A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
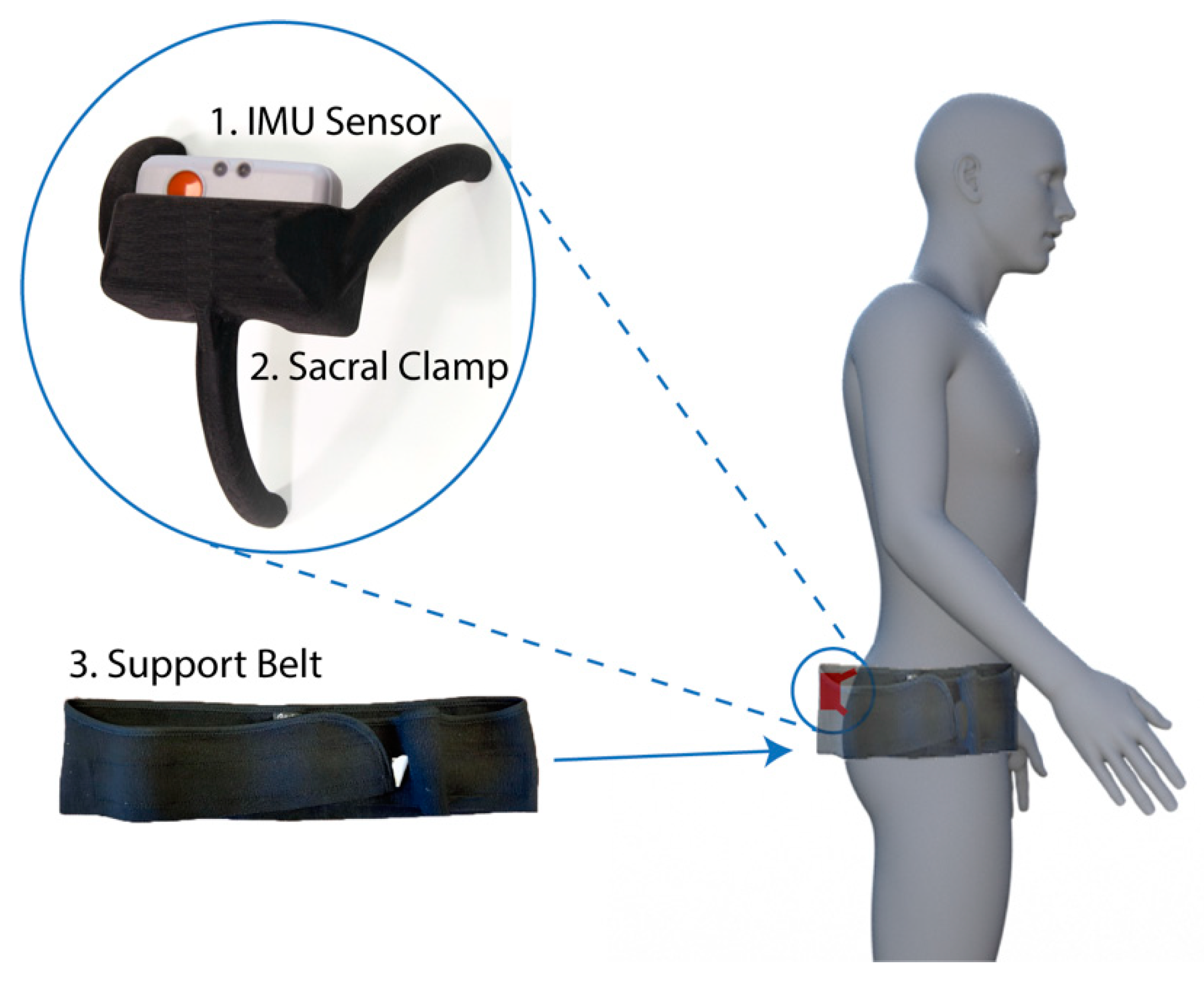
2.1. Pelvis Motion Tracking Device
- Inertial measurement unit (IMU). A research-grade IMU (Shimmer3; Shimmer, Dublin, Ireland) was used to calculate the pelvic tilt. The device (dimensions, 51 × 34 × 14 mm) housed three sensors: An accelerometer, gyroscope, and magnetometer, with the respective measures recorded in 3-axes. For this study, we only used the data from the accelerometer (sensitivity: 660 ± 19.8 mV/g). The device connected to a host computer wirelessly via a Bluetooth serial connection. For these experiments, a script was produced using the Matlab programming language (v2017b; Mathworks Inc., MA, USA) to capture the data with a sampling rate of 200 Hz.
- Sacral clamp. The IMU device was housed in a custom-designed “sacral clamp” which allowed the sensor to lie across the participant’s sacrum. The sacral area was chosen due to it having the least amount of skin/fat thickness between the sensor and the pelvic bone and hence provided a feasible location to best track the tilt of the pelvis accurately.
- Support belt. To hold the sacral clamp securely in place, a wide elasticated belt was fitted around the individual’s waist. The belt used was a pregnancy support belt, made of elasticated material, such that it provided the flexibility for the individual to move freely, while holding the sacral clamp firmly in place.
2.2. Sample
2.3. Ethical Review
2.4. Procedure
2.5. IMU Pelvic Tilt Measure
2.6. Radiographic Measures of Pelvic Tilt
2.7. Analyses
3. Results
3.1. Correlation with Radiograph Measures
3.2. Bland-Altman Analysis
3.3. Error Distribution
3.4. Relationship of Error with Body Mass Index and Gender
3.5. Classification Accuracy
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A—Measuring the Accuracy of the Sensor Device and Algorithm
Appendix A.1. Equipment
- Inertial measurement unit: A research-grade IMU (Shimmer3; Shimmer, Dublin, Ireland) was used, the same as that used to capture the patient data in our main manuscript. The device (dimensions, 51 × 34 × 14 mm) housed three sensors: Accelerometer, gyroscope, and magnetometer, with the respective measures recorded in 3-axes. For calculating tilt, we only used the data from the accelerometer (sensitivity: 660 ± 19.8 mV/g). The device connected to a host computer wirelessly via a Bluetooth serial connection, with data collected at a sampling rate of 200 Hz, using the Matlab programming language (v2017b; Mathworks Inc., Natick, MA, USA).
- Robot arm: An industrial robot arm, KUKA KR10 R900 (KUKA AG, Augsburg, Germany), was used to tilt the device in terms of both pitch and roll (see Figure A1a). The robot had a pose repeatability of ±0.03 mm and complied to the ISO9283 standard (https://www.iso.org/standard/22244.html).
Appendix A.2. Setup

Appendix A.3. Procedure
Appendix A.4. Analysis
Appendix A.5. Results

References
- Falez, F.; Papalia, M.; Favetti, F.; Panegrossi, G.; Casella, F.; Mazzotta, G. Total hip arthroplasty instability in Italy. Int. Orthop. 2017, 41, 635–644. [Google Scholar] [CrossRef] [PubMed]
- de Palma, L.; Procaccini, R.; Soccetti, A.; Marinelli, M. Hospital cost of treating early dislocation following hip arthroplasty. HIP Int. 2012, 22, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lewinnek, G.E.; Lewis, J.L.; Tarr, R.; Compere, C.L.; Zimmerman, J.R. Dislocations after total hip-replacement arthroplasties. J. Bone Jt. Surg. Am. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Seagrave, K.G.; Troelsen, A.; Malchau, H.; Husted, H.; Gromov, K. Acetabular cup position and risk of dislocation in primary total hip arthroplasty. Acta Orthop. 2017, 88, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, Z.C.; Coury, J.G.; Cohen, J.L.; Dorr, L.D. The Current Knowledge on Spinopelvic Mobility. J. Arthroplast. 2018, 33, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; von Roth, P.; Jennings, M.T.; Hanssen, A.D.; Pagnano, M.W. What Safe Zone? The Vast Majority of Dislocated THAs Are Within the Lewinnek Safe Zone for Acetabular Component Position. Clin. Orthop. 2016, 474, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivière, C.; Lazennec, J.-Y.; Van Der Straeten, C.; Auvinet, E.; Cobb, J.; Muirhead-Allwood, S. The influence of spine-hip relations on total hip replacement: A systematic review. Orthop. Traumatol. Surg. Res. 2017, 103, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Y.; Zhang, H. The Influence of Pelvic Tilt on the Anteversion Angle of the Acetabular Prosthesis. Orthop. Surg. 2019, 11, 762–769. [Google Scholar] [CrossRef]
- Pierrepont, J.; Hawdon, G.; Miles, B.P.; Connor, B.O.; Baré, J.; Walter, L.R.; Marel, E.; Solomon, M.; McMahon, S.; Shimmin, A.J. Variation in functional pelvic tilt in patients undergoing total hip arthroplasty. Bone Jt. J. 2017, 99-B, 184–191. [Google Scholar] [CrossRef]
- Schwarzkopf, R.; Muir, J.M.; Paprosky, W.G.; Seymour, S.; Cross, M.B.; Vigdorchik, J.M. Quantifying Pelvic Motion During Total Hip Arthroplasty Using a New Surgical Navigation Device. J. Arthroplast. 2017, 32, 3056–3060. [Google Scholar] [CrossRef]
- Roettges, P.S.; Hannallah, J.R.; Smith, J.L.; Ruth, J.T. Predictability of Pelvic Tilt During Total Hip Arthroplasty Using a Traction Table. J. Arthroplast. 2018, 33, 2556–2559. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y. Measurement of Pelvic Movement during Total Hip Arthroplasty Using a Hip Navigation System. Orthop. Surg. 2019, 4, 4. [Google Scholar]
- Inaba, Y.; Kobayashi, N.; Suzuki, H.; Ike, H.; Kubota, S.; Saito, T. Preoperative planning for implant placement with consideration of pelvic tilt in total hip arthroplasty: Postoperative efficacy evaluation. BMC Musculoskelet. Disord. 2016, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Nakashima, Y.; Yamamoto, T.; Motomura, G.; Kanazawa, M.; Takagishi, K.; Iwamoto, Y. Late Anterior Dislocation Due to Posterior Pelvic Tilt in Total Hip Arthroplasty. Open Orthop. J. 2016, 10, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Shon, W.Y.; Sharma, V.; Oh, J.-K.; Moon, J.G.; Suh, D.H. Can pelvic tilting be ignored in total hip arthroplasty? Int. J. Surg. Case Rep. 2014, 5, 633–636. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiak, M.; Rychlik, M.; Musielak, B.; Chen, B.P.-J.; Idzior, M.; Grzegorzewski, A. An accurate method of radiological assessment of acetabular volume and orientation in computed tomography spatial reconstruction. BMC Musculoskelet. Disord. 2015, 16, 42. [Google Scholar] [CrossRef] [Green Version]
- Tyrakowski, M.; Yu, H.; Siemionow, K. Pelvic incidence and pelvic tilt measurements using femoral heads or acetabular domes to identify centers of the hips: Comparison of two methods. Eur. Spine J. 2015, 24, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Tyler, T.; Zook, L.; Brittis, D.; Gleim, G. A new pelvic tilt detection device: Roentgenographic validation and application to assessment of hip motion in professional ice hockey players. J. Orthop. Sports Phys. Ther. 1996, 24, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Nagahara, R.; Gleadhill, S.; Ishizuka, T.; Ohnuma, H.; Ohgi, Y. Measurement of Pelvic Orientation Angles during Sprinting Using a Single Inertial Sensor. Proceedings 2020, 49, 10. [Google Scholar] [CrossRef]
- King, R. Evaluation of X-ray, Acetabular Guides and CT in THR (EXACT). Available online: https://clinicaltrials.gov/ct2/show/record/NCT03072706 (accessed on 16 October 2019).
- Marel, E.; Walter, L.; Solomon, M.; Shimmin, A.; Pierrepont, J. Patient-specific acetabular cup orientation in functional positions using musculoskeletal modelling: A pre-operative planning tool. Orthop. Proc. 2016, 98-B, 19. [Google Scholar]
- Pierrepont, J.W.; Stambouzou, C.Z.; Miles, B.P.; O’Connor, P.B.; Walter, L.; Ellis, A.; Molnar, R.; Baré, J.V.; Solomon, M.; McMahon, S.; et al. Patient Specific Component Alignment in Total Hip Arthroplasty. Reconstr. Rev. Open Access Orthop. J. Reconstr. Arthroplast. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Ozyagcilar, T. Implementing a Tilt-Compensated Ecompass Using Accelerometer and Magnetometer Sensors; Freescale Semiconductor Application Note AN4248; Freescale Semiconductor: Austin, TX, USA, 2012. [Google Scholar]
- DiGioia, A.M.I.; Hafez, M.A.; Jaramaz, B.; Levison, T.J.; Moody, J.E. Functional Pelvic Orientation Measured from Lateral Standing and Sitting Radiographs. Clin. Orthop. Relat. Res. 2006, 453, 272. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. J. R. Stat. Soc. Ser. Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Lembeck, B.; Mueller, O.; Reize, P.; Wuelker, N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005, 76, 517–523. [Google Scholar] [CrossRef]
- Fourchet, F.; Materne, O.; Rajeb, A.; Horobeanu, C.; Farooq, A. Pelvic Tilt: Reliability of Measuring the Standing Position and Range of Motion in Adolescent Athletes. Br. J. Sports Med. 2014, 48, 594. [Google Scholar] [CrossRef]
- Sprigle, S.; Flinn, N.; Wootten, M.; McCorry, S. Development and testing of a pelvic goniometer designed to measure pelvic tilt and hip flexion. Clin. Biomech. 2003, 18, 462–465. [Google Scholar] [CrossRef]
- Gajdosik, R.; Simpson, R.; Smith, R.; DonTigny, R.L. Pelvic Tilt: Intratester Reliability of Measuring the Standing Position and Range of Motion. Phys. Ther. 1985, 65, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Prasad, A.; Boyce, L.; Dawson-Bowling, S.; Achan, P.; Millington, S.; Hanna, S.A. Total hip arthroplasty outcomes in morbidly obese patients. EFORT Open Rev. 2018, 3, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Blom, A.; Boulton, C.; Brittain, R.; Clark, E.; Craig, R.; Dawson-Bowling, S.; Deere, K.; Esler, C.; Goldberg, A.; et al. The National Joint Registry 16th Annual Report 2019; National Joint Registry Annual Reports; National Joint Registry: London, UK, 2019. [Google Scholar]
- López-Nava, I.H.; Muñoz-Meléndez, A. Wearable Inertial Sensors for Human Motion Analysis: A Review. IEEE Sens. J. 2016, 16, 7821–7834. [Google Scholar] [CrossRef]
- Palit, A.; King, R.; Gu, Y.; Pierrepont, J.; Simpson, D.; Williams, M.A. Subject-Specific Surgical Planning for Hip Replacement: A Novel 2D Graphical Representation of 3D Hip Motion and Prosthetic Impingement Information. Ann. Biomed. Eng. 2019, 47, 1642–1656. [Google Scholar] [CrossRef] [Green Version]
- Palit, A.; Williams, M.A.; Turley, G.A.; Renkawitz, T.; Weber, M. Femur First navigation can reduce impingement severity compared to traditional free hand total hip arthroplasty. Sci. Rep. 2017, 7, 7238. [Google Scholar] [CrossRef] [PubMed]
- Turley, G.A.; Williams, M.A.; Wellings, R.M.; Griffin, D.R. Evaluation of range of motion restriction within the hip joint. Med. Biol. Eng. Comput. 2013, 51, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palit, A.; King, R.; Hart, Z.; Gu, Y.; Pierrepont, J.; Elliott, M.T.; Williams, M.A. Bone-to-Bone and Implant-to-Bone Impingement: A Novel Graphical Representation for Hip Replacement Planning. Ann. Biomed. Eng. 2020, 48, 1354–1367. [Google Scholar] [CrossRef] [Green Version]
- Palit, A.; King, R.; Gu, Y.; Pierrepont, J.; Hart, Z.; Elliott, M.T.; Williams, M.A. Prediction and Visualisation of Bony Impingement for Subject Specific Total Hip Arthroplasty*. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2127–2131. [Google Scholar]
- Allegri, M.; Montella, S.; Salici, F.; Valente, A.; Marchesini, M.; Compagnone, C.; Baciarello, M.; Manferdini, M.E.; Fanelli, G. Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Chaléat-Valayer, E.; Mac-Thiong, J.-M.; Paquet, J.; Berthonnaud, E.; Siani, F.; Roussouly, P. Sagittal spino-pelvic alignment in chronic low back pain. Eur. Spine J. 2011, 20, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Minicozzi, S.J.; Russell, B.S.; Ray, K.J.; Struebing, A.Y.; Owens, E.F. Low Back Pain Response to Pelvic Tilt Position: An Observational Study of Chiropractic Patients. J. Chiropr. Med. 2016, 15, 27–34. [Google Scholar] [CrossRef] [Green Version]






| IMU ≥ 13 Degrees | IMU < 13 Degrees | |
|---|---|---|
| Radiograph ≥ 13 degrees | TP = 11 | FN = 2 |
| Radiograph ≤ 13 degrees | FP = 1 | TN = 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Qureshi, A.; Vepa, A.; Rahman, U.; Palit, A.; Williams, M.A.; King, R.; Elliott, M.T. A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty. Sensors 2020, 20, 6182. https://doi.org/10.3390/s20216182
Wang X, Qureshi A, Vepa A, Rahman U, Palit A, Williams MA, King R, Elliott MT. A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty. Sensors. 2020; 20(21):6182. https://doi.org/10.3390/s20216182
Chicago/Turabian StyleWang, Xueyang, Arham Qureshi, Abhinav Vepa, Usama Rahman, Arnab Palit, Mark A. Williams, Richard King, and Mark T. Elliott. 2020. "A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty" Sensors 20, no. 21: 6182. https://doi.org/10.3390/s20216182
APA StyleWang, X., Qureshi, A., Vepa, A., Rahman, U., Palit, A., Williams, M. A., King, R., & Elliott, M. T. (2020). A Sensor-Based Screening Tool for Identifying High Pelvic Mobility in Patients Due to Undergo Total Hip Arthroplasty. Sensors, 20(21), 6182. https://doi.org/10.3390/s20216182





