Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Material and Fruit Sampling
2.2. Fluorescence Spectroscopy
2.3. Determination of Maturity Indices and Maturity Classes
2.4. Data Analysis and Modelling
2.4.1. Maturity Statistics
2.4.2. Prediction Modelling
- Raw data were smoothed using convolution with a Gaussian kernel with σ = 1.5 units (corresponding to a physical sigma of approximately 3 nm at the centre of the spectrum).
- Spectra were “re-binned” into 72 wavelength bands obtained by adding 4 contiguous wavelength bins.
- The resulting spectra were then mean-centred and scaled to unit variance.
- After these pre-processing steps the spectra were fed in the SA algorithm. The algorithm starts with a random draw of 20 bands (out of the total 72). At each iteration, a subset of two bands were randomly swapped, a PLS model was developed and cross-validated on the collection of bands selected by the SA. The optimum PLS model at each step was obtained by minimising the AIC. The algorithm was run for 5000 iterations.
3. Results
3.1. Fruit Maturity Indices
3.1.1. Comparisons between Cultivars
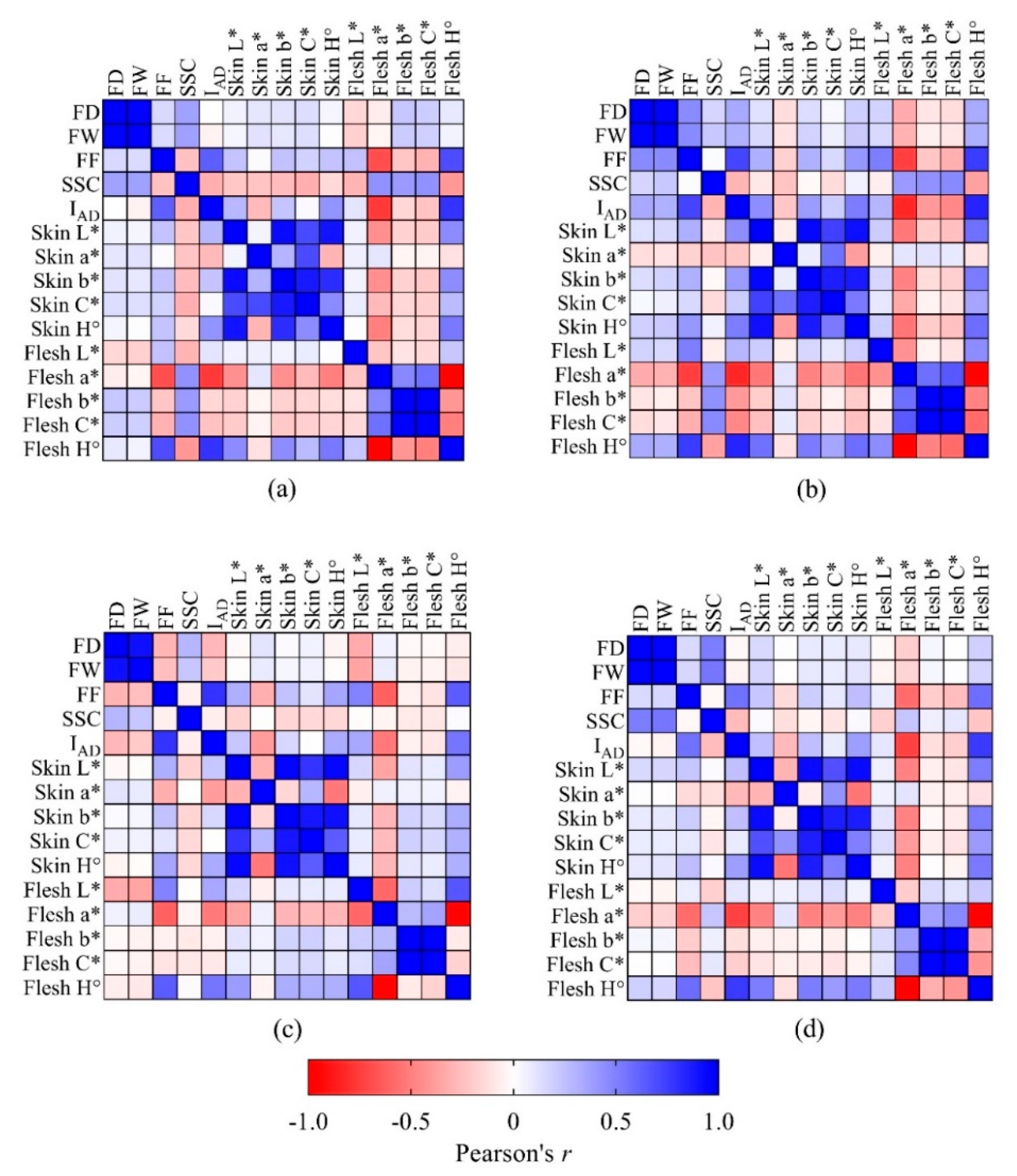
3.1.2. Correlations between Maturity Indices
3.2. Fluorescence Spectra and Maturity Prediction
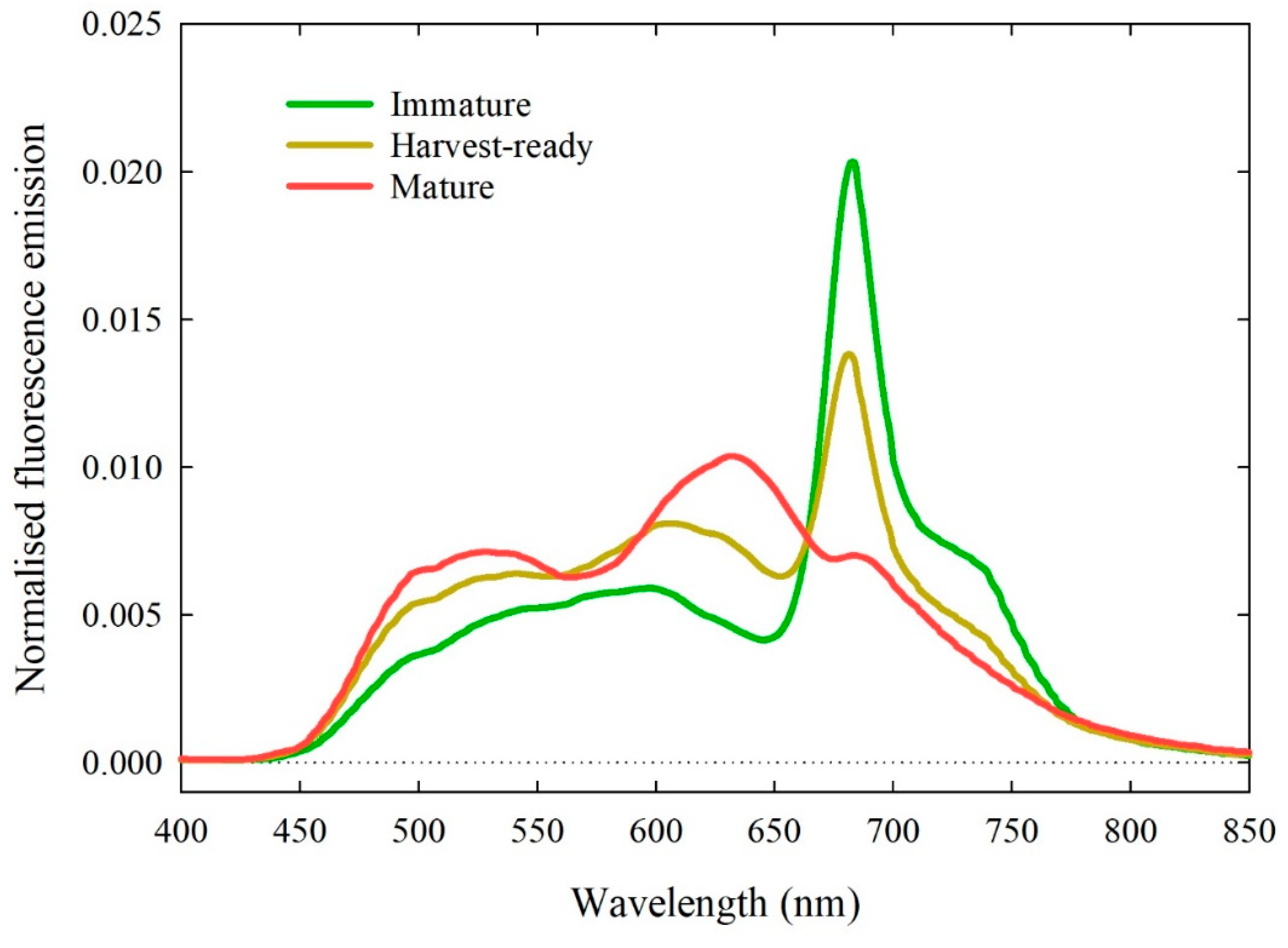
3.2.1. Spectra Characteristics
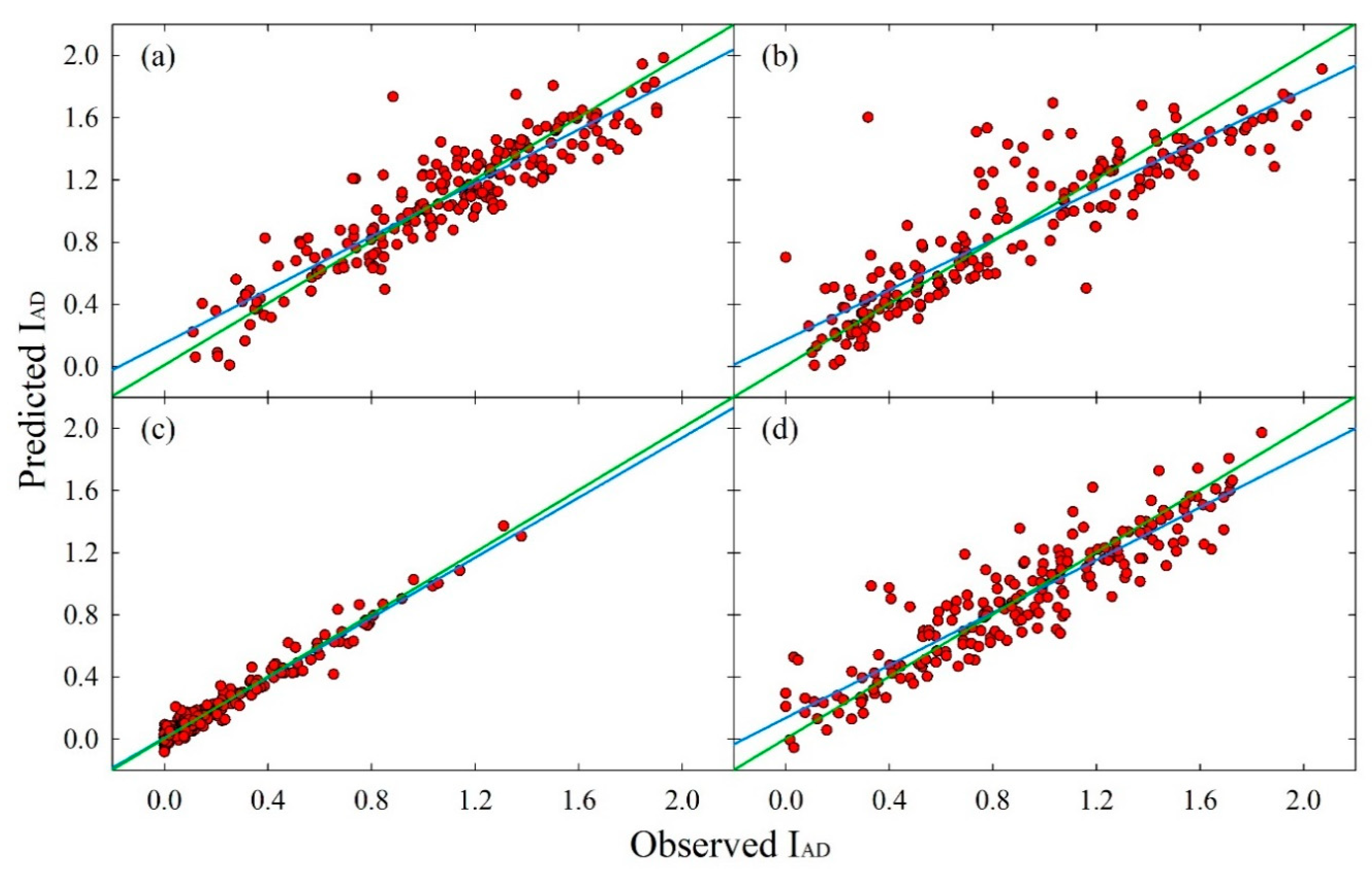
3.2.2. PLS Models
3.2.3. LDA Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crisosto, C.H. Stone fruit maturity indices: A descriptive review. Postharvest News Inf. 1994, 5, 65N–68N. [Google Scholar]
- Kingston, C.M. Maturity Indices for Apple and Pear. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 1992; Volume 13, pp. 407–432. ISBN 978-0-471-57499-6. [Google Scholar]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Betemps, D.L.; Fachinello, J.C.; Galarça, S.P.; Portela, N.M.; Remorini, D.; Massai, R.; Agati, G. Non-destructive evaluation of ripening and quality traits in apples using a multiparametric fluorescence sensor. J. Sci. Food Agric. 2012, 92, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Infante, R. Harvest maturity indicators in the stone fruit industry. Stewart Postharvest Rev. 2012, 8, 1–6. [Google Scholar] [CrossRef]
- Bonora, E.; Stefanelli, D.; Costa, G. Nectarine fruit ripening and quality assessed using the index of absorbance difference (IAD). Int. J. Agron. 2013. [Google Scholar] [CrossRef] [Green Version]
- McGlone, V.A.; Jordan, R.B.; Martinsen, P.J. Vis/NIR estimation at harvest of pre- and post-storage quality indices for “Royal Gala” apple. Postharvest Biol. Technol. 2002. [Google Scholar] [CrossRef]
- Fan, G.; Zha, J.; Du, R.; Gao, L. Determination of soluble solids and firmness of apples by Vis/NIR transmittance. J. Food Eng. 2009. [Google Scholar] [CrossRef]
- Palmer, J.W.; Harker, F.R.; Tustin, D.S.; Johnston, J. Fruit dry matter concentration: A new quality metric for apples. J. Sci. Food Agric. 2010. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, W.; Zhao, C.; Zhang, B. A comparative study for the quantitative determination of soluble solids content, pH and firmness of pears by Vis/NIR spectroscopy. J. Food Eng. 2013. [Google Scholar] [CrossRef]
- Goke, A.; Serra, S.; Musacchi, S. Postharvest dry matter and soluble solids content prediction in d’anjou and bartlett pear using near-infrared spectroscopy. HortScience 2018. [Google Scholar] [CrossRef]
- Harker, F.R.; Carr, B.T.; Lenjo, M.; MacRae, E.A.; Wismer, W.V.; Marsh, K.B.; Williams, M.; White, A.; Lund, C.M.; Walker, S.B.; et al. Consumer liking for kiwifruit flavour: A meta-analysis of five studies on fruit quality. Food Qual. Prefer. 2009. [Google Scholar] [CrossRef]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.M.; Renard, C.M.G.C. Rapid and non-destructive analysis of apricot fruit quality using FT-near-infrared spectroscopy. Food Chem. 2009. [Google Scholar] [CrossRef]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017. [Google Scholar] [CrossRef]
- Minas, I.S.; Blanco-Cipollone, F.; Sterle, D. Accurate non-destructive prediction of peach fruit internal quality and physiological maturity with a single scan using near infrared spectroscopy. Food Chem. 2021. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G. Application of Visible/NIR spectroscopy for the estimation of soluble solids, dry matter and flesh firmness in stone fruits. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Chen, P.; McCarthy, M.J.; Kauten, R. NMR for internal quality evaluation of fruits and vegetables. Trans. Am. Soc. Agric. Eng. 1989. [Google Scholar] [CrossRef]
- Fuleki, T.; Cook, F.I. Relationship of Maturity as Indicated by Flesh Color to Quality of Canned Clingstone Peaches. Can. Inst. Food Sci. Technol. J. 1976. [Google Scholar] [CrossRef]
- Kader, A.A.; Heintz, C.N.; Chordas, A. Postharvest quality of fresh and canned peaches as influenced by genotypes and maturity at harvest. J. Am. Soc. Hortic. Sci. 1982, 107, 947–951. [Google Scholar]
- Delwiche, M.J.; Baumgardner, R.A. Ground color as a peach maturity index. J. Am. Soc. Hortic. Sci. 1985, 110, 53–57. [Google Scholar]
- Tourjee, K.R.; Barrett, D.M.; Romero, M.V.; Gradziel, T.M. Measuring flesh color variability among processing clingstone peach genotypes differing in carotenoid composition. J. Am. Soc. Hortic. Sci. 1998, 123, 433–437. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the chlorophyll absorbance index (IAD) are related to peach fruit maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Greer, D.H. Non-destructive chlorophyll fluorescence and colour measurements of ‘braeburn’ and ‘royal gala’ apple (malus domestica) fruit development throughout the growing season. N. Z. J. Crop Hortic. Sci. 2005, 33, 413–421. [Google Scholar] [CrossRef]
- ISO 11664-4:2008(en), Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11664:-4:ed-1:v1:en (accessed on 24 August 2020).
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Peavey, M.; Goodwin, I.; McClymont, L.; Chandra, S. Effect of shading on red colour and fruit quality in blush pears “ANP-0118” and “ANP-0131”. Plants 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, D.C.; Crisosto, C.H.; Hasey, J.K.; Thompson, J.F. Comparison of instrumental and manual inspection of clingston peaches. Appl. Eng. Agric. 2006, 22, 883–889. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef]
- Slaughter, D.C.; Crisosto, C.H.; Tiwari, G. Nondestructive determination of flesh color in clingstone peaches. J. Food Eng. 2013, 116, 920–925. [Google Scholar] [CrossRef]
- Belefant-Miller, H.; Miller, G.H.; Rutger, J.N. Nondestructive Measurement of Carotenoids in Plant Tissues by Fluorescence Quenching. Crop Sci. 2005, 45, 1786–1789. [Google Scholar] [CrossRef] [Green Version]
- Merzlyak, M.N.; Melø, T.B.; Naqvi, K.R. Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: Signature analysis, assessment, modelling, and relevance to photoprotection. J. Exp. Bot. 2008, 59, 349–359. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Lo Bianco, R. Field non-destructive determination of nectarine quality under deficit irrigation. Acta Hortic. 2021, in press. [Google Scholar]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Solovchenko, A.; Chivkunova, O.; Gitelson, A.; Merzlyak, M. Non-Destructive Estimation Pigment Content, Ripening, Quality and Damage in Apple Fruit with Spectral Reflectance in the Visible Range. Fresh Prod. 2010, 4, 91–102. [Google Scholar]
- Frisina, C.; Bonora, E.; Ceccarelli, A.; Stefanelli, D. DA Meter IAD Maturity Classes: Database—HIN. Available online: http://www.hin.com.au/networks/profitable-stonefruit-research/stonefruit-maturity-and-fruit-quality/da-meter-iad-maturity-classes-database (accessed on 24 August 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 7 September 2020).
- Peters, G. Userfriendlyscience: Quantitative Analysis Made Accessible; R Package Version 0.7.2; 2018; Available online: https://userfriendlyscience.com (accessed on 7 September 2020).
- van Laarhoven, P.J.M.; Aarts, E.H.L. Simulated annealing. In Simulated Annealing: Theory and Applications; Springer: Dordrecht, The Netherlands, 1987; pp. 7–15. ISBN 978-94-015-7744-1. [Google Scholar]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Lin, L.I.-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Pelliccia, D. Wavelength Band Selection with Simulated Annealing. Available online: https://github.com/nevernervous78/nirpyresearch/blob/master/snippets/Wavelengthbandselectionwithsimulatedannealing.ipynb (accessed on 7 September 2020).
- do Nascimento Nunes, M.C. Color Atlas of Postharvest Quality of Fruits and Vegetables; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll fluorescence emission spectrum inside a leaf. Photochem. Photobiol. Sci. 2008, 7, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Matteini, P.; Oliveira, J.; De Freitas, V.; Mateus, N. Fluorescence approach for measuring anthocyanins and derived pigments in red wine. J. Agric. Food Chem. 2013, 61, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC—DAD—ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Sabatino, L.; Muratore, A.; Belligno, A.; Gagliano, G. Phenolic Characterization of Sicilian Yellow Flesh Peach (Prunus persica L.) Cultivars at Different Ripening Stages. J. Food Qual. 2012, 35, 255–262. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Ngomo, O.; Vanderesse, R.; Arnoux, P.; Myrzakhmetov, B.; Frochot, C.; Guiavarc’h, Y. Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods 2017, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. [Google Scholar]

| Cultivar | Maturity Classes (IAD) | ||
|---|---|---|---|
| Immature 1 | Harvest-Ready 2 | Mature 3 | |
| ‘August Flame’ | IAD > 1.3 | 1.3 ≤ IAD ≤ 0.7 | IAD < 0.7 |
| ‘O’Henry’ | IAD > 1.2 | 1.2 ≤ IAD ≤ 0.7 | IAD < 0.7 |
| ‘Redhaven’ | IAD > 1.6 | 1.6 ≤ IAD ≤ 0.6 | IAD < 0.6 |
| ‘September Sun’ | IAD > 1.2 | 1.2 ≤ IAD ≤ 0.8 | IAD < 0.8 |
| Maturity Index/Colour Attribute | Cultivar | |||
|---|---|---|---|---|
| ‘August Flame’ | ‘O’Henry’ | ‘Redhaven’ | ‘September Sun’ | |
| FD (mm) | 62.4 (5.5) c | 64.9 (4.6) b | 52.8 (5.4) d | 70.2 (7.3) a |
| FW (g) | 127.7 (30.4) c | 146.7 (26.3) b | 77.6 (24) d | 186.3 (54.2) a |
| FF (kgf) | 7.2 (1.8) a | 6.2 (2.1) b | 1.9 (1.5) c | 6.1 (1.7) b |
| SSC (°Brix) | 13.2 (2.3) b | 14.8 (2.3) a | 11.9 (1.3) c | 13.4 (2.2) b |
| IAD | 1.1 (0.4) a | 0.8 (0.5) b | 0.1 (0.3) c | 0.9 (0.4) b |
| Skin L* | 48.0 (6.4) c | 51.9 (7.1) b | 56.2 (10.7) a | 52.0 (8.3) b |
| Skin a* | 27.5 (6.5) b | 28.1 (6.4) b | 30.6 (7.7) a | 29.8 (6.7) ab |
| Skin b* | 25.8 (8.3) b | 25.9 (9.0) b | 30.8 (11.6) a | 29.9 (9.1) a |
| Skin C* | 39.1 (8.5) c | 40.2 (8.2) c | 47.6 (9.3) a | 43.5 (7.8) b |
| Skin H° | 40.1 (9.7) b | 39.4 (11.0) ab | 42.4 (13.7) a | 42.4 (11.4) ab |
| Flesh L* | 78.5 (2.8) b | 78.4 (3.1) bc | 81.3 (6.5) a | 77.8 (2.7) c |
| Flesh a* | 4.9 (4.3) bc | 7.4 (5.6) ab | 6.7 (3.1) a | 3.2 (3.8) c |
| Flesh b* | 55.1 (3.5) c | 55.9 (4.2) b | 58.3 (4.2) a | 54.8 (3.2) c |
| Flesh C* | 55.6 (3.7) c | 56.4 (4.5) b | 58.7 (4.2) a | 54.9 (3.4) c |
| Flesh H° | 84.9 (4.3) ab | 82.7 (5.5) bc | 83.3 (2.9) c | 86.5 (3.8) a |
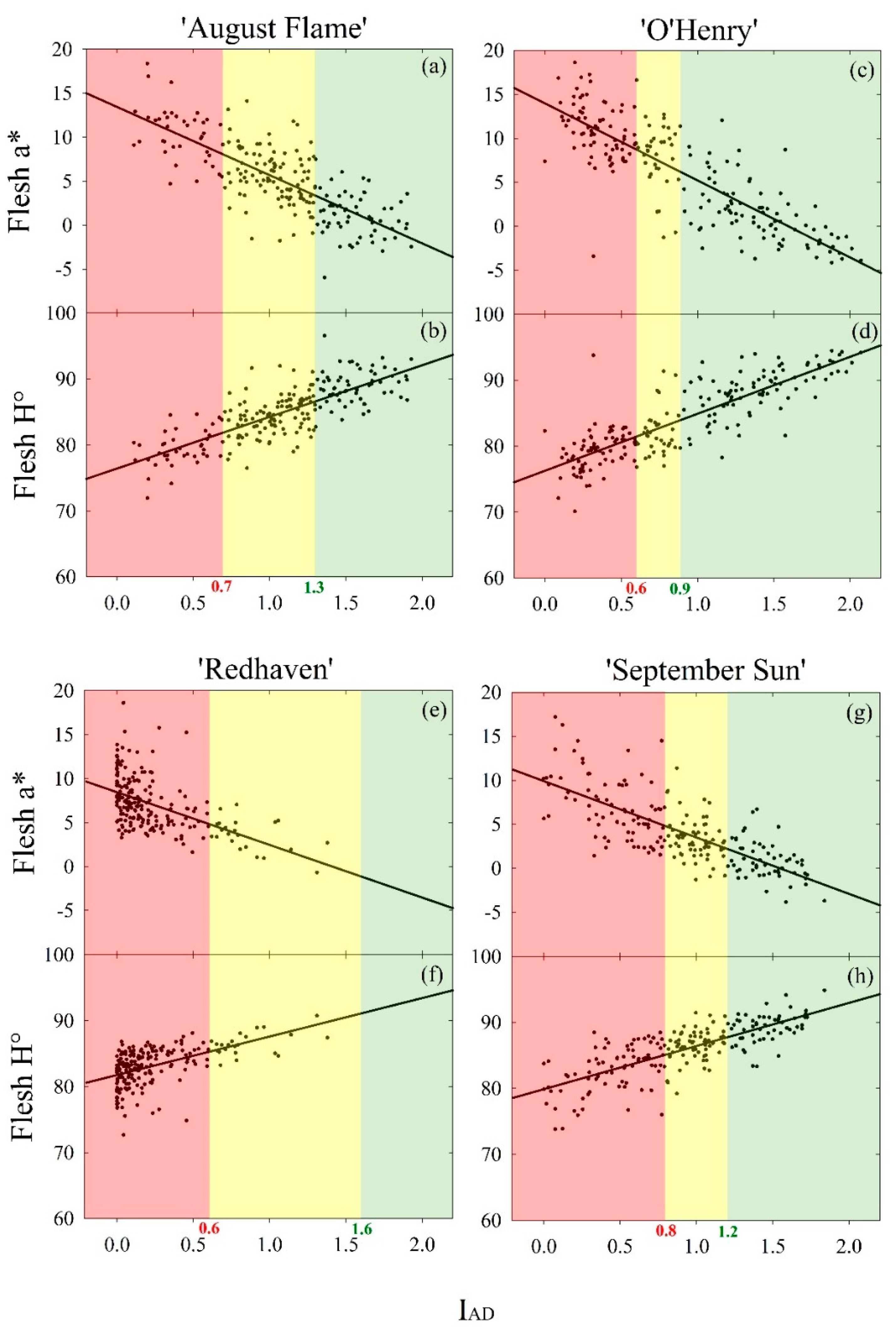
| Cultivar | Flesh Colour Attribute | Linear Regression Models | Predicted Maturity Thresholds | |||
|---|---|---|---|---|---|---|
| Intercept | Slope | R2 | Harvest-Ready | Mature | ||
| ‘August Flame’ | a* | 13.4 (0.5) | −7.8 (0.4) | 0.60 | 3.4 | 8.0 |
| H° | 76.4 (0.5) | 7.8 (0.4) | 0.62 | 86.6 | 81.9 | |
| ‘O’Henry’ | a* | 14.0 (0.4) | −8.8 (0.4) | 0.70 | 3.5 | 7.8 |
| H° | 76.3 (0.4) | 8.6 (0.4) | 0.71 | 86.7 | 82.3 | |
| ‘Redhaven’ | a* | 8.4 (0.2) | −6.0 (0.7) | 0.28 | −1.1 | 4.9 |
| H° | 81.8 (0.2) | 5.8 (0.6) | 0.30 | 91.1 | 85.3 | |
| ‘September Sun’ | a* | 9.9 (0.4) | −6.4 (0.4) | 0.55 | 2.2 | 4.8 |
| H° | 79.9 (0.4) | 6.5 (0.4) | 0.57 | 87.7 | 85.1 | |
| Maturity Index/Colour Attribute | Immature | Harvest-Ready | Mature |
|---|---|---|---|
| FF 1 (kgf) | 8.8 | 6.4 | 5.1 |
| SSC 2 (°Brix) | 12.7 | 12.2 | 12.4 |
| IAD 3 | 1.5 | 1.0 | 0.4 |
| Skin a* | 25.7 | 32.3 | 35.2 |
| Skin H° | 53.7 | 37.3 | 31.6 |
| Flesh a* | − 2.5 | 5.0 | 8.9 |
| Flesh H° | 92.7 | 84.7 | 79.5 |
| Cultivar | Maturity index | R2CV1 | rc2 | AIC3 | RMSECV4 | LV 5 |
|---|---|---|---|---|---|---|
| ‘August Flame’ | FF 1 | 0.69 | 0.82 | 184 | 1.48 | 12 |
| SSC 2 | 0.72 | 0.84 | 301 | 1.9 | 12 | |
| IAD 3 | 0.83 | 0.91 | −655 | 0.18 | 10 | |
| Skin a* | 0.76 | 0.86 | 509 | 3.21 | 12 | |
| Skin H° | 0.77 | 0.87 | 657 | 4.65 | 11 | |
| Flesh a* | 0.76 | 0.87 | 337 | 2.09 | 12 | |
| Flesh H° | 0.80 | 0.89 | 305 | 1.93 | 12 | |
| ‘O’Henry’ | FF | 0.69 | 0.81 | 149 | 1.15 | 12 |
| SSC | 0.75 | 0.86 | 196 | 1.55 | 11 | |
| IAD | 0.80 | 0.89 | −528 | 0.24 | 9 | |
| Skin a* | 0.75 | 0.85 | 467 | 3.21 | 7 | |
| Skin H° | 0.83 | 0.91 | 629 | 4.45 | 9 | |
| Flesh a* | 0.87 | 0.93 | 317 | 2.04 | 10 | |
| Flesh H° | 0.87 | 0.93 | 296 | 1.93 | 9 | |
| ‘Redhaven’ | FF | 0.78 | 0.87 | −20 | 0.71 | 10 |
| SSC | 0.58 | 0.74 | 220 | 1.34 | 7 | |
| IAD | 0.96 | 0.98 | −1126 | 0.05 | 9 | |
| Skin a* | 0.76 | 0.87 | 570 | 3.75 | 7 | |
| Skin H° | 0.88 | 0.94 | 668 | 4.78 | 10 | |
| Flesh a* | 0.46 | 0.62 | 375 | 2.26 | 9 | |
| Flesh H° | 0.49 | 0.65 | 340 | 2.07 | 10 | |
| ‘September Sun’ | FF | 0.50 | 0.67 | 280 | 1.95 | 6 |
| SSC | 0.49 | 0.67 | 382 | 2.01 | 8 | |
| IAD | 0.83 | 0.91 | −651 | 0.18 | 8 | |
| Skin a* | 0.76 | 0.86 | 508 | 3.28 | 7 | |
| Skin H° | 0.79 | 0.88 | 692 | 5.21 | 10 | |
| Flesh a* | 0.73 | 0.84 | 312 | 2.01 | 10 | |
| Flesh H° | 0.74 | 0.85 | 303 | 1.97 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. https://doi.org/10.3390/s20226555
Scalisi A, Pelliccia D, O’Connell MG. Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors. 2020; 20(22):6555. https://doi.org/10.3390/s20226555
Chicago/Turabian StyleScalisi, Alessio, Daniele Pelliccia, and Mark Glenn O’Connell. 2020. "Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer" Sensors 20, no. 22: 6555. https://doi.org/10.3390/s20226555
APA StyleScalisi, A., Pelliccia, D., & O’Connell, M. G. (2020). Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors, 20(22), 6555. https://doi.org/10.3390/s20226555





