A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems
Abstract
1. A Brief History of Glucose Biosensing
2. Enzymatic and Non-Enzymatic Electrochemical Biosensors
2.1. Nanomaterial-Based Electrochemical Enzymatic Glucose Biosensors
2.1.1. Gold Nanostructures and Their Use in Hybrid Glucose Biosensors
2.1.2. Carbon Nanotubes (CNTs) and Their Hybrid-Based Glucose Biosensors
2.1.3. Carbon/Graphene Quantum Dots (CQDs, GQDs)-Based Glucose Biosensors
2.1.4. Hydrogel-Chitosan-Based Glucose Biosensors
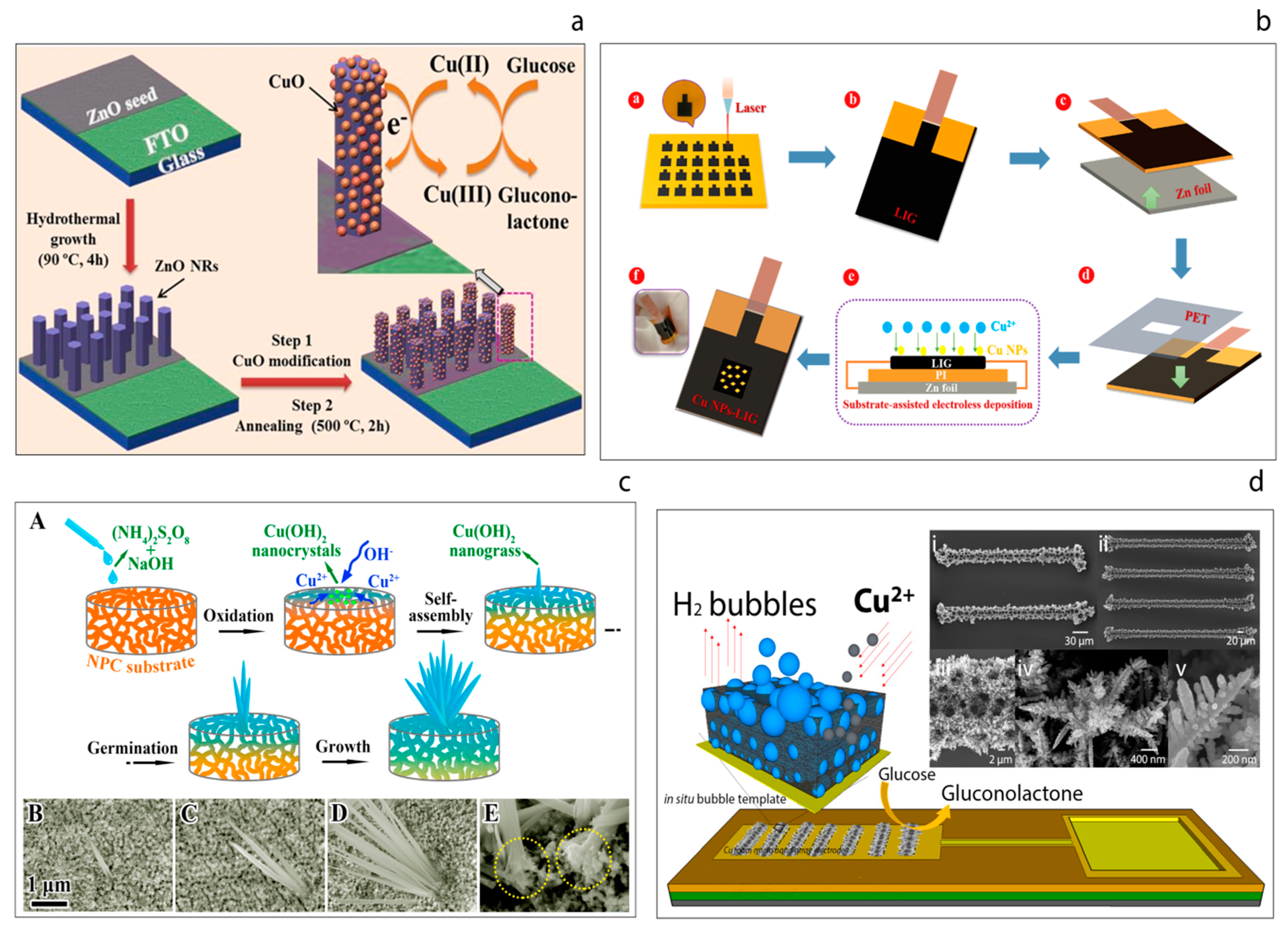
2.2. Non-Enzymatic Detection of Glucose; Direct Glucose Electro Oxidation
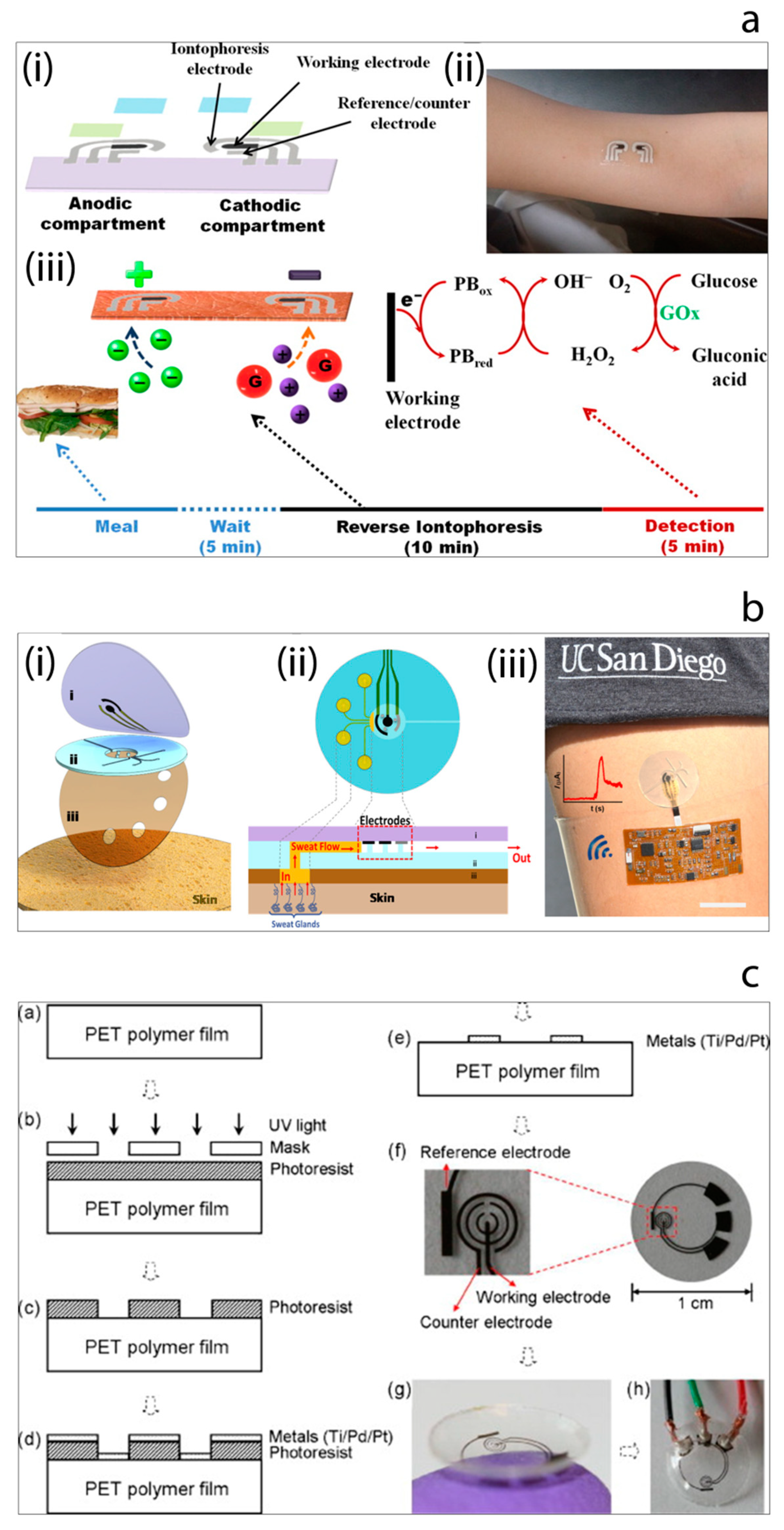
3. Wearable Non-Invasive Electrochemical Glucose Sensors
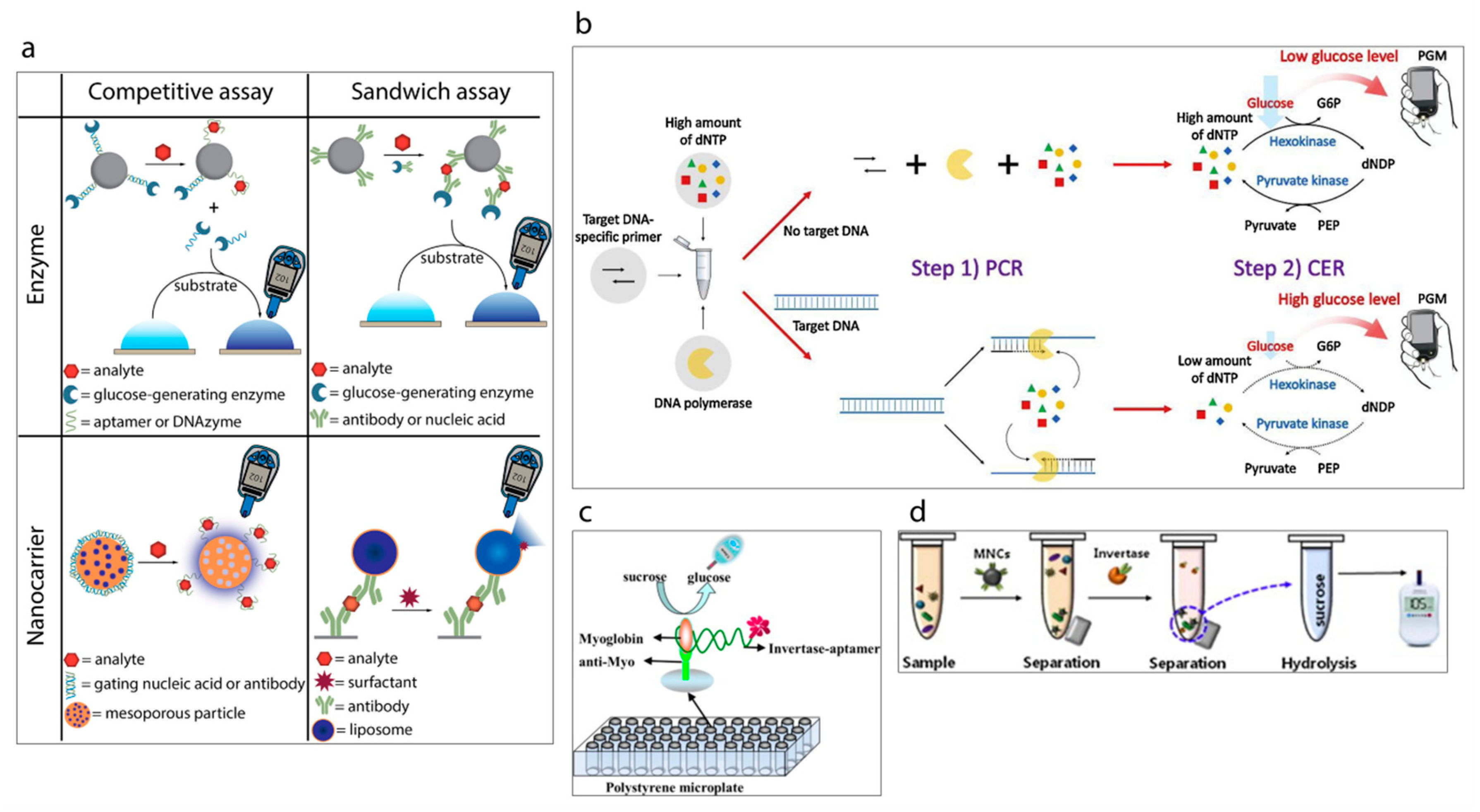
4. Point-of-Care Diagnostic Devices Based on Personal Glucose Meters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef]
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Christwardana, M.; Chung, Y.; Kim, D.-H.; Kwon, Y. Glucose biofuel cells using the two-step reduction reaction of bienzyme structure as cathodic catalyst. J. Ind. Eng. Chem. 2019, 71, 435–444. [Google Scholar] [CrossRef]
- Riklin, A.; Katz, E.; Wiliner, I.; Tocker, A.; Bückmann, A.F. Direct Electrical Communication between Graphite-Electrodes and Surface Adsorbed Glucose-Oxidase Redox Polymer Complexes. Angew. Chem. Int. Ed. Engl. 1990, 29, 82–89. [Google Scholar]
- Degani, Y.; Heller, A. Direct Electrical Communication between Chemically Modified Enzymes and Metal-Electrodes. I. Electron-Transfer from Glucose-Oxidase to Metal-Electrodes Via Electron Relays, Bound Covalently to the Enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- Riklin, A.; Katz, E.; Wiliner, I.; Stocker, A.; Bückmann, A.F. Improving enzyme–Electrode contacts by redox modification of cofactors. Nature 1995, 376, 672–675. [Google Scholar] [CrossRef]
- Rizwan, M.; Elma, S.; Lim, S.A.; Ahmed, M.U. AuNPs/CNOs/SWCNTs/chitosan-nanocomposite modified electrochemical sensor for the label-free detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 107, 211–217. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Zhu, Y.; Wu, J.; Li, K.; Lin, G.; Li, X.; Liu, R.; Liu, X.; Wong, C.-P. Facile one-step fabrication of glucose oxidase loaded polymeric nanoparticles decorating MWCNTs for constructing glucose biosensing platform: Structure matters. Biosens. Bioelectron. 2019, 135, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Z.; He, S.; Zhang, B.; Li, X.; Yao, M. Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens. Bioelectron. 2014, 52, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Pemble, M.E. A dual-enzyme, micro-band array biosensor based on the electrodeposition of carbon nanotubes embedded in chitosan and nanostructured Au-foams on microfabricated gold band electrodes. Analyst 2020, 145, 402–414. [Google Scholar] [CrossRef]
- Feng, X.; Cheng, H.; Pan, Y.; Zheng, H. Development of glucose biosensors based on nanostructured graphene-conducting polyaniline composite. Biosens. Bioelectron. 2015, 70, 411–417. [Google Scholar] [CrossRef]
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mater. Sci. Eng. C 2020, 107, 110273. [Google Scholar] [CrossRef]
- Bharath, G.; Madhu, R.; Chen, S.M.; Veeramani, V.; Balamurugan, A.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, C. Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J. Mater. Chem. B 2015, 3, 1360–1370. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef]
- Buk, V.; Pemble, M.E. A highly sensitive glucose biosensor based on a micro disk array electrode design modified with carbon quantum dots and gold nanoparticles. Electrochim. Acta 2019, 298, 97–105. [Google Scholar] [CrossRef]
- Buk, V.; Pemble, M.E.; Twomey, K. Fabrication and evaluation of a carbon quantum dot/gold nanoparticle nanohybrid material integrated onto planar micro gold electrodes for potential bioelectrochemical sensing applications. Electrochim. Acta 2019, 293, 307–317. [Google Scholar] [CrossRef]
- Buk, V.; Emregul, E.; Emregul, K.C. Alginate copper oxide nano-biocomposite as a novel material for amperometric glucose biosensing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Walcarius, A.; Pemble, M.E. Cu Nanodendrite Foams on Integrated Band Array Electrodes for the Nonenzymatic Detection of Glucose. ACS Appl. Nano Mater. 2019, 2, 5878–5889. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Wang, H.; Hou, S. Gold nanoparticles decorated on single layer graphene applied for electrochemical ultrasensitive glucose biosensor. J. Electroanal. Chem. 2019, 855, 113495. [Google Scholar] [CrossRef]
- Lin, J.; He, C.; Zhao, Y.; Zhang, S. One-step synthesis of silver nanoparticles/carbon nanotubes/chitosan film and its application in glucose biosensor. Sens. Actuators B Chem. 2009, 137, 768–773. [Google Scholar] [CrossRef]
- Şenel, M.; Nergiz, C.; Çevik, E. Novel reagentless glucose biosensor based on ferrocene cored asymmetric PAMAM dendrimers. Sens. Actuators B Chem. 2013, 176, 299–306. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Yang, X.; Li, C. Amperometric biosensor based on carbon nanotubes coated with polyaniline/dendrimer-encapsulated Pt nanoparticles for glucose detection. Mater. Sci. Eng. C 2009, 29, 1306–1310. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Liu, Y.; Jiang, X. A sensitive hydrogen peroxide and glucose biosensor based on gold/silver core–shell nanorods. Electrochim. Acta 2013, 108, 39–44. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, Y.; Li, J.; Zhang, Y.; Hu, X. Facile synthesis of tetragonal columnar-shaped TiO2 nanorods for the construction of sensitive electrochemical glucose biosensor. Biosens. Bioelectron. 2014, 54, 528–533. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Prokhorov, E.; Bahena, D.; Esparza, R.; Meyyappan, M. Chitosan-Covered Pd@Pt Core–Shell Nanocubes for Direct Electron Transfer in Electrochemical Enzymatic Glucose Biosensor. ACS Omega 2017, 2, 1896–1904. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Siontorou, C.; Karapetis, S.; Bratakou, S.; Tzamtzis, N. Chapter 6-Nanobiosensors Based on Graphene Electrodes: Recent Trends and Future Applications. In Applications of Nanomaterials; Bhagyaraj, S.M., Ed.; Woodhead Publishing: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2018; pp. 161–177. [Google Scholar]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Lawal, A.T. Progress in utilisation of graphene for electrochemical biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [PubMed]
- Scognamiglio, V.; Arduini, F. The technology tree in the design of glucose biosensors. TrAC Trends Anal. Chem. 2019, 120, 115642. [Google Scholar] [CrossRef]
- Willner, B.; Katz, E.; Willner, I. Electrical contacting of redox proteins by nanotechnological means. Curr. Opin. Biotechnol. 2006, 17, 589–596. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-Functionalized Carbon Nanotubes: Applications in Nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Functional Hybrid Devices of Proteins and Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 5796–5800. [Google Scholar] [CrossRef]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Chou, A.; Rahmat, W.; Paddon-Row, M.N.; Gooding, J.J. Achieving direct electrical connection to glucose oxidase using aligned single walled carbon nanotube arrays. Electroanalysis 2005, 17, 38–46. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.J.; Yang, D.W.; Xiao, H.L.; Fu, Y.C.; Tan, Y.M.; Yao, S.Z. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Wu, C.; Sun, H.; Li, Y.; Liu, X.; Du, X.; Wang, X.; Xu, P. Biosensor based on glucose oxidase-nanoporous gold co-catalysis for glucose detection. Biosens. Bioelectron. 2015, 66, 350–355. [Google Scholar] [CrossRef]
- Eguílaz, M.; Villalonga, R.; Pingarrón, J.M.; Ferreyra, N.F.; Rivas, G.A. Functionalization of bamboo-like carbon nanotubes with 3-mercaptophenylboronic acid-modified gold nanoparticles for the development of a hybrid glucose enzyme electrochemical biosensor. Sens. Actuators B Chem. 2015, 216, 629–637. [Google Scholar] [CrossRef]
- Parlak, O.; İncel, A.; Uzun, L.; Turner, A.P.F.; Tiwari, A. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens. Bioelectron. 2017, 89, 545–550. [Google Scholar] [CrossRef]
- Ma, E.; Wang, P.; Yang, Q.; Yu, H.; Pei, F.; Li, Y.; Liu, Q.; Dong, Y. Electrochemical immunosensor based on MoS2 NFs/Au@AgPt YNCs as signal amplification label for sensitive detection of CEA. Biosens. Bioelectron. 2019, 142, 111580. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Zhang, X.; Feng, J.; Kong, L.; Wang, P.; Chen, Z.; Dong, Y.; Wei, Q. Ultrasensitive electrochemical immunosensor for quantitative detection of HBeAg using Au@Pd/MoS2@MWCNTs nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2018, 102, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, D.; Wei, Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf. B Biointerfaces 2020, 186, 110683. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, S.; Wei, Y.; Liu, X.; Jiao, S.; Yang, J. Ultrasensitive detection of miRNA-155 based on controlled fabrication of AuNPs@MoS2 nanostructures by atomic layer deposition. Biosens. Bioelectron. 2019, 144, 111660. [Google Scholar] [CrossRef]
- Paul, A.; Vyas, G.; Paul, P.; Srivastava, D.N. Gold-Nanoparticle-Encapsulated ZIF-8 for a Mediator-Free Enzymatic Glucose Sensor by Amperometry. ACS Appl. Nano Mater. 2018, 1, 3600–3607. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef] [PubMed]
- Chernova, T.; Murphy, F.A.; Galavotti, S.; Sun, X.-M.; Powley, I.R.; Grosso, S.; Schinwald, A.; Zacriacas-Cabeza, J.; Dudek, K.M.; Dinsdale, D.; et al. Long-Fiber Carbon Nanotubes Replicate Asbestos-Induced Mesothelioma with Disruption of the Tumor Suppressor Gene Cdkn2a (Ink4a/Arf). Curr. Biol. 2017, 27, 3302–3314.e6. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, P.; Eriksson, B.; Davodi, F.; Buan, M.E.M.; Sorsa, O.; Kallio, T.; Lindstrom, R.W. Carbon corrosion properties and performance of multi-walled carbon nanotube support with and without nitrogen-functionalization in fuel cell electrodes. Electrochim. Acta 2020, 332, 135384. [Google Scholar] [CrossRef]
- Yan, H.; Tang, X.; Zhu, X.; Zeng, Y.; Lu, X.; Yin, Z.; Lu, Y.; Yang, Y.; Li, L. Sandwich-type electrochemical immunosensor for highly sensitive determination of cardiac troponin I using carboxyl-terminated ionic liquid and helical carbon nanotube composite as platform and ferrocenecarboxylic acid as signal label. Sens. Actuators B Chem. 2018, 277, 234–240. [Google Scholar] [CrossRef]
- Wei, A.; Mu, J.; He, R.; Bai, X.; Liu, Z.; Zhang, L.; Zhang, L.H.; Liu, Z.; Wang, Y. Preparation of Li4Ti5O12/carbon nanotubes composites and LiCoO2/Li4Ti5O12 full-cell with enhanced electrochemical performance for high-power lithium-ion batteries. J. Phys. Chem. Solids 2020, 138, 109303. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.-M. Direct electrochemistry and electrocatalysis of glucose oxidase based poly(l-arginine)-multi-walled carbon nanotubes. RSC Adv. 2014, 4, 50771–50781. [Google Scholar] [CrossRef]
- Luo, P.J.G.; Sahu, S.; Yang, S.T.; Sonkar, S.K.; Wang, J.P.; Wang, H.F.; LeCroy, G.E.; Cao, L.; Sun, Y.-P. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan Mediated Targeted Drug Delivery System: A Review. J. Pharm. Pharm. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef]
- Safda, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef]
- Morris, G.A.; Kök, S.M.; Harding, S.E.; Adams, G.G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 257–284. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Dang, J.M.; Leong, K.W. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Di, M.A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar]
- Zhang, Y.; Li, Y.; Wu, W.; Jiang, Y.; Hu, B. Chitosan coated on the layers’ glucose oxidase immobilized on cysteamine/Au electrode for use as glucose biosensor. Biosens. Bioelectron. 2014, 60, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Anusha, J.R.; Raj, C.J.; Cho, B.-B.; Fleming, A.T.; Yu, K.-H.; Kim, B.C. Amperometric glucose biosensor based on glucose oxidase immobilized over chitosan nanoparticles from gladius of Uroteuthis duvauceli. Sens. Actuators B Chem. 2015, 215, 536–543. [Google Scholar] [CrossRef]
- Şenel, M. Simple method for preparing glucose biosensor based on in-situ polypyrrole cross-linked chitosan/glucose oxidase/gold bionanocomposite film. Mater. Sci. Eng. C 2015, 48, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sun, J.; Zhang, Y.; Bian, C.; Xia, S.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, X.; Xing, L.; Liu, X.; Luo, S.; Wei, W.; Kong, B.; Li, Y. Electrodeposition of chitosan–ionic liquid–glucose oxidase biocomposite onto nano-gold electrode for amperometric glucose sensing. Biosens. Bioelectron. 2009, 24, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Farjami, F. Electrodeposition of gold–Platinum alloy nanoparticles on ionic liquid–Chitosan composite film and its application in fabricating an amperometric cholesterol biosensor. Biosens. Bioelectron. 2011, 26, 2547–2552. [Google Scholar] [CrossRef]
- Che, X.; Yuan, R.; Chai, Y.; Li, J.; Song, Z.; Li, W.; Zhong, X. A glucose biosensor based on chitosan–Prussian blue–multiwall carbon nanotubes–hollow PtCo nanochains formed by one-step electrodeposition. Colloids Surf. B Biointerfaces 2011, 84, 454–461. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene–carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devadas, B.; Chen, S.-M. Direct electrochemistry of glucose oxidase at electrochemically reduced graphene oxide-multiwalled carbon nanotubes hybrid material modified electrode for glucose biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rafighi, P.; Tavahodi, M.; Haghighi, B. Fabrication of a third-generation glucose biosensor using graphene-polyethyleneimine-gold nanoparticles hybrid. Sens. Actuators B Chem. 2016, 232, 454–461. [Google Scholar] [CrossRef]
- Palsaniya, S.; Nemade, H.B.; Dasmahapatra, A.K. Mixed Surfactant-Mediated Synthesis of Hierarchical PANI Nanorods for an Enzymatic Glucose Biosensor. ACS Appl. Polym. Mater. 2019, 1, 647–656. [Google Scholar]
- Comba, F.N.; Romero, M.R.; Garay, F.S.; Baruzzi, A.M. Mucin and carbon nanotube-based biosensor for detection of glucose in human plasma. Anal. Biochem. 2018, 550, 34–40. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Rabiee, M.; Abdolrahim, M.; Tahriri, M.; Vashaee, D.; Tayebi, L. A novel electrochemical biosensor based on Fe3O4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose. Anal. Biochem. 2017, 519, 19–26. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Wang, G.-J. Highly sensitive glucose biosensor based on Au–Ni coaxial nanorod array having high aspect ratio. Biosens. Bioelectron. 2014, 56, 204–209. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, X.; Li, Z.; Fu, Y.; Qin, C.; Wu, F.; Su, Z.; Ma, M.; Xie, Q.; Yao, S.; et al. Fabrication of a chitosan/glucose oxidase–poly(anilineboronic acid)–Aunano/Au-plated Au electrode for biosensor and biofuel cell. Biosens. Bioelectron. 2012, 31, 357–362. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly(3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, D.; Guo, Y.; Guo, Y.; Chen, Q. Simple one-pot preparation of chitosan-reduced graphene oxide-Au nanoparticles hybrids for glucose sensing. Sens. Actuators B Chem. 2015, 221, 265–272. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.-W.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.P.; Chen, X.J. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Meng, S.J.; Wu, M.Y.; Wang, Q.; Dai, Z.Y.; Si, W.L.; Huang, W.; Dong, X. Cobalt oxide nanosheets wrapped onto nickel foam for non-enzymatic detection of glucose. Nanotechnology 2016, 27, 344001. [Google Scholar] [CrossRef]
- Long, M.; Tan, L.; Liu, H.; He, Z.; Tang, A. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef]
- Xu, H.; Xia, C.; Wang, S.; Han, F.; Akbari, M.K.; Hai, Z.; Zhuiykov, S. Electrochemical non-enzymatic glucose sensor based on hierarchical 3D Co3O4/Ni heterostructure electrode for pushing sensitivity boundary to a new limit. Sens. Actuators B Chem. 2018, 267, 93–103. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.-H.; Kwon, D.-W.; Yang, H.-Y.; Hahn, Y.-B. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Lu, Z. High-performance hybrid electrode decorated by well-aligned nanograss arrays for glucose sensing. Biosens. Bioelectron. 2018, 102, 288–295. [Google Scholar] [CrossRef]
- Coyle, V.E.; Kandjani, A.E.; Field, M.R.; Hartley, P.; Chen, M.; Sabri, Y.M.; Bhargava, S.K. Co3O4 needles on Au honeycomb as a non-invasive electrochemical biosensor for glucose in saliva. Biosens. Bioelectron. 2019, 141, 111479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Long, M.; Tan, L.; Zhang, Y.; Ouyang, J.; Liu, P.; Tang, A. Helical TiO2 Nanotube Arrays Modified by Cu–Cu2O with Ultrahigh Sensitivity for the Nonenzymatic Electro-oxidation of Glucose. ACS Appl. Mater. Interfaces 2015, 7, 12719–12730. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Su, W.-R.; Yeh, Y.-C. Functional Channel of SWCNTs/Cu2O/ZnO NRs/Graphene Hybrid Electrodes for Highly Sensitive Nonenzymatic Glucose Sensors. ACS Appl. Mater. Interfaces 2020, 12, 32905–32914. [Google Scholar] [CrossRef] [PubMed]
- Esmaeeli, A.; Ghaffarinejad, A.; Zahedi, A.; Vahidi, O. Copper oxide-polyaniline nanofiber modified fluorine doped tin oxide (FTO) electrode as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 266, 294–301. [Google Scholar] [CrossRef]
- Qian, Q.; Hu, Q.; Li, L.; Shi, P.; Zhou, J.; Kong, J.; Zhang, X.; Sun, G.; Huang, W. Sensitive fiber microelectrode made of nickel hydroxide nanosheets embedded in highly-aligned carbon nanotube scaffold for nonenzymatic glucose determination. Sens. Actuators B Chem. 2018, 257, 23–28. [Google Scholar] [CrossRef]
- Ghanbari, K.; Babaei, Z. Fabrication and characterization of non-enzymatic glucose sensor based on ternary NiO/CuO/polyaniline nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef]
- Liu, X.; Long, L.; Yang, W.; Chen, L.; Jia, J. Facilely electrodeposited coral-like copper micro-/nano-structure arrays with excellent performance in glucose sensing. Sens. Actuators B Chem. 2018, 266, 853–860. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Xu, S.; Cai, J.; Zhu, X.; Zhu, Y.; Wei, W.; Liu, X.; Luo, J. Molecularly imprinted polymeric nanoparticles decorated with Au NPs for highly sensitive and selective glucose detection. Biosens. Bioelectron. 2018, 100, 497–503. [Google Scholar] [CrossRef]
- Mani, S.; Vediyappan, V.; Chen, S.-M.; Madhu, R.; Pitchaimani, V.; Chang, J.-Y.; Liu, S.-B. Hydrothermal synthesis of NiWO4 crystals for high performance non-enzymatic glucose biosensors. Sci. Rep. 2016, 6, 24128. [Google Scholar] [CrossRef]
- Lang, X.-Y.; Fu, H.-Y.; Hou, C.; Han, G.-F.; Yang, P.; Liu, Y.-B.; Jiang, Q. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat. Commun. 2013, 4, 2169. [Google Scholar] [CrossRef]
- Mishra, A.K.; Jarwal, D.K.; Mukherjee, B.; Kumar, A.; Ratan, S.; Tripathy, M.R.; Jit, S. Au nanoparticles modified CuO nanowireelectrode based non-enzymatic glucose detection with improved linearity. Sci. Rep. 2020, 10, 11451. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.M.V.; Rajendran, V.; Mishra, R.K.; Jayaraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAC Trends Anal. Chem. 2020, 131, 116024. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Liu, B.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.C.; Shin, A.; Liu, X.; Wang, J. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef]
- Lisi, F.; Peterson, J.R.; Gooding, J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020, 148, 111835. [Google Scholar] [CrossRef]
- Drain, K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Lan, T.; Zhang, J.J.; Lu, Y. Transforming the blood glucose meter into a general healthcare meter for in vitro diagnostics in mobile health. Biotechnol. Adv. 2016, 34, 331–341. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ahn, J.K.; Park, K.S.; Park, H.G. Portable glucose meter-based label-free strategy for target DNA detection. Sens. Actuators B Chem. 2020, 310, 127808. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Yang, X.; Wang, K.; Liu, F.; Zhao, Q.; Liu, P.; Liu, R. Multiplex detection of nucleic acids using a low cost microfluidic chip and a personal glucose meter at the point-of-care. Chem. Commun. 2014, 50, 3824–3826. [Google Scholar] [CrossRef]
- Xu, X.-T.; Liang, K.-Y.; Zeng, J.-Y. Portable and sensitive quantitative detection of DNA using personal glucose meters and exonuclease III-assisted signal amplification. Analyst 2014, 139, 4982–4986. [Google Scholar]
- Zhu, X.; Kou, F.; Xu, H.; Lin, L.; Yang, G.; Lin, Z. A highly sensitive aptamer-immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sens. Actuators B Chem. 2017, 253, 660–665. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H.; Xu, H.; Lin, R.; Han, Y.; Yang, G.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. A reusable and portable immunosensor using personal glucose meter as transducer. Anal. Methods 2014, 6, 5264–5268. [Google Scholar] [CrossRef]
- Yang, W.; Lu, X.; Wang, Y.; Sun, S.; Liu, C.; Li, Z. Portable and sensitive detection of protein kinase activity by using commercial personal glucose meter. Sens. Actuators B Chem. 2015, 210, 508–512. [Google Scholar] [CrossRef]
- Joo, J.; Kwon, D.; Shin, H.H.; Park, K.-H.; Cha, H.J.; Jeon, S. A facile and sensitive method for detecting pathogenic bacteria using personal glucose meters. Sens. Actuators B Chem. 2013, 188, 1250–1254. [Google Scholar] [CrossRef]
- Wan, Y.; Qi, P.; Zeng, Y.; Sun, Y.; Zhang, D. Invertase-mediated system for simple and rapid detection of pathogen. Sens. Actuators B Chem. 2016, 233, 454–458. [Google Scholar] [CrossRef]
- Su, J.; Xu, J.; Chen, Y.; Xiang, Y.; Yuan, R.; Chai, Y. Sensitive detection of copper(II) by a commercial glucometer using click chemistry. Biosens. Bioelectron. 2013, 45, 219–222. [Google Scholar] [CrossRef]
- Xu, X.-T.; Liang, K.-Y.; Zeng, J.-Y. Highly sensitive and portable mercury(ii) ion sensor using personal glucose meter. Anal. Methods 2015, 7, 81–85. [Google Scholar]
- Wang, Q.; Liu, F.; Yang, X.; Wang, K.; Wang, H.; Deng, X. Sensitive point-of-care monitoring of cardiac biomarker myoglobin using aptamer and ubiquitous personal glucose meter. Biosens. Bioelectron. 2015, 64, 161–164. [Google Scholar] [CrossRef]
- Bruch, R.; Baaske, J.; Chatelle, C.; Meirich, M.; Madlener, S.; Weber, W.; Dincer, C.; Urban, G.A. Biosensors: CRISPR/Cas13a-Powered Electrochemical Microfluidic Biosensor for Nucleic Acid Amplification-Free miRNA Diagnostics. Adv. Mater. 2019, 31, 1905311. [Google Scholar] [CrossRef] [PubMed]






| Enzymatic Glucose Sensors | Sensitivity | Linear Range | Detection Limit | Reproducibility | Life Time | Ref. |
|---|---|---|---|---|---|---|
| GR–CNT–ZnO–GOx | 5.36 (±0.072) µA mM−1 cm−2 | 0.01–6.5 mM | 4.5 (±0.08) µM | RSD 3.24% (n = 5) | 94.6% peak current was retained even after 4 weeks at 4 °C | [82] |
| ERGOc–MWCNTd/GOx/Nf | 7.95 µA mM−1 cm−2 | 0.01–6.5 mM | 4.7 µM | RSD 205% (n = 7) | The biosensor retained its 90% peak current after one month | [83] |
| Fc/GOD/Au/SLG | - | 0.0005–5000 µM | 0.0001 µM | RSD 3.8% (n = 6) | After two weeks, the current response retained 81% of the initial response | [24] |
| Au/GNS-PEI-AuNPs/Glu-GOx | 93 μA mM−1 cm−2 | 1–100 µM | 0.32 µM | RSD 6.7% (n = 5) | The biosensor was kept in PBS (0.1 M, pH 7.0) at 4 °C while not in use. The current response maintained 88% of its initial value after 10 days. | [84] |
| PANI-SDS-F127(1:1)/GOx | 485.787 μA mM−1 cm−2 | 5–50 mM | 3.202 μM | - | - | [85] |
| Pt-CNT-muc 50% | 15 mA M−1 cm−2 | 0.002–3.2 mM | 3 μM | RSD, 2.2% for the set of evaluated samples | 300 days | [86] |
| GOx/PVA-Fe3O4/Sn | 9.36 µA mM−1 | 0.005–30 mM | 8 μM | RSD 4.2% (n = 5) | The current response of biosensor is maintained about 81% of its initial response after a month. | [87] |
| Au–Ni coaxial nanorad array/GOx | 778.2 μA mM−1 cm−2 | 0.0275–27.75 mM | 5.5 μM | - | The measured peak current dropped by approximately 13% after 30 days storage at 4 °C | [88] |
| CS/GOx–PABA–Aunano/Au-plated Au | 97.7 μA mM−1 cm−2 | 0.002–3.7 mM | 0.1 μM | RSD 4.2% (n = 5) | The current response of the sensor maintains 85% of the initial current response after 1 month. | [89] |
| GOx/Pt/rGO/P3ABA | 22.01 μA mM−1 cm−2 | 0.25–6.00 mM | 44.3 μM | RSD 2.58% (n = 5) | After 7 days, the storage electrode retained the current response of ca. 86% of the initial response | [90] |
| GOD/CS-rGO/AuNPs/Pt electrode | 102.4 μA mM−1 cm−2 | 0.01–2.13 mM | 1.7 μM | RSD 3.2% (n = 5) | After one month, 15% loss of its initial current response was observed | [91] |
| GOx/PtNP/PANI/PtE | 96.1 μA mM−1 cm−2 | 0.01–8 mM | 0.7 μM | - | - | [92] |
| GOx/gold/MoS2/gold nanofilm on the polymer electrode | - | 10–500 nM | 10 nM | - | - | [93] |
| Non-Enzymatic Glucose Sensors | Sensitivity | Linear Range | Detection Limit | Reproducibility | Life Time | Ref. |
|---|---|---|---|---|---|---|
| SWCNTs/Cu2O/ZnO nanorods (NRs)/graphene | 466.1 & 203.1 µA mM−1 cm−2 | 0–5.556 & 5.556–11.111 mM | - | - | - | [104] |
| CuO/PANI-NF/FTO | 2800 & 1359 µA mM−1 cm−2 | 0.00025–0.28 & 0.28–4.6 mM | 0.24 µM | RSD 3.6% | after 11 days, the electrode showed ∼90% of its initial signal | [105] |
| Ni(OH)2/CNT fiber microelectrode | 12.2 mA cm−2 mM−1 | 20 µM–10.5 mM | 0.645 μM | - | - | [106] |
| CuO/NiO/PANI/GCE | - | 20–2500 μM | 2 μM | RSD 3.8% (n = 5) | a loss of only approximately 10.8% in current response after 15 days | [107] |
| coral-like Cu micro-/nano-structure arrays | 3826 μA mM−1 cm−2 | 0.20 μM–1.90 mM | 0.04 μM | RSD 2.51% (n = 6) | 14 days; 11.5% loss in current response | [108] |
| Au@MIP sensor | - | 10−10 to 10−8 mol L−1 and 10−8 to 100 mol L−1 | 3 × 10−12 mol L−1 | Although the response of the sensor was nearly identical for the electrode originating from same copolymer and Au@MIPNs solution, these copolymers and Au@MIPNs were individually handmade, resulting that there was imperfect reproducibility from one batch to another. | 12 days with only a slight decrease in the current | [109] |
| NiWO4-modified GCE | 269.6 μA mM−1 cm−2 | 0.004 μM–4.1 mM | 0.18 μM | RSD 2.7% % (n = 5) | After 50 consecutive CV cycles, it was found that the glucose sensor retained ca. 97.2% of its initial oxidation peak potential value | [110] |
| S/NPG/Co3O4 hybrid microelectrode | 12.5 mA mM−1 cm−2 | 1 μM–10 mM | 5 nM | - | The aging tolerance ensures the S/NPG/Co3O4 hybrid microelectrode to retain ~99.5% of its original current response over a storage period of 15 days at room temperature. Even the S/NPG/Co3O4 hybrid microelectrode stored at room temperature for 4 months still maintains the high capability to analyze serum sample at ultralow concentrations | [111] |
| CuO NWs with Au NPs | 1591.44 μA mM−1 cm−2 | 0.001 mM–44.36 mM | 0.3 μA | RSD 5% (n = 10) | The sensor response retains 95% (measured on day 30) of its original response (measured on day 0). | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. https://doi.org/10.3390/s20216013
Juska VB, Pemble ME. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors. 2020; 20(21):6013. https://doi.org/10.3390/s20216013
Chicago/Turabian StyleJuska, Vuslat B., and Martyn E. Pemble. 2020. "A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems" Sensors 20, no. 21: 6013. https://doi.org/10.3390/s20216013
APA StyleJuska, V. B., & Pemble, M. E. (2020). A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors, 20(21), 6013. https://doi.org/10.3390/s20216013






