, 4866–4877. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet printing proteins and hybrid cell-containing materials and structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Esfandyarpour, R.; Didonato, M.J.; Yang, Y.; Durmus, N.G.; Harris, J.S.; Davis, R.W. Multifunctional, inexpensive, and reusable nanoparticle-printed biochip for cell manipulation and diagnosis. Proc. Natl. Acad. Sci. USA 2017, 114, E1306–E1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garma, L.D.; Ferrari, L.M.; Scognamiglio, P.; Greco, F.; Santoro, F. Inkjet-printed PEDOT:PSS multi-electrode arrays for low-cost: In vitro electrophysiology. Lab Chip 2019, 19, 3776–3786. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Adly, N.Y.; Schnitker, J.; Yakushenko, A.; Rinklin, P.; Offenhäusser, A.; Wolfrum, B. All-inkjet-printed gold microelectrode arrays for extracellular recording of action potentials. Flex. Print. Electron. 2017, 2, 035003. [Google Scholar] [CrossRef]
- Adly, N.; Weidlich, S.; Seyock, S.; Brings, F.; Yakushenko, A.; Offenhäusser, A.; Wolfrum, B. Printed microelectrode arrays on soft materials: From PDMS to hydrogels. npj Flex. Electron. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Tonello, S.; Borghetti, M.; Lopomo, N.F.; Serpelloni, M.; Sardini, E.; Marziano, M.; Serzanti, M.; Uberti, D.; DELL’ERA, P.; Inverardi, N.; et al. Ink-jet printed stretchable sensors for cell monitoring under mechanical stimuli: A feasibility study. J. Mech. Med. Biol. 2019, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Plotczyk, M.; Higgins, C.A. Skin biology. In Biomaterials for Skin Repair and Regeneration; Elsevier: Amsterdam, The Netherlands, 2019; vol. 1, pp. 3–25. [Google Scholar]
- Boukamp, P.; Petrussevska, R.I.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusening, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Berezin, O.; Kamyshny, A. Triggering the sintering of silver nanoparticles at room temperature. ACS Nano 2010, 4, 1943–1948. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Mihailescu, C.F.; Anghel, D.F.; Magdassi, S. Conductive inks with a ‘built-in’ mechanism that enables sintering at room temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar] [CrossRef]
- Han, D.-W.; Woo, Y.I.; Lee, M.H.; Lee, J.H.; Lee, J.; Park, J. In-vivo and in-vitro biocompatibility evaluations of silver nanoparticles with antimicrobial activity. J. Nanosci. Nanotechnol. 2012, 12, 5205–5209. [Google Scholar] [CrossRef]
- Greulich, C.; Kittler, S.; Epple, M.; Muhr, G.; Köller, M. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs). Langenbeck’s Arch. Surg. 2009, 394, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 71, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Solly, K.; Wang, X.; Xu, X.; Strulovici, B.; Zheng, W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev. Technol. 2004, 2, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Vedula, S.R.K.; Ravasio, A.; Lim, C.T.; Ladou, B. Collective cell migration: A mechanistic perspective. Physiology 2013, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Yang, Y.; Zhang, D.D.; Wong, P.K. Advances in wound-healing assays for probing collective cell migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef] [Green Version]
- Pennacchio, F.A.; Garma, L.D.; Matino, L.; Santoro, F. Bioelectronics goes 3D: New trends in cell-chip interface engineering. J. Mater. Chem. B 2018, 6, 7096–7101. [Google Scholar] [CrossRef]
- De Araujo, A.L.A.; Claudel, J.; Kourtiche, D.; Nadi, M. Use of an insulation layer on the connection tracks of a biosensor with coplanar electrodes to increase the normalized impedance variation. Biosensors 2019, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Schwan, H.P. Electric characteristics of tissues. Biophysik 1963, 1, 198–208. [Google Scholar] [CrossRef]
- Caviglia, C.; Zor, K.; Canepa, S.; Carminati, M.A.R.C.O.; Larsen, L.B.; Raiteri, R.; Andresen, T.L.; Heiskanen, A.; Emnéus, J. Interdependence of initial cell density, drug concentration and exposure time revealed by real-time impedance spectroscopic cytotoxicity assay. Analyst 2015, 140, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; An, Y.; Jin, T.; Zhang, F.; He, P. Real-time monitoring of skin wound healing on nano-grooves topography using electric cell-substrate impedance sensing (ECIS). Sens. Actuators B Chem. 2017, 250, 461–468. [Google Scholar] [CrossRef]
- Patel, P.; Markx, G.H. Dielectric measurement of cell death. Enzyme Microb. Technol. 2008, 43, 463–470. [Google Scholar] [CrossRef]
- Mazlan, N.S.; Ramli, M.M.; Abdullah, M.M.A.B.; Halin, D.C.; Isa, S.M.; Talip, L.F.A.; Danial, N.S.; Murad, S.Z. Interdigitated electrodes as impedance and capacitance biosensors: A review. AIP Conf. Proc. 2017, 1885, 020276. [Google Scholar]
- Igreja, R.; Dias, C.J. Analytical evaluation of the interdigital electrodes capacitance for a multi-layered structure. Sens. Actuators A Phys. 2004, 112, 291–301. [Google Scholar] [CrossRef]
- Igreja, R.; Dias, C.J. Extension to the analytical model of the interdigital electrodes capacitance for a multi-layered structure. Sens. Actuators A Phys. 2011, 172, 392–399. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. Chapter 9—Data and Models, 3rd ed.; Academic Press: Oxford, UK, 2015; pp. 329–404. [Google Scholar]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Sele, C.W.; von Werne, T.; Friend, R.H.; Sirringhaus, H. Lithography-free, self-aligned inkjet printing with sub-hundred-nanometer resolution. Adv. Mater. 2005, 17, 997–1001. [Google Scholar] [CrossRef]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sandison, M.E.; Anicet, N.; Glidle, A.; Cooper, J.M. Optimization of the geometry and porosity of microelectrode arrays for sensor design. Anal. Chem. 2002, 74, 5717–5725. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Baeumner, A.J. Characterization and optimization of interdigitated ultramicroelectrode arrays as electrochemical biosensor transducers. Electroanalysis 2004, 16, 724–729. [Google Scholar] [CrossRef]
- Pejcic, B.; de Marco, R. Impedance spectroscopy: Over 35 years of electrochemical sensor optimization. Electrochim. Acta 2006, 51, 6217–6229. [Google Scholar] [CrossRef]
- Radke, S.M.; Alocilja, E.C. Design and fabrication of a microimpedance biosensor for bacterial detection. IEEE Sens. J. 2004, 4, 434–440. [Google Scholar] [CrossRef]
- Price, D.T.; Rahman, A.R.A.; Bhansali, S. Design rule for optimization of microelectrodes used in electric cell-substrate impedance sensing (ECIS). Biosens. Bioelectron. 2009, 24, 2071–2076. [Google Scholar] [CrossRef]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojena-Medina, D.; Hubl, M.; Bäuscher, M.; Jorcano, J.L.; Ngo, H.-D.; Acedo, P. Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors 2020, 20, 5711. https://doi.org/10.3390/s20195711
Mojena-Medina D, Hubl M, Bäuscher M, Jorcano JL, Ngo H-D, Acedo P. Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors. 2020; 20(19):5711. https://doi.org/10.3390/s20195711
Chicago/Turabian StyleMojena-Medina, Dahiana, Moritz Hubl, Manuel Bäuscher, José Luis Jorcano, Ha-Duong Ngo, and Pablo Acedo. 2020. "Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors" Sensors 20, no. 19: 5711. https://doi.org/10.3390/s20195711
APA StyleMojena-Medina, D., Hubl, M., Bäuscher, M., Jorcano, J. L., Ngo, H.-D., & Acedo, P. (2020). Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors, 20(19), 5711. https://doi.org/10.3390/s20195711





