Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation
Abstract
1. Introduction
2. Materials and Methods
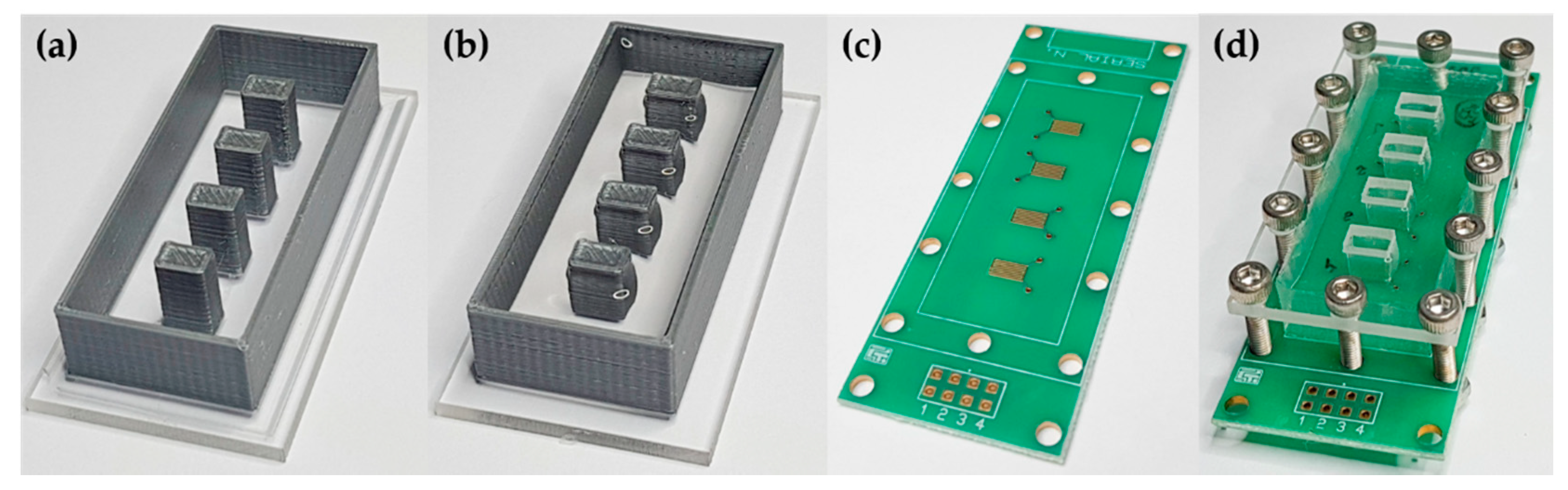
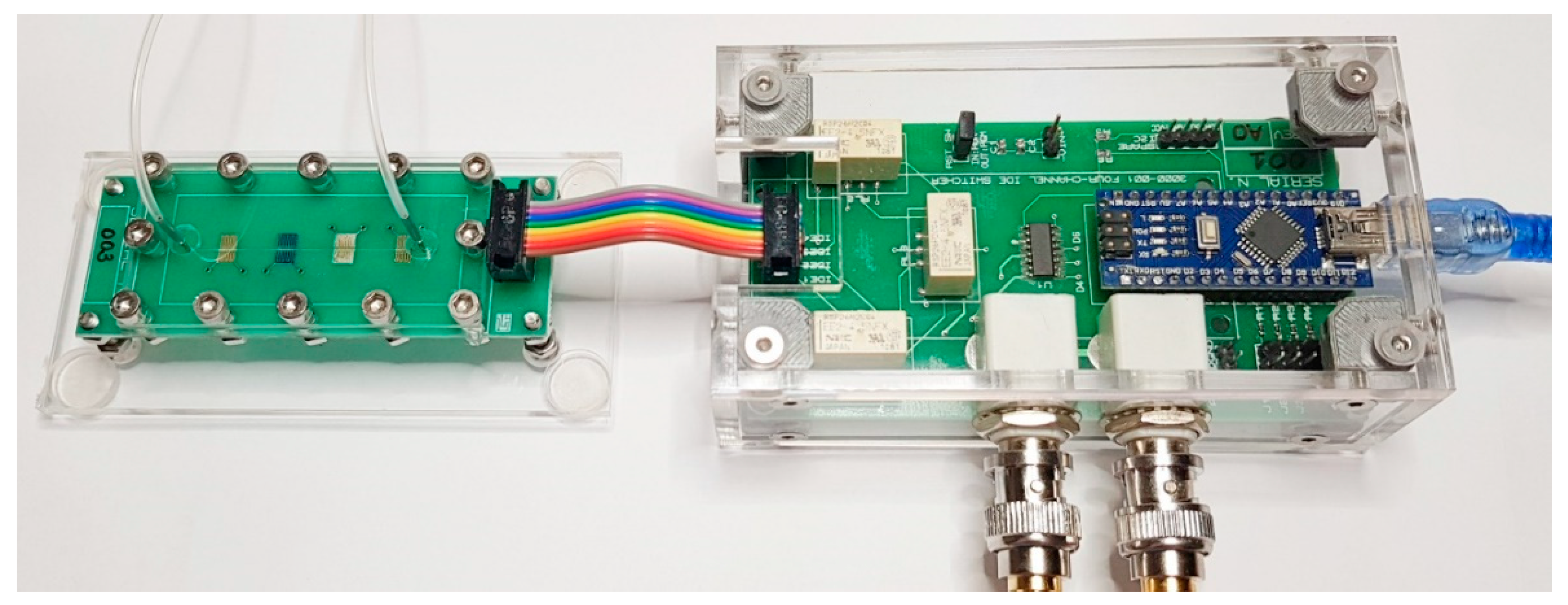
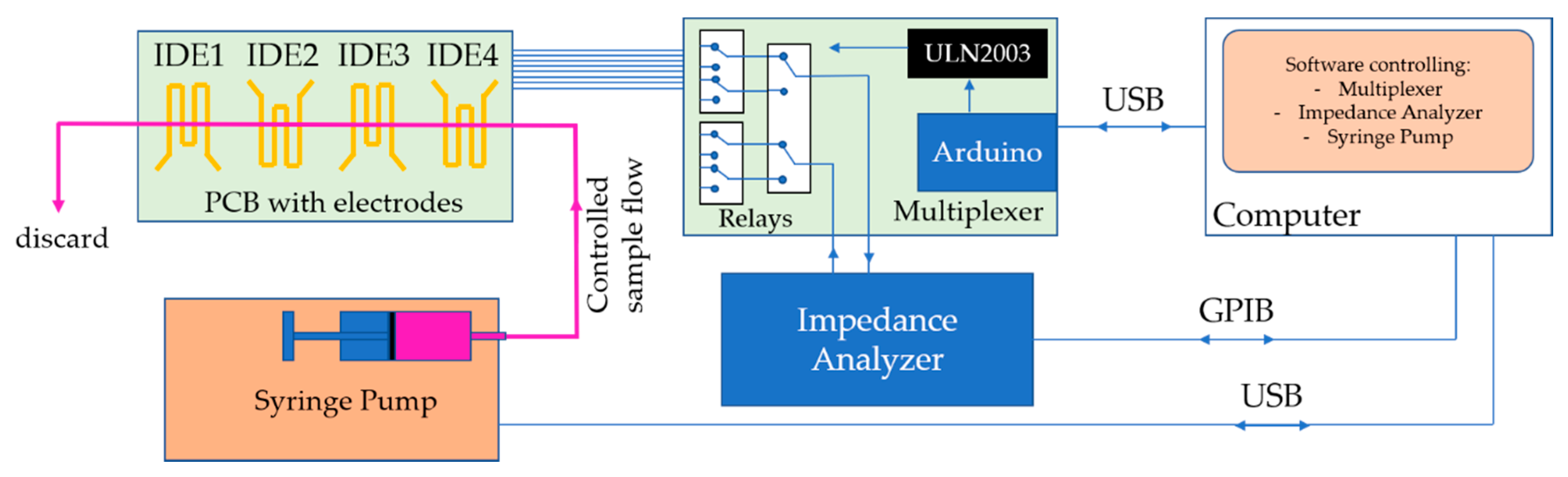
2.1. Collinear Electrodes and Automated Multiplexing
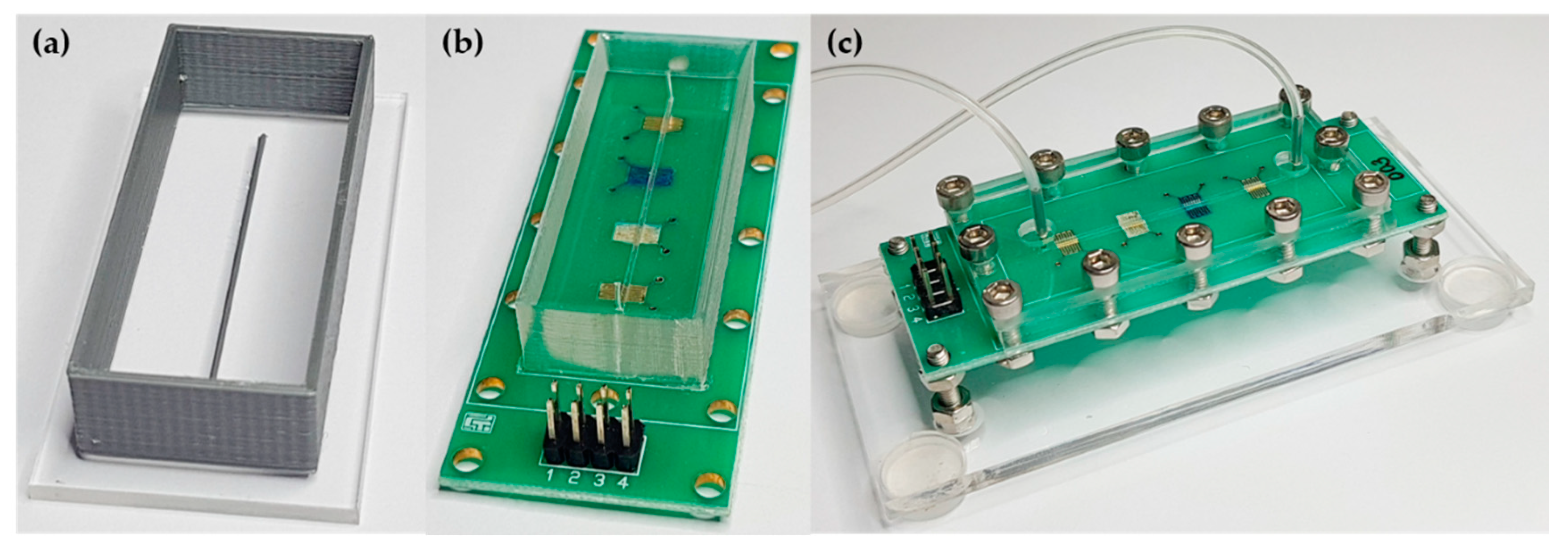
2.2. Layer-by-Layer Deposition onto Collinear Electrodes
2.3. Fabrication of Elastomer Channels and Device Assembling
2.4. Impedance Measurements on the Automated Microfluidic e-Tongue
3. Results and Discussion
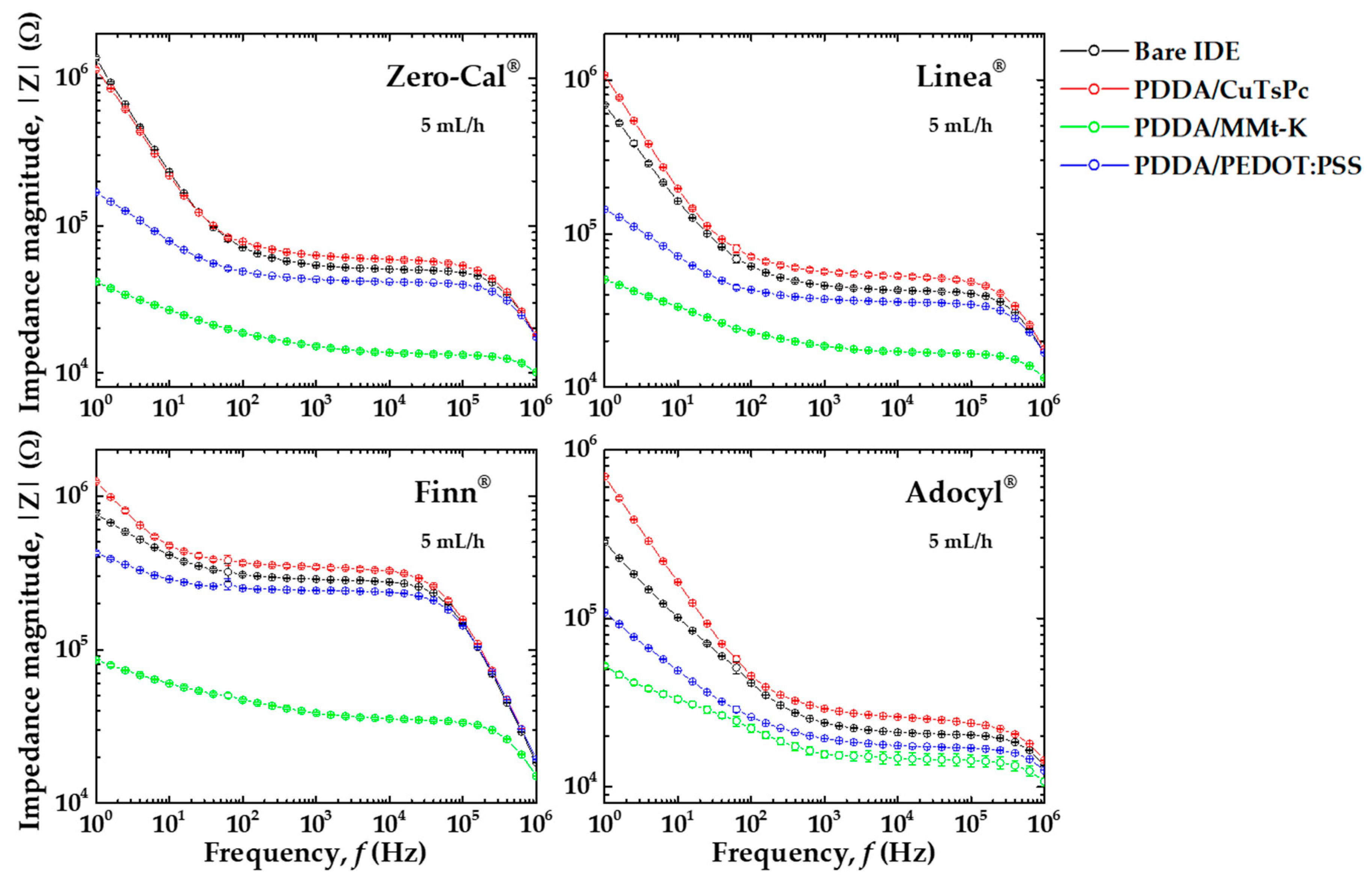
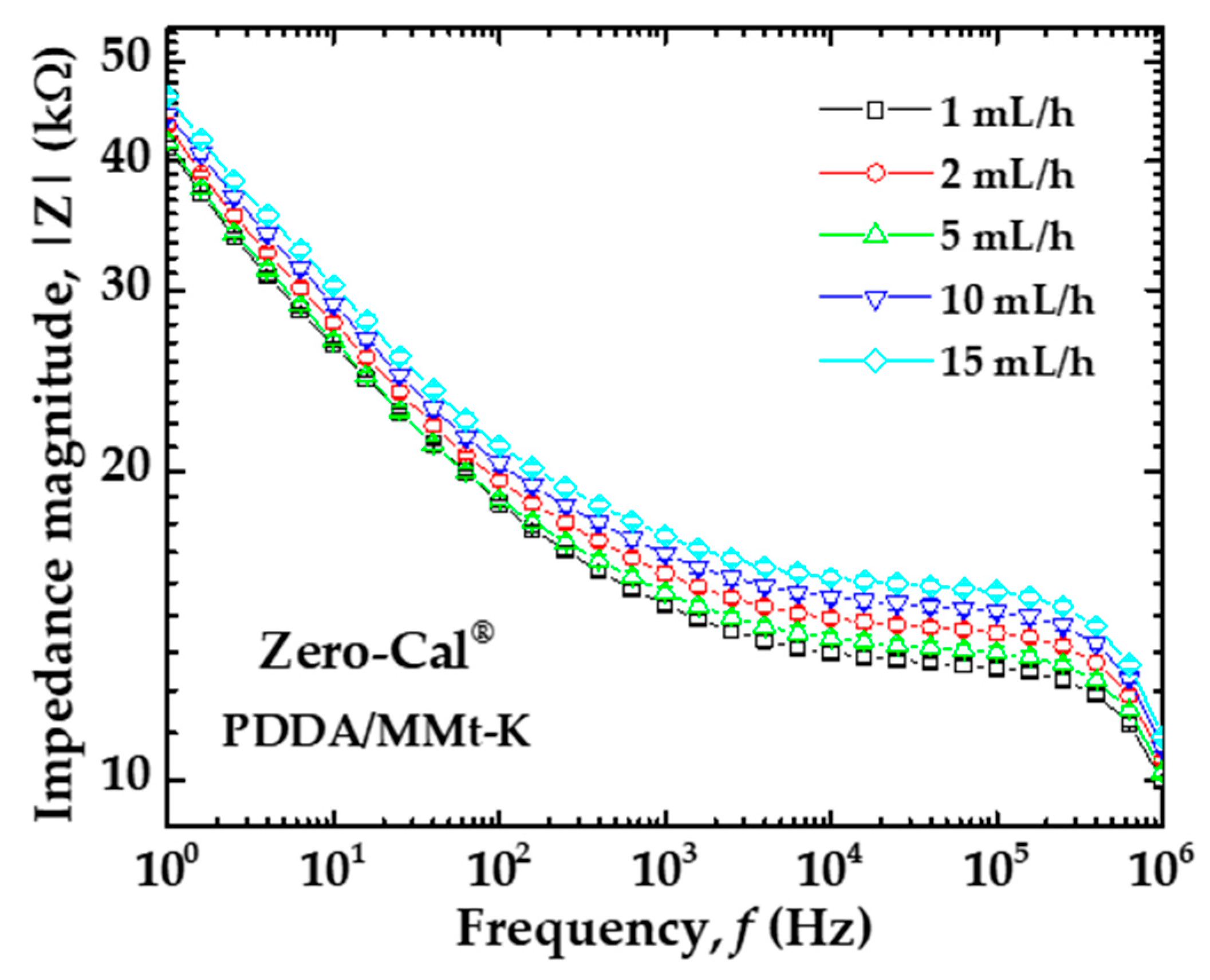
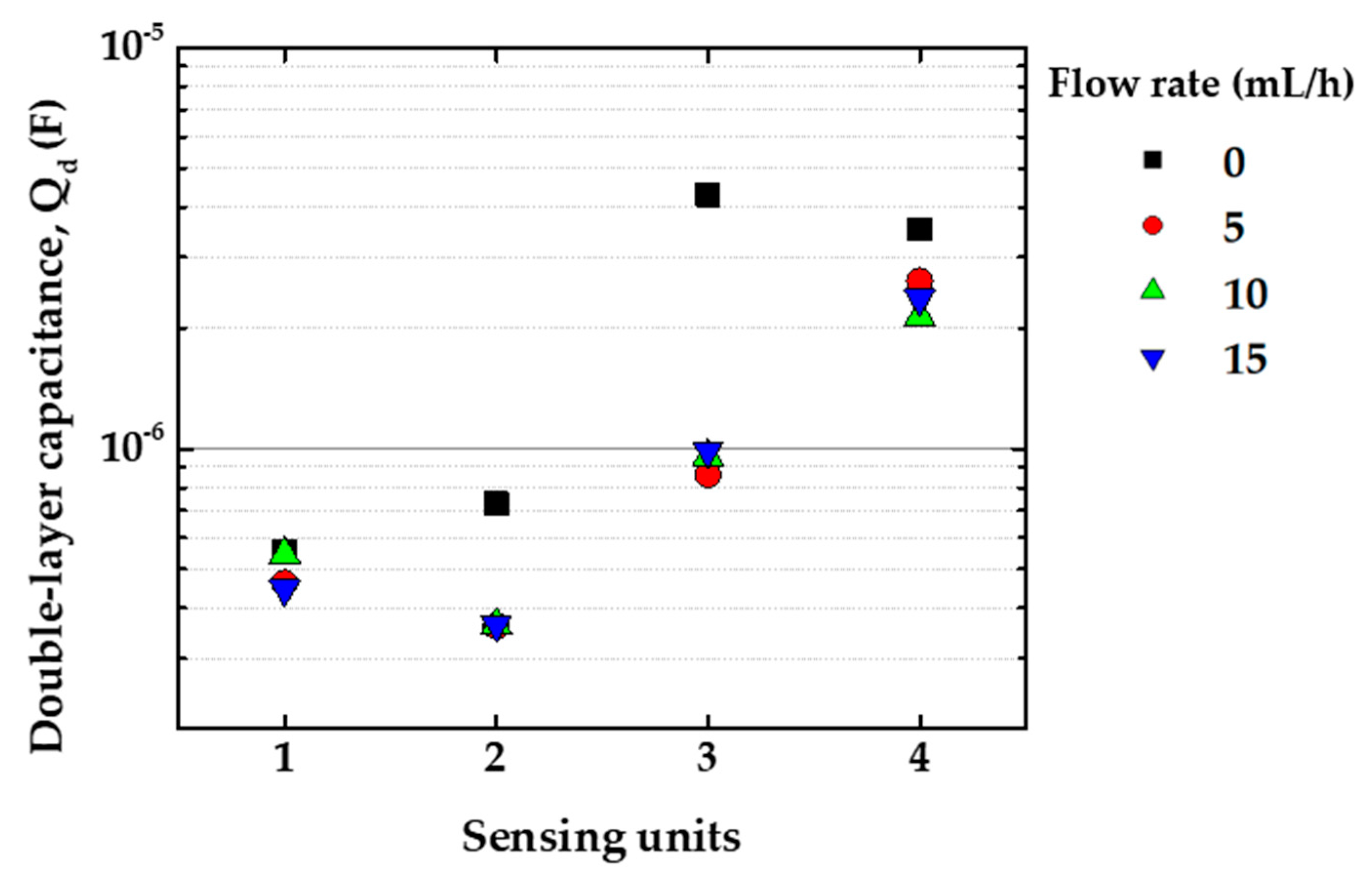
3.1. Electrical Impedance Measurements in Distinct Flow Rates
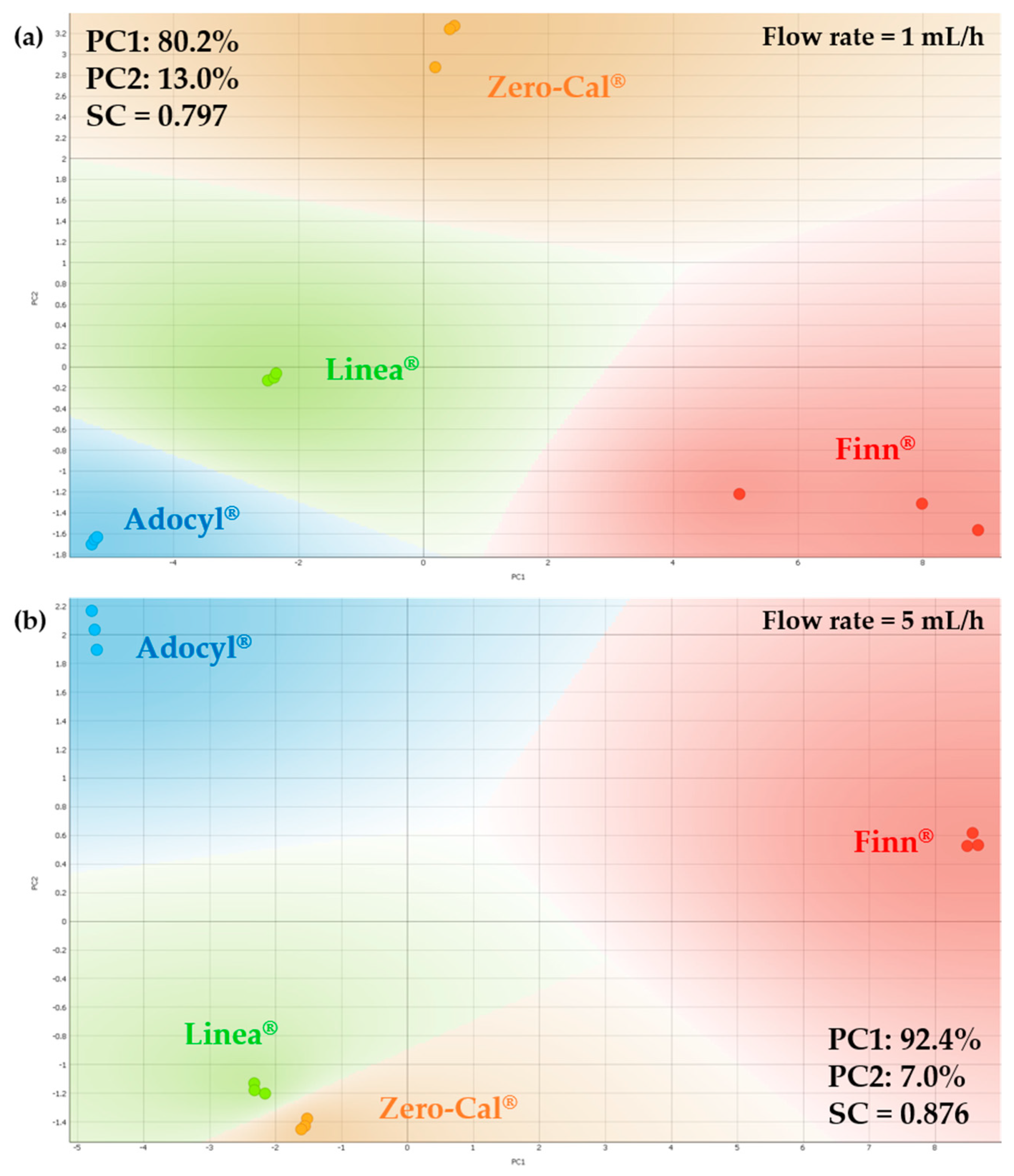
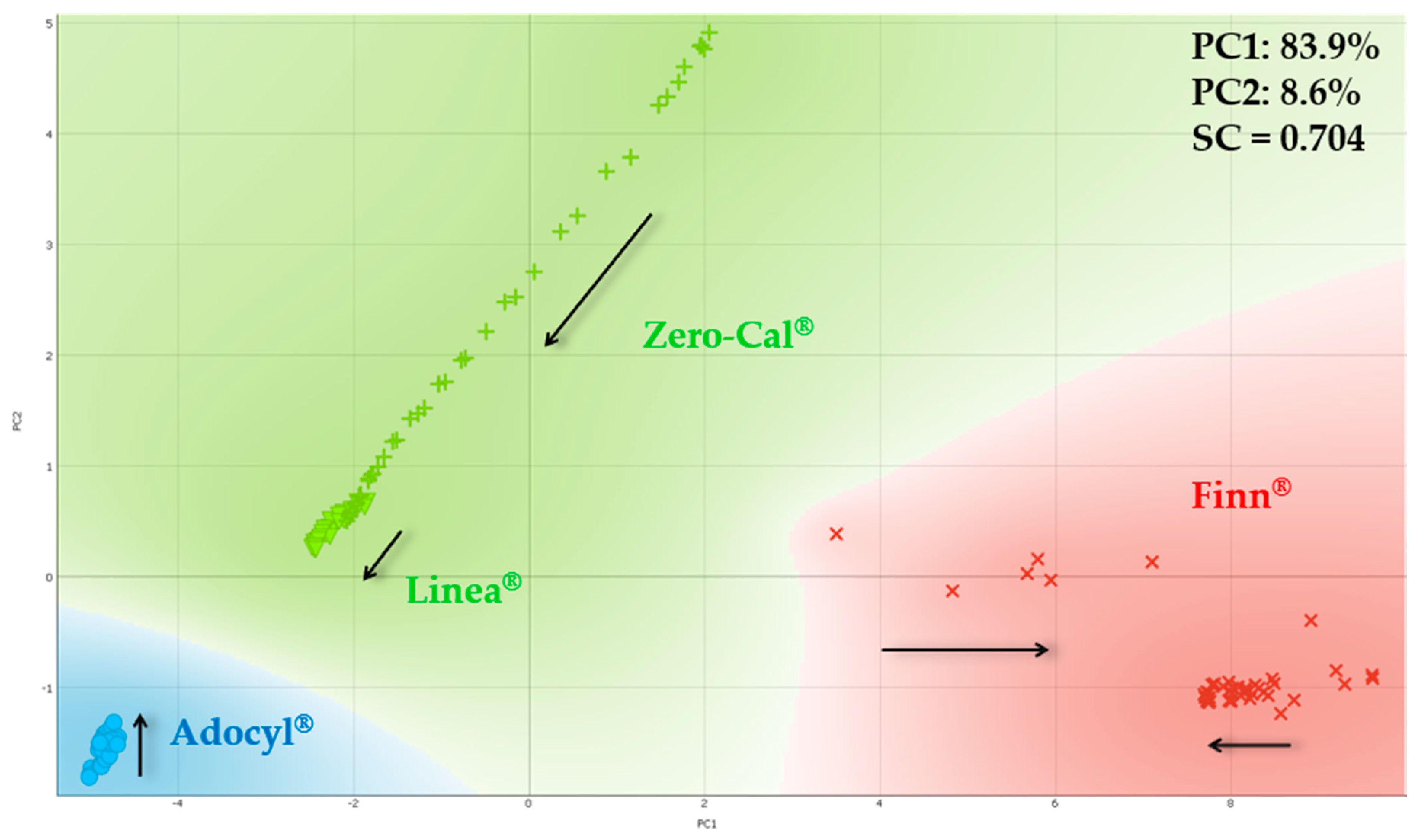
3.2. Principal Component Analysis for Electrical Impedance Data
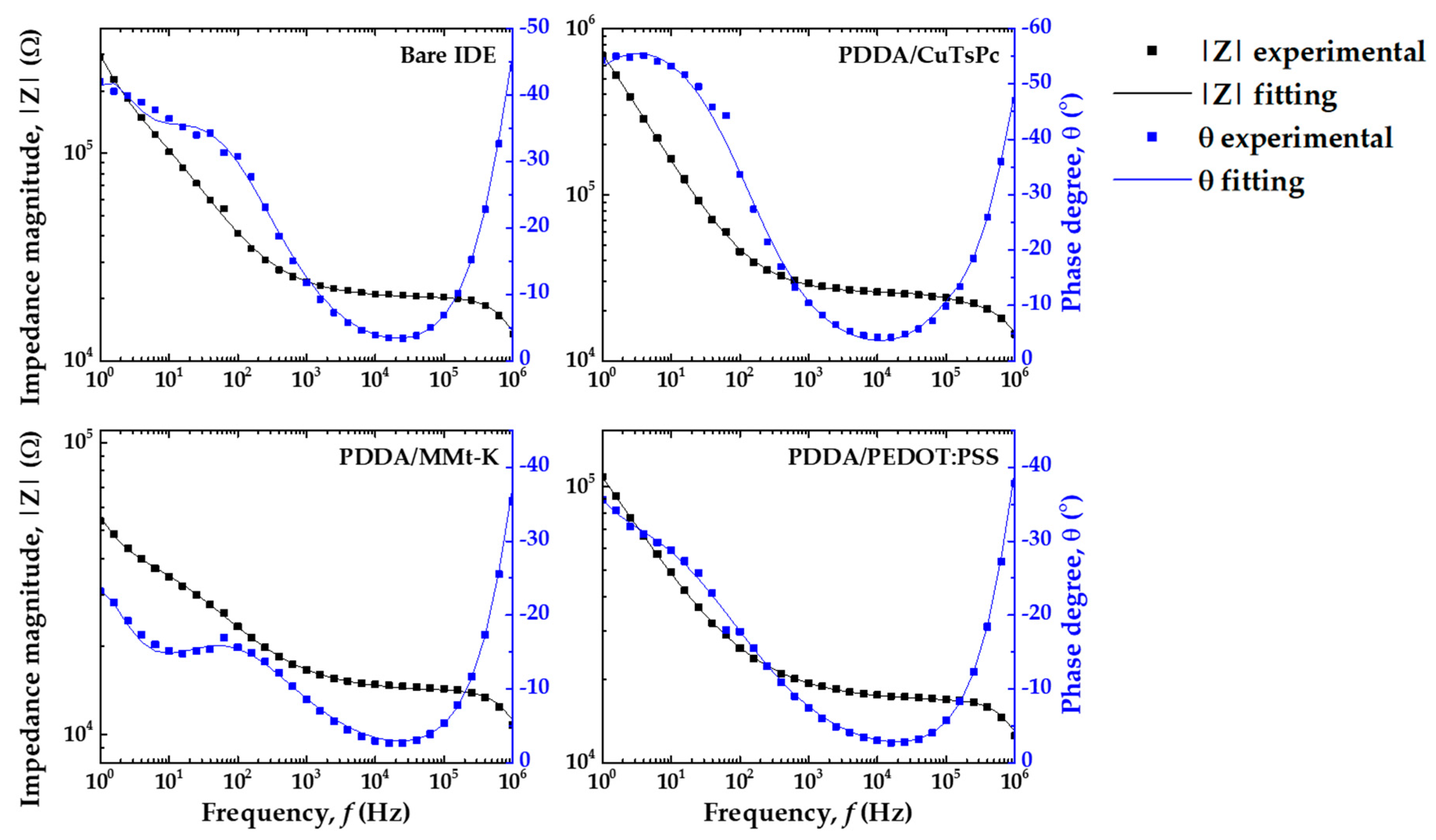
3.3. Analysis of Electrical Impedance Data by Equivalent Circuit Model
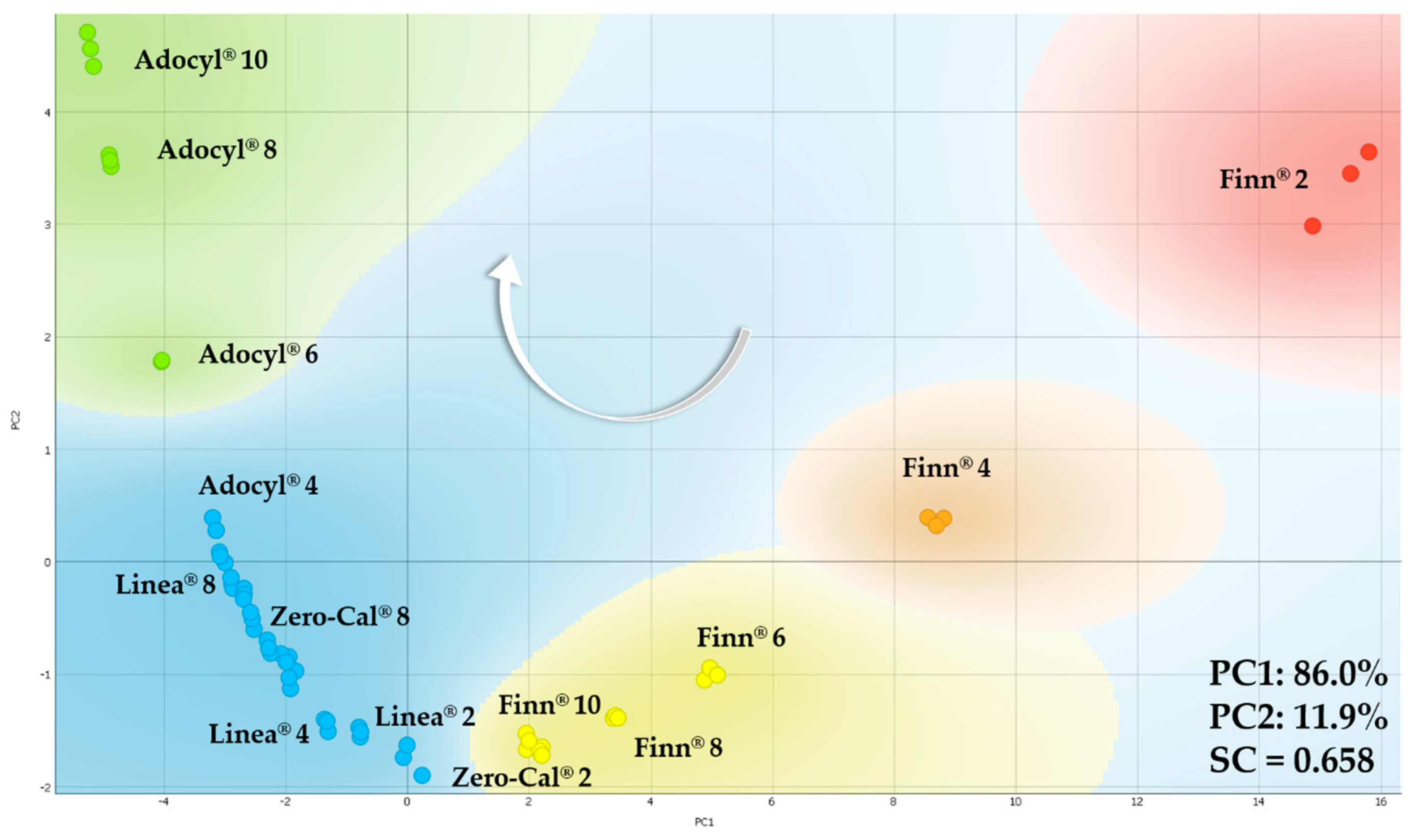
3.4. Measurements of Sucralose-Based Sweeteners at Distinct Concentrations
3.4.1. Analysis in Deionized Water
3.4.2. Analysis in Espresso Coffee
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids. Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Shimizu, F.M.; Braunger, M.L.; Riul, A., Jr.; Oliveira, O.N., Jr. Electronic Tongues. In Smart Sensors for Environmental and Medical Applications; Hallil, H., Heidari, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 61–80. ISBN 9781119587422. [Google Scholar]
- de Morais, T.C.B.; Rodrigues, D.R.; de Carvalho Polari Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Elamine, Y.; Inácio, P.M.C.; Lyoussi, B.; Anjos, O.; Estevinho, L.M.; da Graça Miguel, M.; Gomes, H.L. Insight into the sensing mechanism of an impedance based electronic tongue for honey botanic origin discrimination. Sens. Actuators B Chem. 2019, 285, 24–33. [Google Scholar] [CrossRef]
- Paup, V.D.; Barnett, S.M.; Diako, C.; Ross, C.F. Detection of Spicy Compounds Using the Electronic Tongue. J. Food Sci. 2019, 84, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Chacón, A.; Torabi, F.; Faridbod, F.; Ghasemi, J.B.; González-Calabuig, A.; Del Valle, M. Voltammetric electronic tongue for the simultaneous determination of three benzodiazepines. Sensors 2019, 19, 5002. [Google Scholar] [CrossRef] [PubMed]
- Facure, M.H.M.; Schneider, R.; dos Santos, D.M.; Correa, D.S. Impedimetric electronic tongue based on molybdenum disulfide and graphene oxide for monitoring antibiotics in liquid media. Talanta 2020, 217, 121039. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Dantas, C.A.R.; Volpati, D.; Constantino, C.J.L.; Piazzetta, M.H.O.; Gobbi, A.L.; Taylor, D.M.; Oliveira, O.N., Jr.; Riul, A., Jr. Microfluidic electronic tongue. Sens. Actuators B Chem. 2015, 207, 1129–1135. [Google Scholar] [CrossRef]
- Braunger, M.; Fier, I.; Rodrigues, V.; Arratia, P. Microfluidic Mixer with Automated Electrode Switching for Sensing Applications. Chemosensors 2020, 8, 13. [Google Scholar] [CrossRef]
- Braunger, M.L.; Shimizu, F.M.; Jimenez, M.J.M.; Amaral, L.R.; Piazzetta, M.H.; Gobbi, Â.L.; Magalhães, P.S.G.; Rodrigues, V.; Oliveira, O.N., Jr.; Riul, A., Jr. Microfluidic electronic tongue applied to soil analysis. Chemosensors 2017, 5, 14. [Google Scholar] [CrossRef]
- Shimizu, F.M.; Todão, F.R.; Gobbi, A.L.; Oliveira, O.N.; Garcia, C.D.; Lima, R.S. Functionalization-free microfluidic Electronic tongue based on a single response. ACS Sensors 2017, 2, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Daikuzono, C.M.; Shimizu, F.M.; Manzoli, A.; Riul, A.; Piazzetta, M.H.O.; Gobbi, A.L.; Correa, D.S.; Paulovich, F.V.; Oliveira, O.N. Information Visualization and Feature Selection Methods Applied to Detect Gliadin in Gluten-Containing Foodstuff with a Microfluidic Electronic Tongue. ACS Appl. Mater. Interfaces 2017, 9, 19646–19652. [Google Scholar] [CrossRef]
- Habara, M.; Ikezaki, H.; Toko, K. Study of sweet taste evaluation using taste sensor with lipid/polymer membranes. Biosens. Bioelectron. 2004, 19, 1559–1563. [Google Scholar] [CrossRef]
- Dyminski, D.S.; Paterno, L.G.; Takeda, H.H.; Bolini, H.M.A.; Mattoso, L.H.C.; Cândido, L.M.B. Correlation between human panel and electronic tongue responses on the analysis of commercial sweeteners. Sens. Lett. 2006, 4, 403–408. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Kirsanov, D.; Frolova, Y.; Clapham, D.; Caricofe, R. Assessment of bitterness intensity and suppression effects using an electronic tongue. AIP Conf. Proc. 2009, 1137, 271–274. [Google Scholar]
- Choi, D.H.; Kim, N.A.; Nam, T.S.; Lee, S.; Jeong, S.H. Evaluation of taste-masking effects of pharmaceutical sweeteners with an electronic tongue system. Drug Dev. Ind. Pharm. 2014, 40, 308–317. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Delaney, C.; Morrin, A.; Diamond, D.; Florea, L.; Oliveira, O.N. Paper based electronic tongue-a low-cost solution for the distinction of sugar type and apple juice brand. Analyst 2019, 144, 2827–2832. [Google Scholar] [CrossRef]
- Carniel Beltrami, M.; Döring, T.; De Dea Lindner, J. Sweeteners and sweet taste enhancers in the food industry. Food Sci. Technol. 2018, 38, 181–187. [Google Scholar] [CrossRef]
- de Lourdes Samaniego-Vaesken, M.; Partearroyo, T.; Cano, A.; Urrialde, R.; Varela-Moreiras, G. Novel database of declared low- and no-calorie sweeteners from foods and beverages available in Spain. J. Food Compos. Anal. 2019, 82, 103234. [Google Scholar] [CrossRef]
- Braunger, M.L.; Daikuzono, C.M.; Riul, A., Jr. A Microfluidic E-Tongue System Using Layer-by-Layer Films Deposited onto Interdigitated Electrodes Inside a Polydimethylsiloxane Microchannel. In Biomimetic Sensing: Methods and Protocols, Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 2027, pp. 141–150. [Google Scholar]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 2491. [Google Scholar] [CrossRef]
- Americo da Silva, T.; Braunger, M.L.; Neris Coutinho, M.A.; Rios do Amaral, L.; Rodrigues, V.; Riul, A. 3D-Printed Graphene Electrodes Applied in an Impedimetric Electronic Tongue for Soil Analysis. Chemosensors 2019, 7, 50. [Google Scholar] [CrossRef]
- Kovacs, Z.; Szöllősi, D.; Zaukuu, J.-L.Z.; Bodor, Z.; Vitális, F.; Aouadi, B.; Zsom-Muha, V.; Gillay, Z. Factors Influencing the Long-Term Stability of Electronic Tongue and Application of Improved Drift Correction Methods. Biosensors 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Orange Data Mining. Available online: https://orange.biolab.si/ (accessed on 30 August 2020).
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Struyf, A.; Hubert, M.; Rousseeuw, P. Clustering in an Object-Oriented Environment. J. Stat. Softw. 1996, 1, 1–30. [Google Scholar] [CrossRef]
- Riul, A., Jr.; dos Santos, D.S., Jr.; Wohnrath, K.; Di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N., Jr.; Taylor, D.M.; Mattoso, L.H.C. Artificial taste sensor: Efficient combination of sensors made from Langmuir-Blodgett films of conducting polymers and a ruthenium complex and self-assembled films of an azobenzene-containing polymer. Langmuir 2002, 18, 239–245. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Soto, A.M.G.; Mello, S.V.; Bone, S.; Taylor, D.M.; Mattoso, L.H.C. An electronic tongue using polypyrrole and polyaniline. Synth. Met. 2003, 132, 109–116. [Google Scholar] [CrossRef]
- Taylor, D.M.; Macdonald, A.G. AC admittance of the metal/insulator/electrolyte interface. J. Phys. D Appl. Phys. 1987, 20, 1277–1283. [Google Scholar] [CrossRef]
- ZView Software. Available online: https://www.ameteksi.com/products/software/zview-software (accessed on 15 September 2020).
- Valsa, J.; Dvořák, P.; Friedl, M. Network model of the CPE. Radioengineering 2011, 20, 619–626. [Google Scholar]
- Lee, Y.S. Intermolecular and Colloidal Forces. In Self-Assembly and Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 21–46. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; ISBN 978-1-4614-8932-0. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of Detection: A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- De Barros, A.; Constantino, C.J.L.; da Cruz, N.C.; Bortoleto, J.R.R.; Ferreira, M. High performance of electrochemical sensors based on LbL films of gold nanoparticles, polyaniline and sodium montmorillonite clay mineral for simultaneous detection of metal ions. Electrochim. Acta 2017, 235, 700–708. [Google Scholar] [CrossRef]

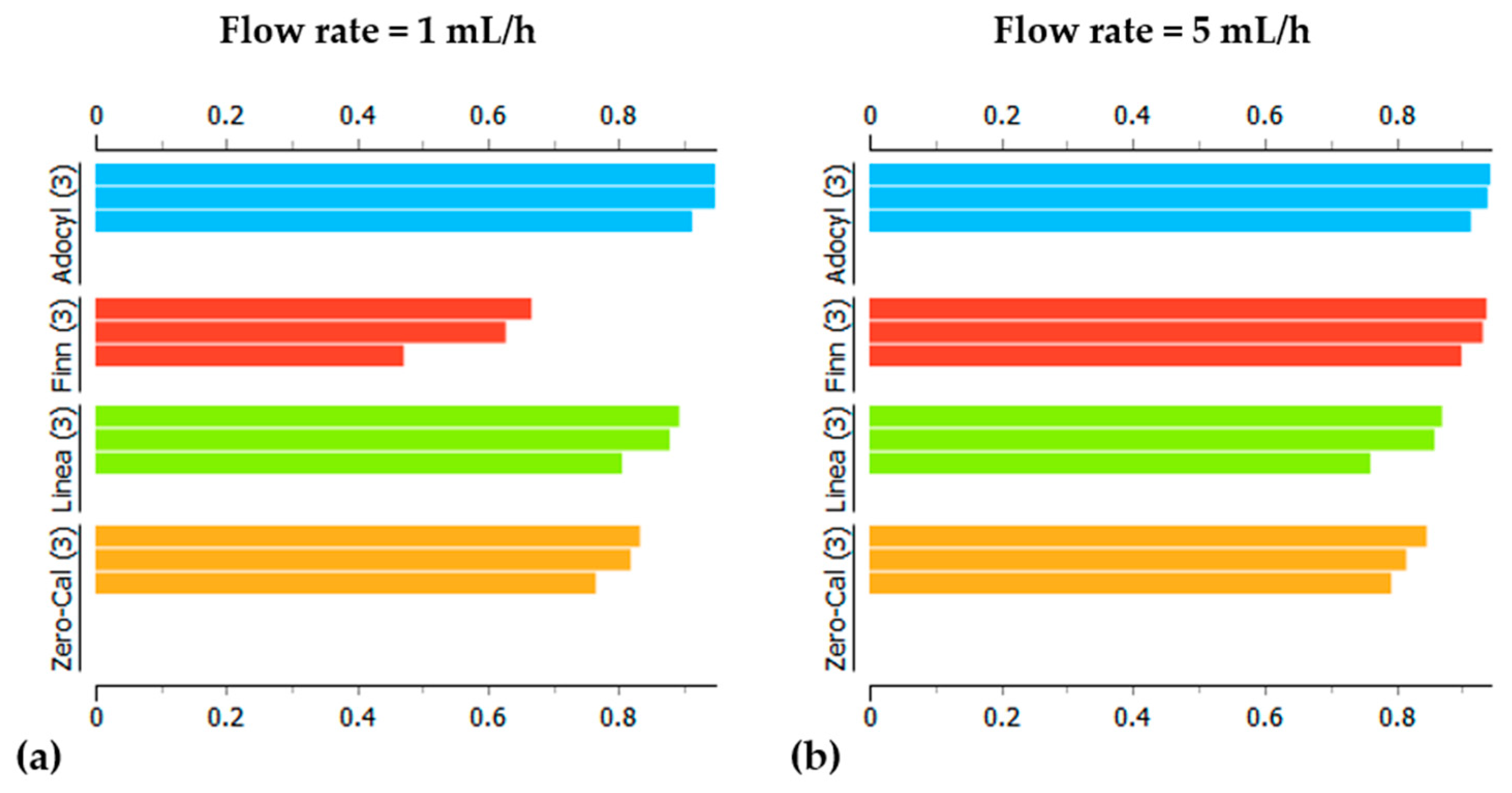



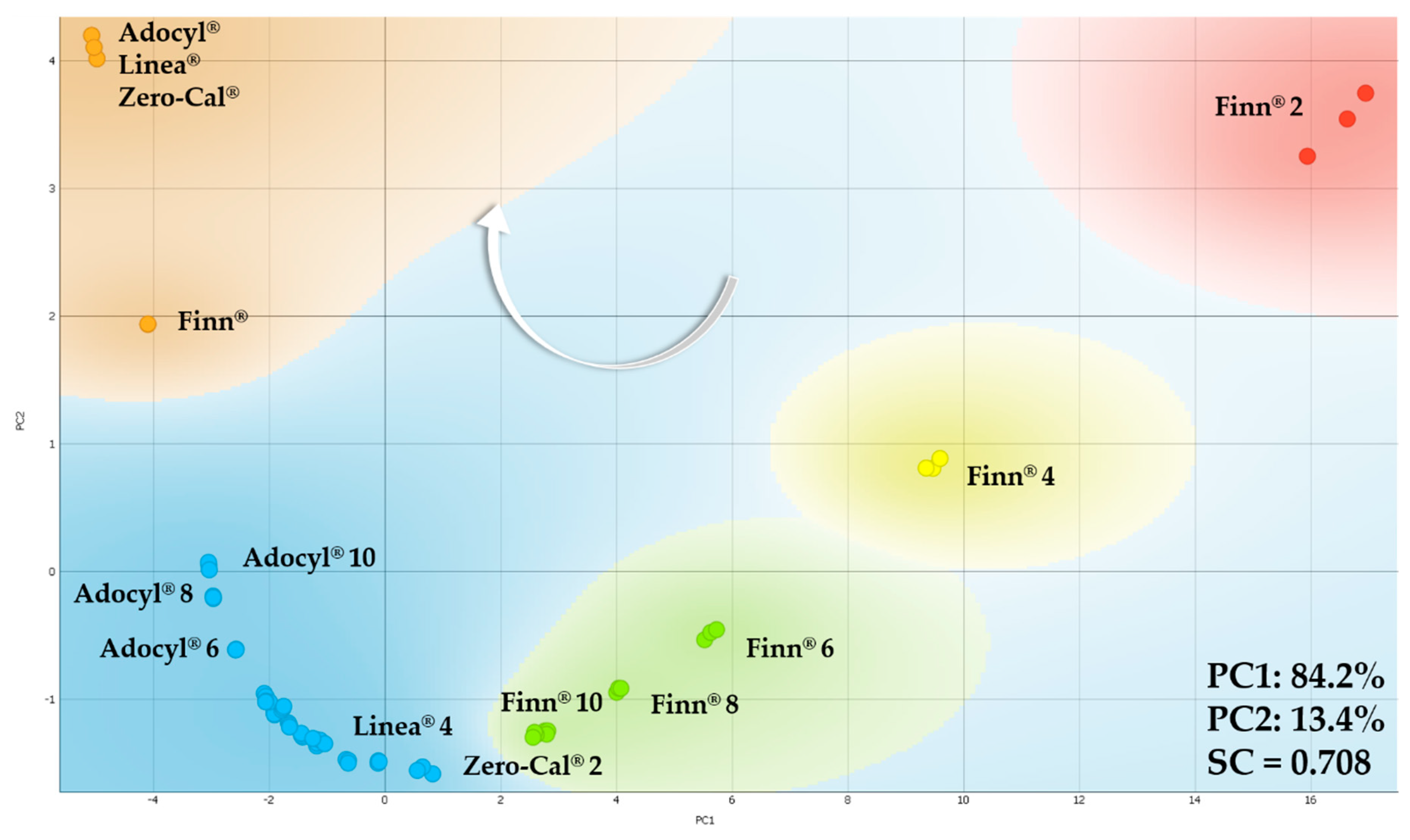
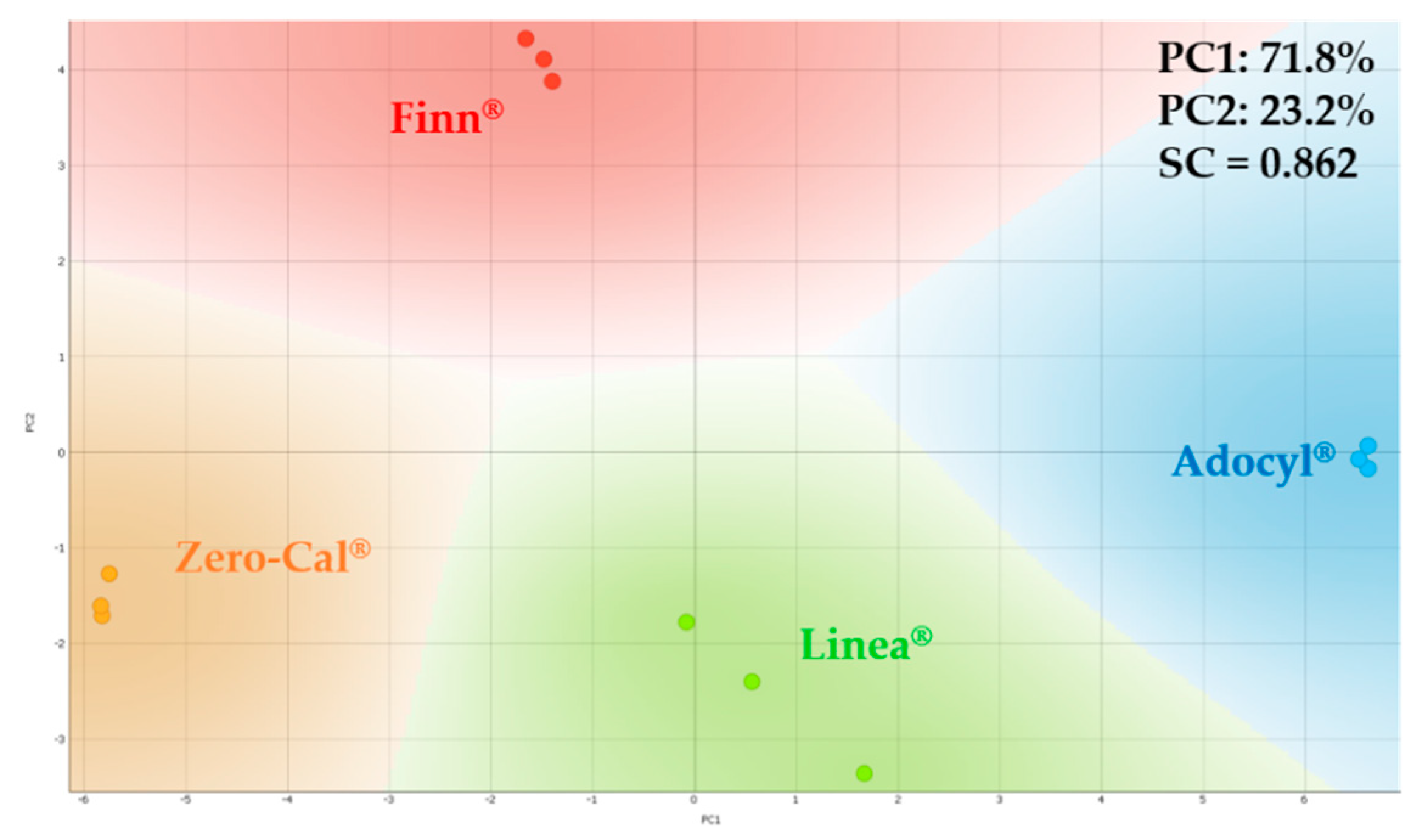
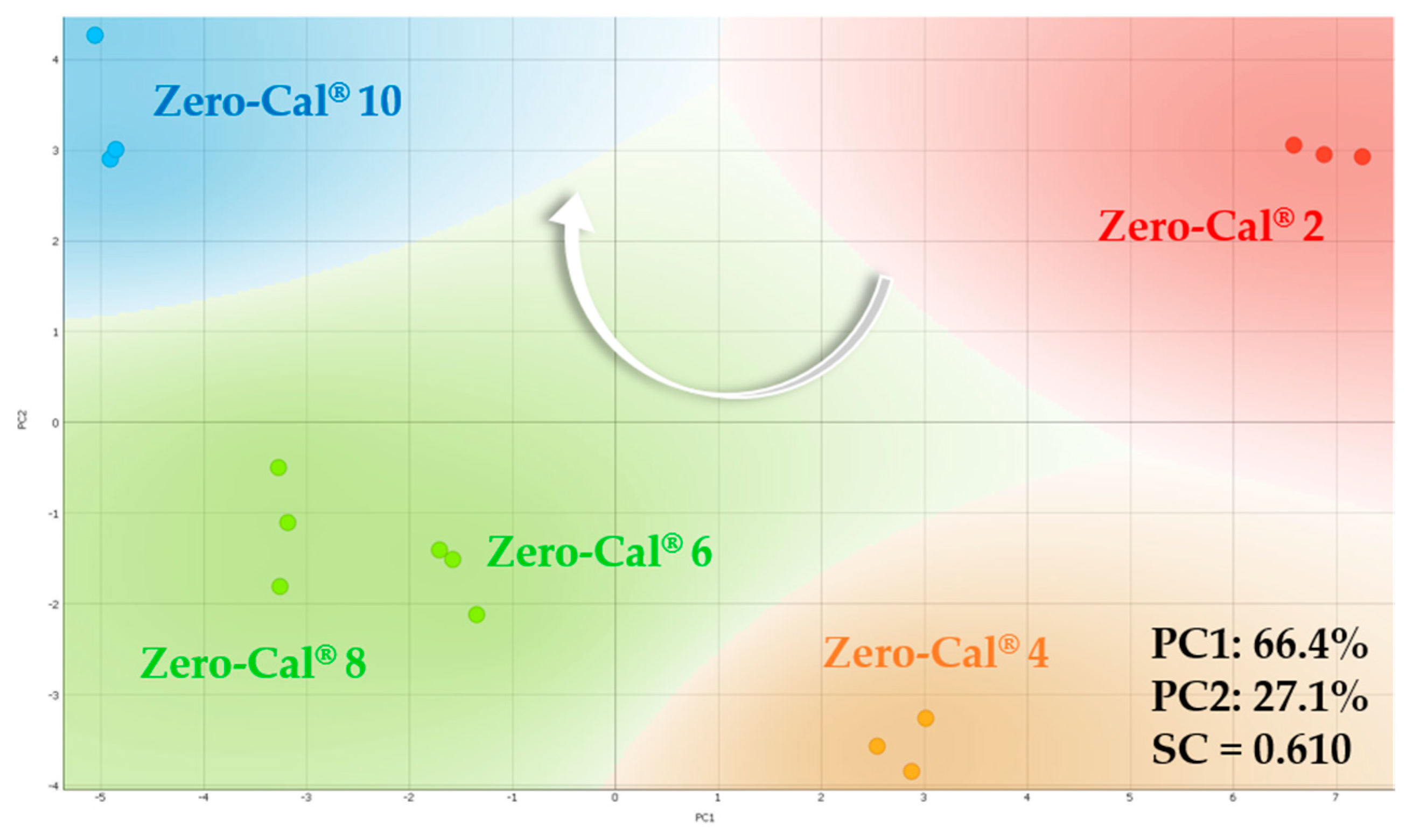
| Sensing Units | LD, LQ, and Sensitivity * | Zero-Cal® | Linea® | Finn® | Adocyl® |
|---|---|---|---|---|---|
| Bare IDE | LD | 0.12 | 0.11 | 0.05 | 0.21 |
| LQ | 0.39 | 0.36 | 0.18 | 0.70 | |
| Sensitivity | 0.91 | 0.79 | 0.79 | 0.78 | |
| PDDA/CuTsPc | LD | 0.10 | 0.11 | 0.02 | 0.25 |
| LQ | 0.32 | 0.37 | 0.05 | 0.82 | |
| Sensitivity | 0.90 | 0.80 | 0.82 | 0.87 | |
| PDDA/MMt-K | LD | 0.04 | 0.18 | 0.05 | 0.12 |
| LQ | 0.12 | 0.60 | 0.18 | 0.41 | |
| Sensitivity | 0.96 | 0.77 | 0.86 | 0.81 | |
| PDDA/PEDOT:PSS | LD | 0.10 | 0.09 | 0.11 | 0.11 |
| LQ | 0.32 | 0.31 | 0.38 | 0.35 | |
| Sensitivity | 0.94 | 0.78 | 1.02 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braunger, M.L.; Fier, I.; Shimizu, F.M.; de Barros, A.; Rodrigues, V.; Riul, A., Jr. Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation. Sensors 2020, 20, 6194. https://doi.org/10.3390/s20216194
Braunger ML, Fier I, Shimizu FM, de Barros A, Rodrigues V, Riul A Jr. Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation. Sensors. 2020; 20(21):6194. https://doi.org/10.3390/s20216194
Chicago/Turabian StyleBraunger, Maria L., Igor Fier, Flávio M. Shimizu, Anerise de Barros, Varlei Rodrigues, and Antonio Riul, Jr. 2020. "Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation" Sensors 20, no. 21: 6194. https://doi.org/10.3390/s20216194
APA StyleBraunger, M. L., Fier, I., Shimizu, F. M., de Barros, A., Rodrigues, V., & Riul, A., Jr. (2020). Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation. Sensors, 20(21), 6194. https://doi.org/10.3390/s20216194






