Degradability of Biodegradable Soil Moisture Sensor Components and Their Effect on Maize (Zea mays L.) Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sensor Component Materials
2.2. Crop Management
2.3. Plant and Material Data Collection
2.4. Statistical Analysis
3. Results
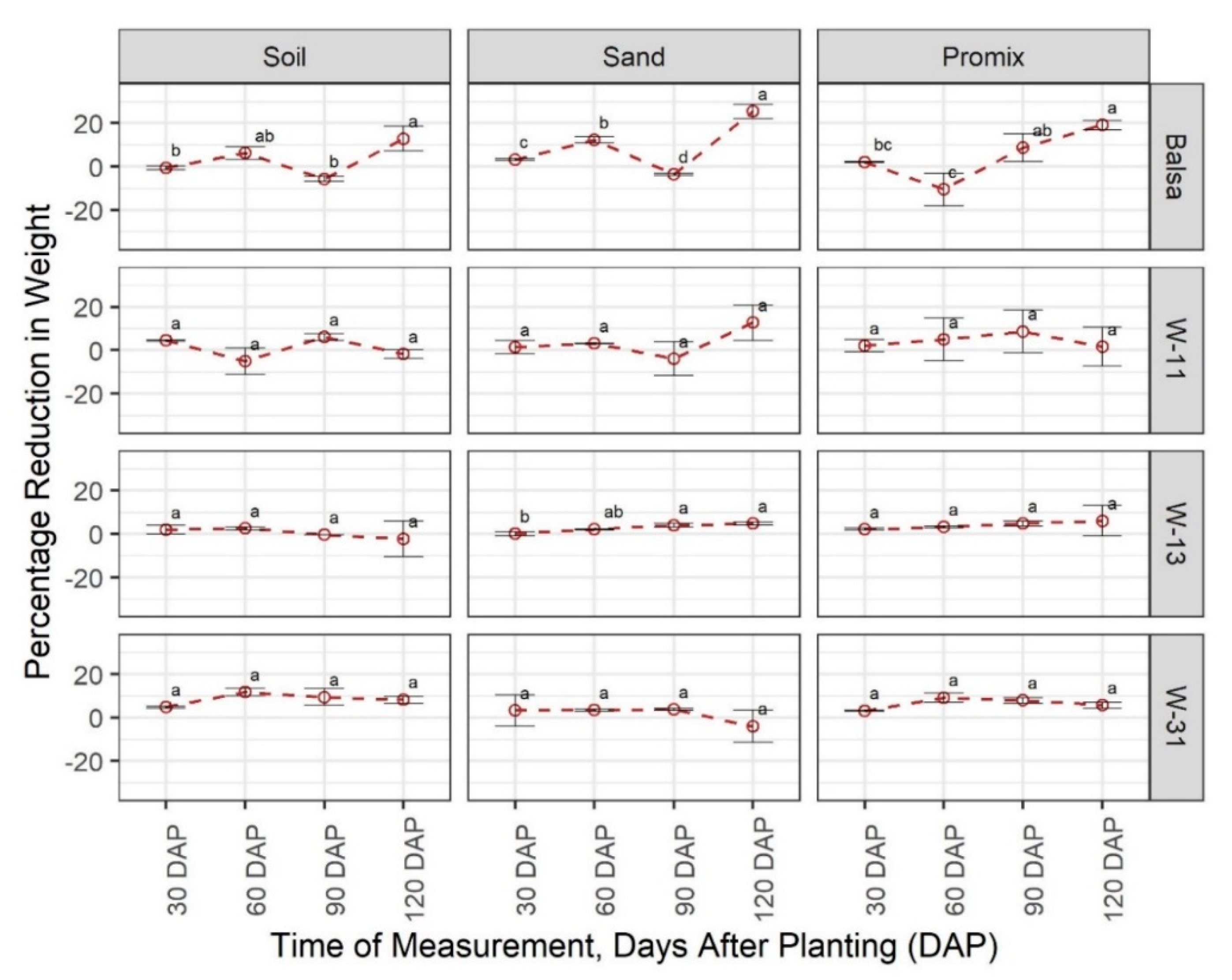
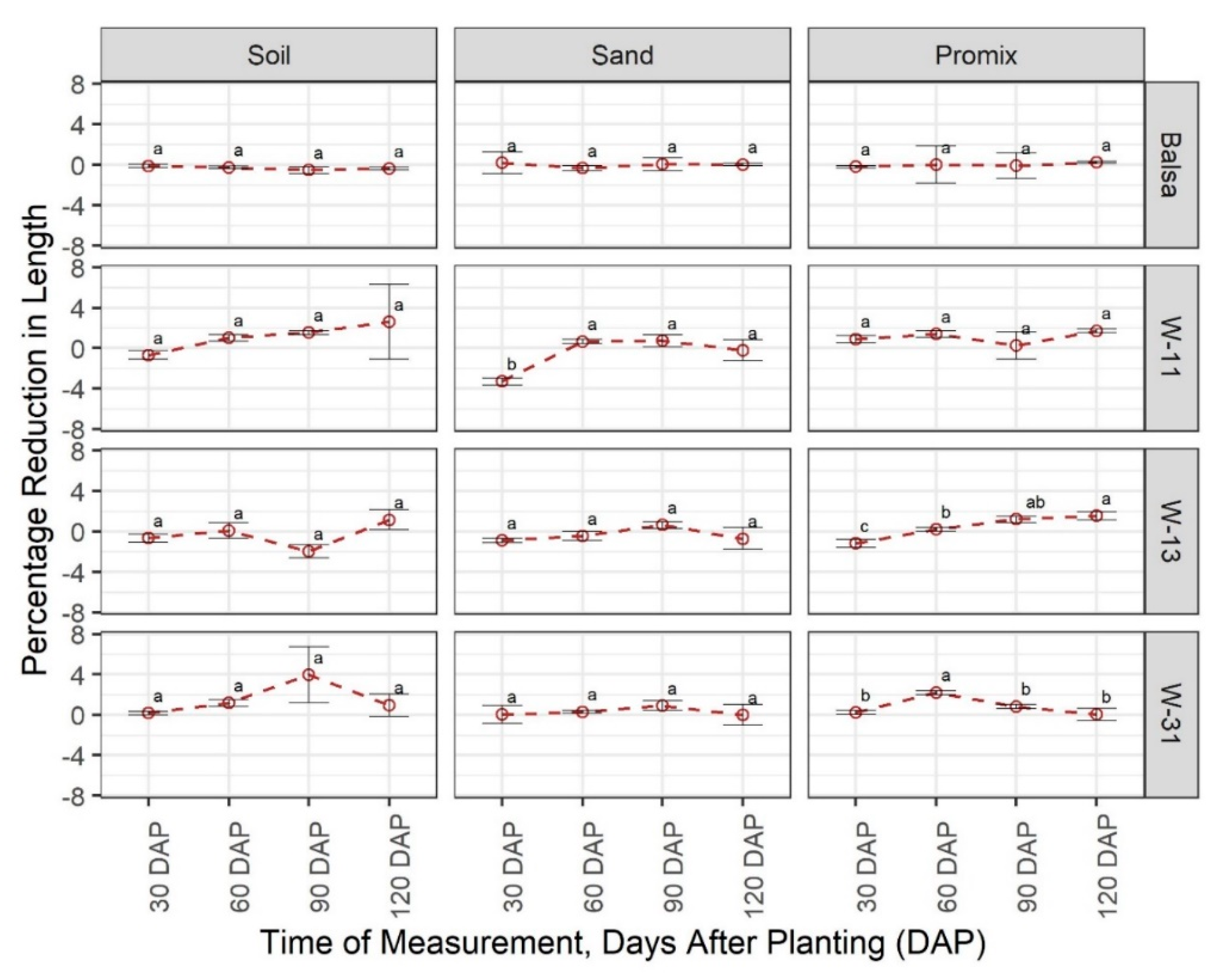
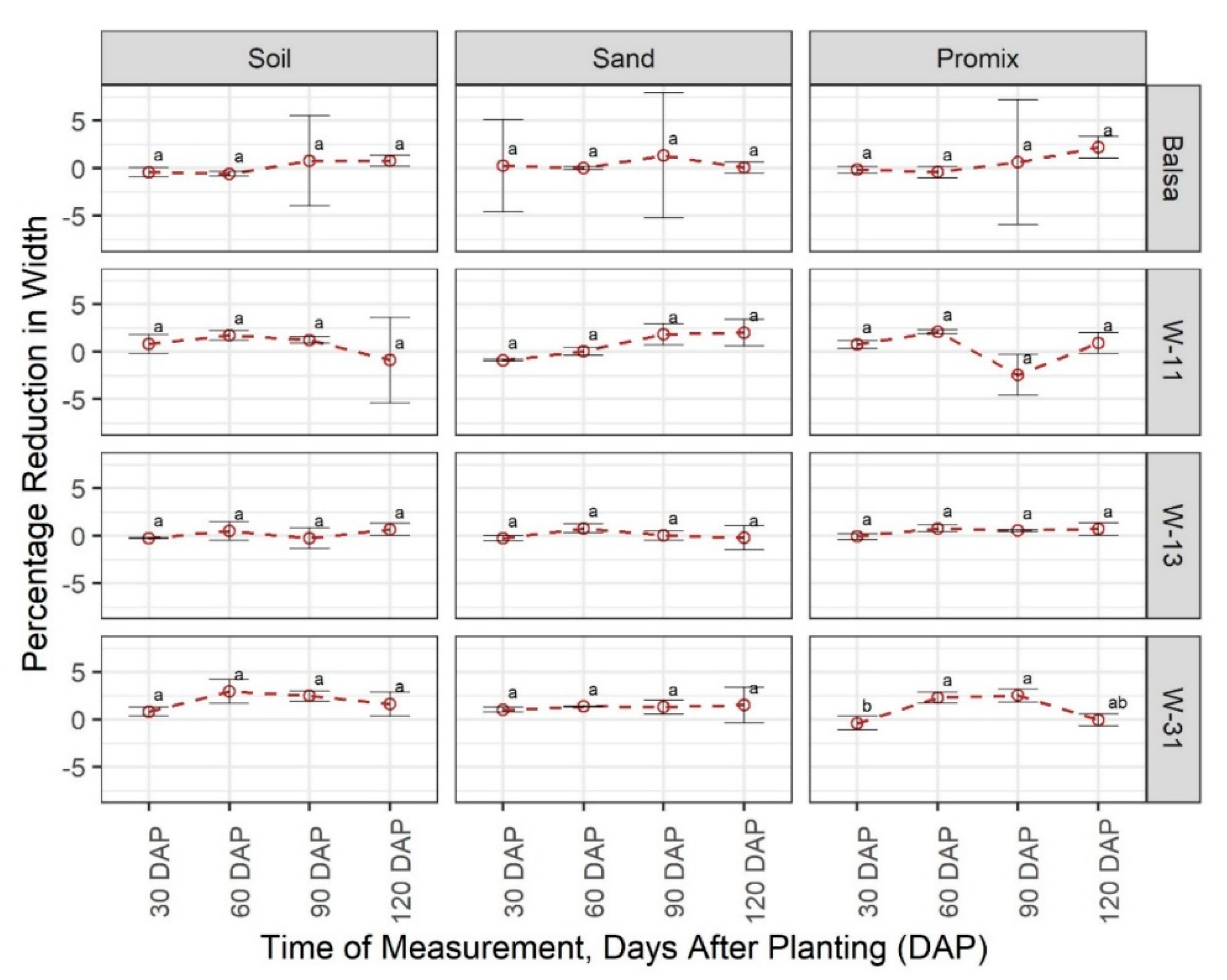
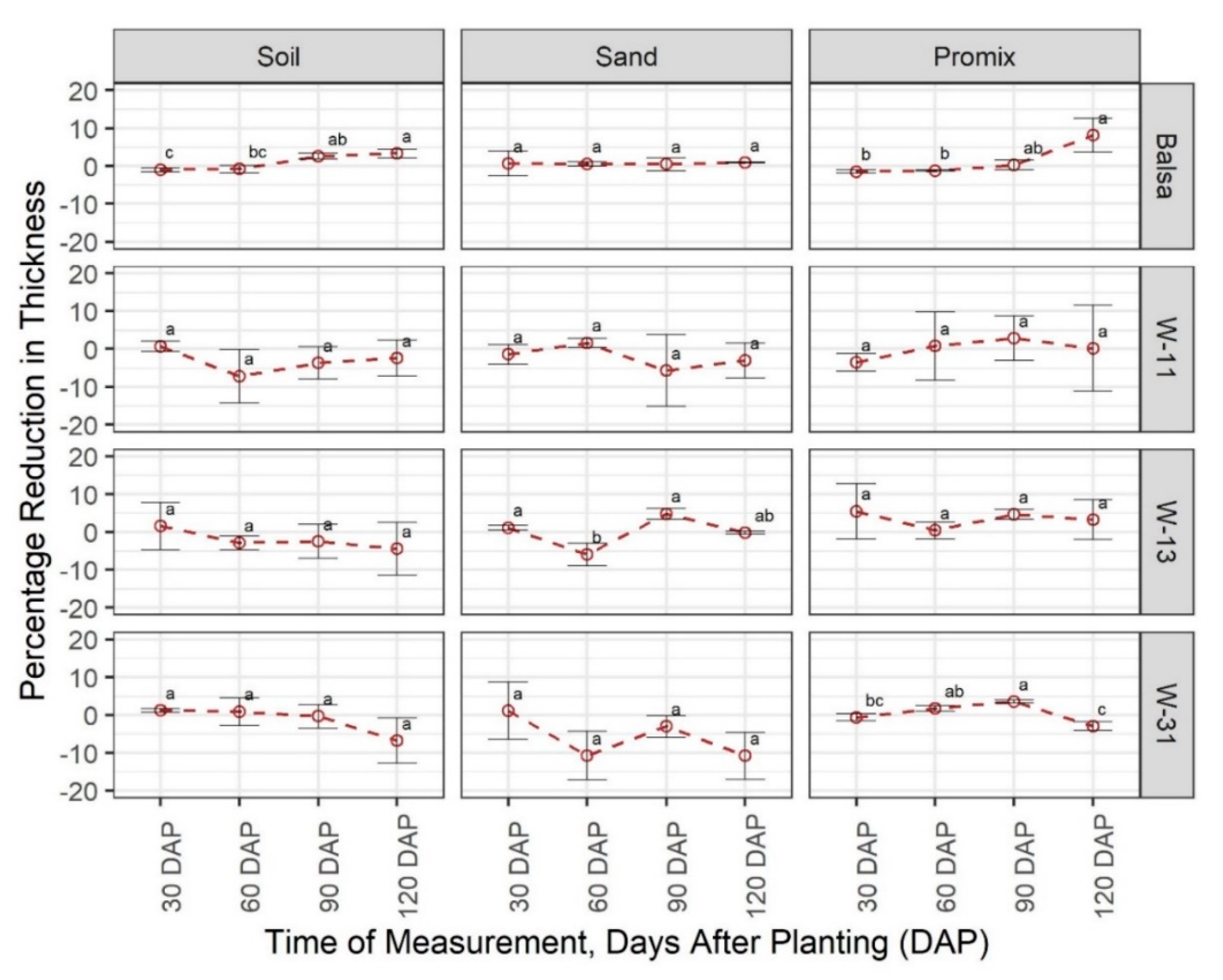
3.1. Degradability of Materials
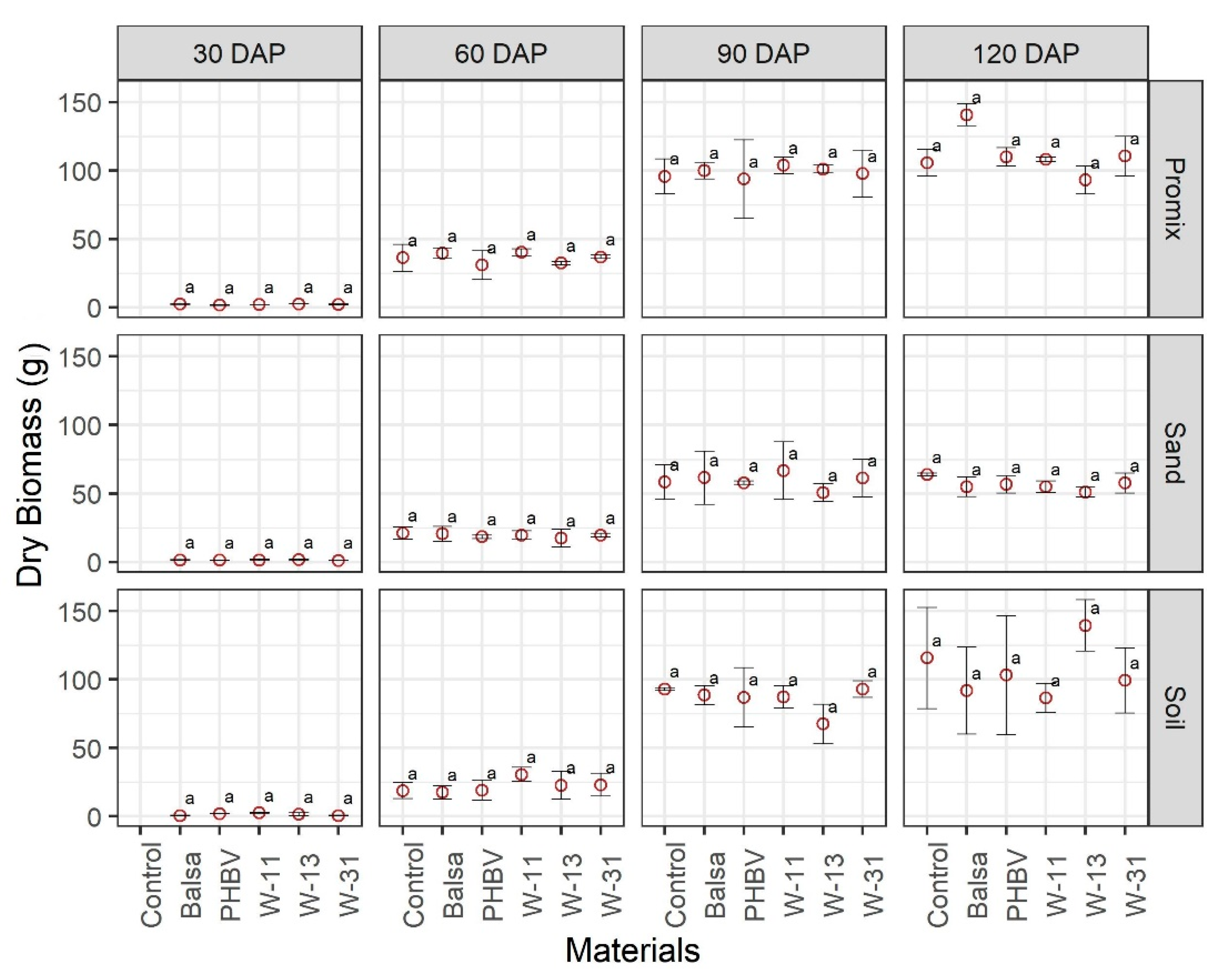
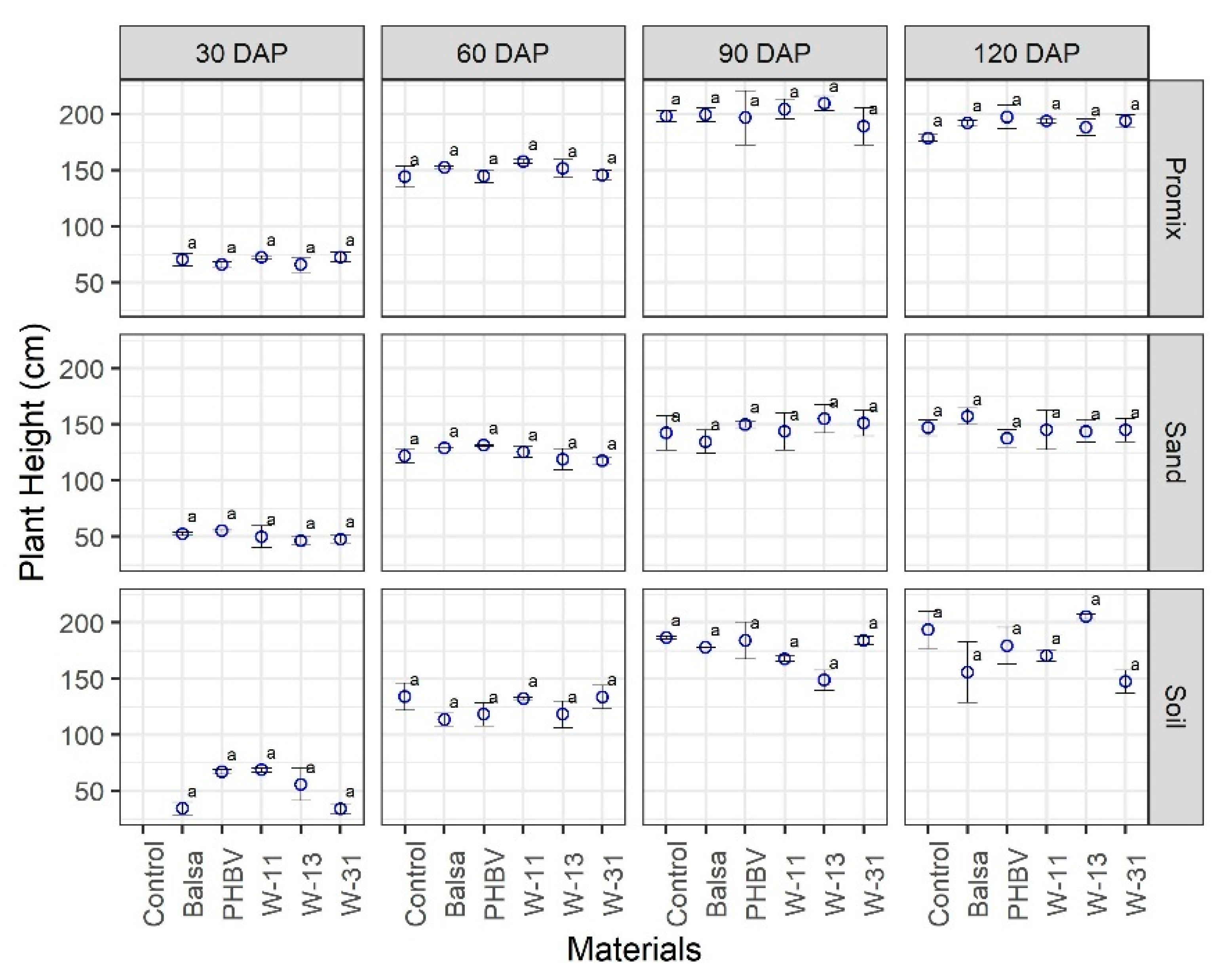
3.2. Effect of Materials on Growth and Development of Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, J.; McCulloch, J. Soil moisture estimation by the neutron scattering method in Britain. J. Hydrol. 1966, 4, 254–263. [Google Scholar] [CrossRef]
- Van Bavel, C. Neutron measurement of surface soil moisture. J. Geophys. Res. 1961, 66, 4193–4198. [Google Scholar] [CrossRef]
- Edokossi, K.; Calabia, A.; Jin, S.; Molina, I. GNSS-Reflectometry and Remote Sensing of Soil Moisture: A Review of Measurement Techniques, Methods, and Applications. Remote Sens. 2020, 12, 614. [Google Scholar] [CrossRef] [Green Version]
- Ledieu, J.; De Ridder, P.; De Clerck, P.; Dautrebande, S. A method of measuring soil moisture by time-domain reflectometry. J. Hydrol. 1986, 88, 319–328. [Google Scholar] [CrossRef]
- Kaleita, A.L.; Tian, L.F.; Hirschi, M.C. Relationship between soil moisture content and soil surface reflectance. Trans. ASAE 2005, 48, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- SU, S.L.; Singh, D.; Baghini, M.S. A critical review of soil moisture measurement. Measurement 2014, 54, 92–105. [Google Scholar] [CrossRef]
- Ridley, A.; Burland, J. A new instrument for the measurement of soil moisture suction. Géotechnique 1993, 43, 321–324. [Google Scholar] [CrossRef]
- Anderson, N.W. Non-Toxic, Biodegradable Sensor Nodes for Use with a Wireless Network. U.S. Patent 8,063,774, 22 November 2011. [Google Scholar]
- Bauer-Reich, C.; Hoey, J.; Sailer, R.; Schneck, N.; Ulven, C. Biodegradable Soil Sensor, System and Method. U.S. Patent 9,964,532, 8 May 2018. [Google Scholar]
- Allen, M.G. Microfabricated implantable wireless microsystems: Permanent and biodegradable implementations. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 1–4. [Google Scholar]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef]
- Luo, M.; Martinez, A.W.; Song, C.; Herrault, F.; Allen, M.G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromechanical Syst. 2013, 23, 4–13. [Google Scholar] [CrossRef]
- MacLeod, J.; Rosei, F. Photonic crystals: Sustainable sensors from silk. Nat. Mater. 2013, 12, 98–100. [Google Scholar] [CrossRef]
- Schusser, S.; Poghossian, A.; Bäcker, M.; Leinhos, M.; Wagner, P.; Schöning, M.J. Characterization of biodegradable polymers with capacitive field-effect sensors. Sens. Actuators B Chem. 2013, 187, 2–7. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, G.A.; Sülzle, J.; Dalla Valle, F.; Cantarella, G.; Robotti, F.; Jokic, P.; Knobelspies, S.; Daus, A.; Büthe, L.; Petti, L. Biodegradable and highly deformable temperature sensors for the internet of things. Adv. Funct. Mater. 2017, 27, 1702390. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, W.; Rahimi, R.; Ziaie, B. A Biodegradable Sensor Housed in 3D Printed Porous Tube for in-Situ Soil Nitrate Detection. In Proceedings of the Hilton Head Workshop 2018: A Solid-State Sensors, Actuators and Microsystems Workshop, Hilton Head Island, SC, USA, 3–7 June 2018; pp. 148–151. [Google Scholar]
- Barouti, G.; Jaffredo, C.G.; Guillaume, S.M. Advances in drug delivery systems based on synthetic poly (hydroxybutyrate)(co) polymers. Prog. Polym. Sci. 2017, 73, 1–31. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Olkhov, A.; Siracusa, V.; Iordanskii, A. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P. Applications of PHB-a microbially produced biodegradable thermoplastic. Phys. Technol. 1985, 16, 32. [Google Scholar] [CrossRef]
- Pouton, C.W.; Akhtar, S. Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv. Drug Deliv. Rev. 1996, 18, 133–162. [Google Scholar] [CrossRef]
- McDonough, K.; Itrich, N.; Casteel, K.; Menzies, J.; Williams, T.; Krivos, K.; Price, J. Assessing the biodegradability of microparticles disposed down the drain. Chemosphere 2017, 175, 452–458. [Google Scholar] [CrossRef]
- Alves, L.P.S.; do Amaral, F.P.; Kim, D.; Bom, M.T.; Gavídia, M.P.; Teixeira, C.S.; Holthman, F.; de Oliveira Pedrosa, F.; de Souza, E.M.; Chubatsu, L.S. Importance of poly-3-hydroxybutyrate metabolism to the ability of Herbaspirillum seropedicae to promote plant growth. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Hatzinger, P.B.; Alexander, M. Biodegradation of organic compounds sequestered in organic solids or in nanopores within silica particles. Environ. Toxicol. Chem. 1997, 16, 2215–2221. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T. Chemical modification of partially hydrogenated vegetable oil to improve its functional properties for candles. J. Am. Oil Chem. Soc. 2007, 84, 1149–1159. [Google Scholar] [CrossRef]
- Rezaei, K.; Wang, T.; Johnson, L.A. Hydrogenated vegetable oils as candle wax. J. Am. Oil Chem. Soc. 2002, 79, 1241–1247. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Grimaldi, R.; Gioielli, L.A.; dos Santos, A.O.; Cardoso, L.P.; Gonçalves, L.A.G. Thermal behavior, microstructure, polymorphism, and crystallization properties of zero trans fats from soybean oil and fully hydrogenated soybean oil. Food Biophys. 2009, 4, 106–118. [Google Scholar] [CrossRef]
- Hita, C.; Parlanti, E.; Jambu, P.; Joffre, J.; Ambles, A. Triglyceride degradation in soil. Org. Geochem. 1996, 25, 19–28. [Google Scholar] [CrossRef]
- Sousa, D.Z.; Pereira, M.A.; Stams, A.J.; Alves, M.M.; Smidt, H. Microbial communities involved in anaerobic degradation of unsaturated or saturated long-chain fatty acids. Appl. Environ. Microbiol. 2007, 73, 1054–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasi, R.J.; Dalrymple, G.W. Biostimulation Agent for Bioremediation and Methods Therefor. U.S. Patent 12/090,927, 11 June 2009. [Google Scholar]
- Da Silva, A.; Kyriakides, S. Compressive response and failure of balsa wood. Int. J. Solids Struct. 2007, 44, 8685–8717. [Google Scholar] [CrossRef] [Green Version]
- TranVan, L.; Legrand, V.; Jacquemin, F. Thermal decomposition kinetics of balsa wood: Kinetics and degradation mechanisms comparison between dry and moisturized materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Staff, S.S. Soil Survey of Larimer County, Colorado; USDA Coop. Soil Survey: Denver, CO, USA, 1980. [Google Scholar]
- Shaver, T.; Khosla, R.; Westfall, D. Evaluation of Two Ground-Based Active Crop Canopy Sensors in Maize: Growth Stage, Row Spacing, and Sensor Movement Speed. Soil Sci. Soc. Am. J. 2010, 74, 2101–2108. [Google Scholar] [CrossRef]
- Longchamps, L.; Khosla, R. Early detection of nitrogen variability in maize using fluorescence. Agron. J. 2014, 106, 511–518. [Google Scholar] [CrossRef]
- Siqueira, R.; Longchamps, L.; Dahal, S.; Khosla, R. Use of Fluorescence Sensing to Detect Nitrogen and Potassium Variability in Maize. Remote Sens. 2020, 12, 1752. [Google Scholar] [CrossRef]
- Fauci, M.F.; Dick, R. Plant Response to Organic Amendments and Decreasing Inorganic Nitrogen Rates in Soils froma Long-Term Experiment. Soil Sci. Soc. Am. J. 1994, 58, 134–138. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Pearson: Boston, MA, USA, 1977; Volume 2. [Google Scholar]
- R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-104R Development Core Team. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 16 June 2020).

| Material | Abbreviation | Function in Sensor | Description |
|---|---|---|---|
| Balsa wood | B | Structural substrate | Lightweight natural substance |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | PHBV | Printing substrate | Rapidly biodegradable carbon polymer |
| 1:1 Beeswax:soy wax blend | W-1:1 | Encapsulant | Beeswax, natural wax produced by bees. Denser than soy wax |
| 1:3 Beeswax:soy wax blend | W-1:3 | Encapsulant | Soy wax, natural wax produced from soybean. Softer than beeswax |
| 3:1 Beeswax:soy wax blend | W-3:1 | Encapsulant |
| Material | Attribute | Mean | SD |
|---|---|---|---|
| W-11 | Length (mm) | 14.991 | 0.092 |
| Width (mm) | 15.021 | 0.124 | |
| Thickness (mm) | 5.238 | 0.531 | |
| Weight (g) | 1.068 | 0.109 | |
| W-13 | Length (mm) | 14.977 | 0.099 |
| Width (mm) | 14.967 | 0.104 | |
| Thickness (mm) | 5.473 | 0.499 | |
| Weight (g) | 1.081 | 0.097 | |
| W-31 | Length (mm) | 15.181 | 0.141 |
| Width (mm) | 15.171 | 0.082 | |
| Thickness (mm) | 5.118 | 0.661 | |
| Weight (g) | 1.066 | 0.130 | |
| Balsa | Length (mm) | 50.525 | 0.627 |
| Width (mm) | 24.963 | 1.101 | |
| Thickness (mm) | 6.530 | 0.123 | |
| Weight (g) | 1.174 | 0.162 |
| Soil | Promix | Sand | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W-31-H1 | B-H1 | W-11-H3 | W-11-H4 | W-31-H2 | W-11-H2 | W-13-H2 | B-H1 | W-11-H1 | W-13-H1 | C1 | |
| C2 | W-31-H2 | B-H3 | PHBV-H3 | W-31-H4 | W-31-H1 | C2 | W-11-H4 | B-H4 | PHBV-H1 | PHBV-H3 | W-31-H3 |
| W-13-H2 | W-13-H1 | W-31-H3 | PHBV-H2 | W-31-H3 | PHBV-H1 | PHBV-H3 | PHBV-H4 | B-H2 | W-11-H4 | W-11-H2 | C2 |
| W-31-H4 | C1 | W-13-H4 | B-H4 | B-H2 | W-13-H4 | B-H4 | C3 | W-13-H3 | W-31-H1 | W-11-H3 | |
| W-11-H2 | C3 | PHBV-H4 | W-11-H1 | W-11-H3 | W-13-H3 | C3 | W-13-H4 | B-H3 | W-13-H2 | P HBV-H2 | |
| PHBV-H1 | B-H2 | W-13-H3 | W-11-H1 | C1 | PHBV-H2 | W-13-H1 | B-H3 | W-31-H4 | B-H1 | W-31-H2 | PHBV-H4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, S.; Yilma, W.; Sui, Y.; Atreya, M.; Bryan, S.; Davis, V.; Whiting, G.L.; Khosla, R. Degradability of Biodegradable Soil Moisture Sensor Components and Their Effect on Maize (Zea mays L.) Growth. Sensors 2020, 20, 6154. https://doi.org/10.3390/s20216154
Dahal S, Yilma W, Sui Y, Atreya M, Bryan S, Davis V, Whiting GL, Khosla R. Degradability of Biodegradable Soil Moisture Sensor Components and Their Effect on Maize (Zea mays L.) Growth. Sensors. 2020; 20(21):6154. https://doi.org/10.3390/s20216154
Chicago/Turabian StyleDahal, Subash, Wubengeda Yilma, Yongkun Sui, Madhur Atreya, Samantha Bryan, Valerie Davis, Gregory Lewis Whiting, and Raj Khosla. 2020. "Degradability of Biodegradable Soil Moisture Sensor Components and Their Effect on Maize (Zea mays L.) Growth" Sensors 20, no. 21: 6154. https://doi.org/10.3390/s20216154







