Effects of Wearable Devices with Biofeedback on Biomechanical Performance of Running—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Inclusion and Exclusion Criteria
2.2. Search Strategy and Study Selection
2.3. Data Extraction
3. Results
3.1. Search Results
3.2. Subjects
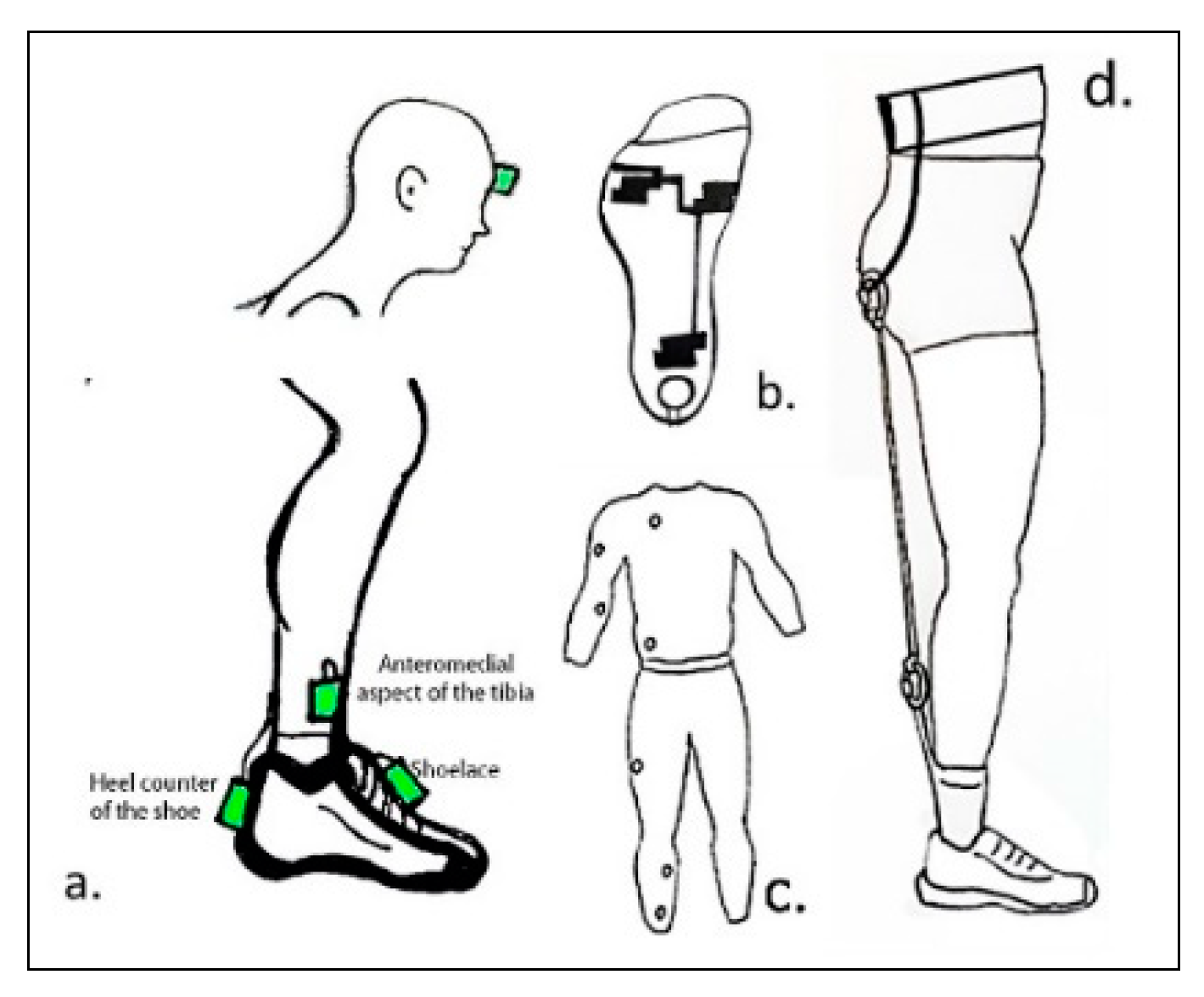
3.3. Components of Wearable Biofeedback Devices
3.3.1. Use of Wearables Sensors
3.3.2. Use of Feedback Modalities
3.3.3. Instructions to Participants
3.4. Outcomes Measures of Running
3.4.1. Equipment
3.4.2. Study Environment
3.5. Effects of the Use of Biofeedback on Running Performance
3.5.1. Peak Positive Acceleration (PPA)
3.5.2. Stride Frequencies (SF)
3.5.3. Other Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PPA | Peak positive acceleration measured during the stance phase at heel strike |
| VILR | Vertical instantaneous loading rate in the slope from 20% to 80% of impact phase |
| VALR | Vertical average loading rate in the slope from 20% to 80% of impact phase |
| SF | Stride frequency or cadence. Number of steps taken per minute |
| COP | Center of pressure at the foot |
| COM | Center of mass of the body |
| CGT | Contact ground time. Time that the foot is on the ground during the stance phase |
References
- Barnes, K.R.; Kilding, A.E. Running economy: Measurement, norms, and determining factors. Sports Med. Open 2015, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugan, S.A.; Bhat, K.P. Biomechanics and Analysis of Running Gait. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Ounpuu, S. The biomechanics of running: A kinematic and kinetic analysis. Instr. Course Lect. 1990, 39, 305–318. [Google Scholar] [PubMed]
- Lenhart, R.; Thelen, D.; Heiderscheit, B. Hip Muscle Loads During Running at Various Step Rates. J. Orthop. Sports Phys. Ther. 2014, 44, A764–A766. [Google Scholar] [CrossRef]
- Novacheck, T.F. The biomechanics of running. Gait Posture 1998, 7, 77–95. [Google Scholar] [CrossRef]
- Hamner, S.R.; Seth, A.; Delp, S.L. Muscle contributions to propulsion and support during running. J. Biomech. 2010, 43, 2709–2716. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.W.; Rudins, A. Foot Biomechanics During Walking and Running. Mayo Clin. Proc. 1994, 69, 448–461. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.B. An Evidence-Based Videotaped Running Biomechanics Analysis. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 217–236. [Google Scholar] [CrossRef] [Green Version]
- Reenalda, J.; Maartens, E.; Buurke, J.H.; Gruber, A.H. Kinematics and shock attenuation during a prolonged run on the athletic track as measured with inertial magnetic measurement units. Gait Posture 2019, 68, 155–160. [Google Scholar] [CrossRef]
- Tian, F.; Li, N.; Zheng, Z.; Huang, Q.; Zhu, T.; Li, Q.; Wang, W.; Tsai, T.-Y.; Wang, S. The effects of marathon running on three-dimensional knee kinematics during walking and running in recreational runners. Gait Posture 2020, 75, 72–77. [Google Scholar] [CrossRef]
- Anderson, T. Biomechanics and Running Economy. Sports Med. 1996, 22, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Ae, M. The next steps for expanding and developing sport biomechanics. Sports Biomech. 2020, 19, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Keogh, J.; Kos, A.; Santuz, A.; Umek, A.; Urbanczyk, C.; van der Kruk, E.; Rossi, S. Sport Biomechanics Applications Using Inertial, Force, and EMG Sensors: A Literature Overview. Appl. Bionics Biomech. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Jenny, D.F.; Jenny, P. On the mechanical power output required for human running—Insight from an analytical model. J. Biomech. 2020, 110, 109948. [Google Scholar] [CrossRef] [PubMed]
- Kyröläinen, H.; Belli, A.; Komi, P.V. Biomechanical factors affecting running economy. Med. Sci. Sports Exerc. 2001, 33, 1330–1337. [Google Scholar] [CrossRef]
- Cheung, R.T.; Davis, I.S. Landing pattern modification to improve patellofemoral pain in runners: A case series. J. Orthop. Sports Phys. 2011, 41, 914–919. [Google Scholar] [CrossRef]
- Monte, A.; Maganaris, C.; Baltzopoulos, V.; Zamparo, P. The influence of Achilles tendon mechanical behaviour on “apparent” efficiency during running at different speeds. Eur. J. Appl. Physiol. 2020, 120, 2495–2505. [Google Scholar] [CrossRef]
- Kuhman, D.; Melcher, D.; Paquette, M.R. Ankle and knee kinetics between strike patterns at common training speeds in competitive male runners. Eur. J. Sport Sci. 2016, 16, 433–440. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Warrener, A.G.; Wang, J.; Castillo, E.R. Effects of stride frequency and foot position at landing on braking force, hip torque, impact peak force and the metabolic cost of running in humans. J. Exp. Biol. 2015, 218, 3406–3414. [Google Scholar] [CrossRef] [Green Version]
- Schache, A.G.; Blanch, P.D.; Murphy, A.T. Relation of anterior pelvic tilt during running to clinical and kinematic measures of hip extension. Br. J. Sports Med. 2000, 34, 279. [Google Scholar] [CrossRef] [Green Version]
- Moore, I. Is There an Economical Running Technique? A Review of Modifiable Biomechanical Factors Affecting Running Economy. Sports Med. 2016, 46, 793–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hooren, B.; Goudsmit, J.; Vos, S.; Restrepo, J. Real-time feedback by wearables in running: Current approaches, challenges and suggestions for improvements. J. Sports Sci. 2020, 38, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.G.; Kempf, J.; Heiderscheit, B.C. Influence of Stride Frequency and Length on Running Mechanics: A Systematic Review. Sports Health A Multidiscip. Approach 2014, 6, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, W.W.; Ting, K.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.S.; Davis, I.S.; So, W.K.Y.; Chan, R.H.M.; Cheung, R.T.H. Neurophysiological Correlates of Gait Retraining With Real-Time Visual and Auditory Feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1341–1349. [Google Scholar] [CrossRef]
- Townshend, A.D.; Franettovich Smith, M.M.; Creaby, M.W. The energetic cost of gait retraining: A pilot study of the acute effect. Phys. Ther. Sport 2017, 23, 113–117. [Google Scholar] [CrossRef]
- Demircan, E. A pilot study on locomotion training via biomechanical models and a wearable haptic feedback system. ROBOMECH J. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Moran, M.F.; Wager, J.C. Influence of Gait Retraining on Running Economy: A Review and Potential Applications. Strength Cond. J. 2020, 42, 12–23. [Google Scholar] [CrossRef]
- Denadai, B.; Aguiar, R.; Lima, L.; Greco, C.; Caputo, F. Explosive Training and Heavy Weight Training are Effective for Improving Running Economy in Endurance Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 545–554. [Google Scholar] [CrossRef]
- Lake, M.J.; Cavanagh, P.R. Six weeks of training does not change running mechanics or improve running economy. Med. Sci. Sports Exerc. 1996, 28, 860–869. [Google Scholar] [CrossRef]
- Tamburini, P.; Storm, F.; Buckley, C.; Bisi, M.C.; Stagni, R.; Mazzà, C. Moving from laboratory to real life conditions: Influence on the assessment of variability and stability of gait. Gait Posture 2018, 59, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Jung, A.P. The impact of resistance training on distance running performance. Sports Med. 2003, 33, 539–552. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, M.P.; Haaf, D.S.M.; van Cingel, R.; de Wijer, A.; Nijhuis-van der Sanden, M.W.G.; Staal, J.B. Injuries in runners; a systematic review on risk factors and sex differences. PLoS ONE 2015, 10, e0114937. [Google Scholar] [CrossRef] [PubMed]
- Simon, S. Quantification of human motion: Gait analysis - Benefits and limitations to its application to clinical problems. J. Biomech. 2005, 37, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, S.J.M.; Benbasat, A.Y.; Scarborough, D.M.; Krebs, D.E.; Paradiso, J.A. Gait Analysis Using a Shoe-Integrated Wireless Sensor System. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Düking, P.; Hotho, A.; Holmberg, H.C.; Fuss, F.K.; Sperlich, B. Comparison of non-invasive individual monitoring of the training and health of athletes with commercially available wearable technologies. Front. Physiol. 2016, 7, 71. [Google Scholar] [CrossRef]
- Shull, P.B.; Damian, D.D. Haptic wearables as sensory replacement, sensory augmentation and trainer—A review. J. Neuroeng. Rehabil. 2015, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Cesini, I.; Martini, E.; Filosa, M.; Spigler, G.; Sabatini, A.M.; Vitiello, N.; Oddo, C.M.; Crea, S. Perception of Time-Discrete Haptic Feedback on the Waist is Invariant with Gait Events. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1595–1604. [Google Scholar] [CrossRef]
- Yalcin, B.; Bayram, H.A.; Bayram, M.B.; Isik, A. Investigation of Biological Feedback Influence on Posture and Knee Biomechanics During Running. In Proceedings of the 21st National Biomedical Engineering Meeting (BIYOMUT), Istanbul, Turkey, 24 November–26 December 2017; pp. i–iv. [Google Scholar]
- Xu, J.; Bao, T.; Lee, U.H.; Kinnaird, C.; Carender, W.; Huang, Y.; Sienko, K.H.; Shull, P.B. Configurable, wearable sensing and vibrotactile feedback system for real-time postural balance and gait training: Proof-of-concept. J. Neuroeng. Rehabil. 2017, 14, 102. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, M.; Uva, A.E.; Foglia, M.M.; Bevilacqua, V. Asymmetry measurement for vibroactive correction in lower limbs mobility. Comput. Sci. Inf. Syst. 2013, 10, 1387–1406. [Google Scholar] [CrossRef]
- Futrell, E.E.; Gross, K.D.; Reisman, D.; Mullineaux, D.R.; Davis, I.S. Transition to forefoot strike reduces load rates more effectively than altered cadence. J. Sport Health Sci. 2020, 9, 248–257. [Google Scholar] [CrossRef]
- Afzal, M.R.; Lee, H.; Eizad, A.; Lee, C.H.; Oh, M.; Yoon, J. Effects of Vibrotactile Biofeedback Coding Schemes on Gait Symmetry Training of Individuals With Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.H.; Lee, W.C.C. A wearable vibrotactile biofeedback system improves balance control of healthy young adults following perturbations from quiet stance. Hum. Mov. Sci. 2017, 55, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.H.; Wong, D.W.; Ma, C.Z.; Zhang, M.; Lee, W.C. Wearable Vibrotactile Biofeedback Device Allowing Identification of Different Floor Conditions for Lower-Limb Amputees. Arch. Phys. Med. Rehabil. 2016, 97, 1210–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalai, A.A.; Senanayake, S.M.N.A. A Wearable Real-Time Intelligent Posture Corrective System Using Vibrotactile Feedback. IEEE/ASME Trans. Mechatron. 2011, 16, 827–834. [Google Scholar] [CrossRef]
- Koyama, Y.; Watanabe, K. Perceptive sportswear system with auditory feedback based on hetero-core optical fiber for running motion support. Procedia Eng. 2014, 87, 552–555. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.J.; Parker, J.; McCullagh, P.; Zheng, H.; Nugent, C.; Black, N.D.; Mawson, S. A Personalized Self-Management Rehabilitation System for Stroke Survivors: A Quantitative Gait Analysis Using a Smart Insole. JMIR Rehabil. Assist. Technol. 2016, 3, e11. [Google Scholar] [CrossRef] [Green Version]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Validation of inertial measurement units with an optoelectronic system for whole-body motion analysis. Med. Biol. Eng. Comput. 2017, 55, 609–619. [Google Scholar] [CrossRef]
- Warmerdam, E.; Romijnders, R.; Welzel, J.; Hansen, C.; Schmidt, G.; Maetzler, W. Quantification of arm swing during walking in healthy adults and parkinson’s disease patients: Wearable sensor-based algorithm development and validation. Sensors 2020, 20, 5963. [Google Scholar] [CrossRef]
- Duncan, M.J.; Dobell, A.; Noon, M.; Clark, C.C.T.; Roscoe, C.M.P.; Faghy, M.A.; Stodden, D.; Sacko, R.; Eyre, E.L.J. Calibration and cross-validation of accelerometery for estimating movement skills in children aged 8–12 years. Sensors 2020, 20, 2776. [Google Scholar] [CrossRef]
- Mahoney, J.M.; Rhudy, M.B. Methodology and validation for identifying gait type using machine learning on IMU data. J. Med. Eng. Technol. 2019, 43, 25–32. [Google Scholar] [CrossRef]
- Staunton, C.; Wundersitz, D.; Gordon, B.; Kingsley, M. Construct Validity of Accelerometry-Derived Force to Quantify Basketball Movement Patterns. Int. J. Sports Med. 2017, 38, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Wouda, F.J.; Giuberti, M.; Bellusci, G.; Maartens, E.; Reenalda, J.; Van Beijnum, B.-J.F.; Veltink, P.H. Estimation of Vertical Ground Reaction Forces and Sagittal Knee Kinematics During Running Using Three Inertial Sensors. Front. Physiol. 2018, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Fusca, M.; Negrini, F.; Perego, P.; Magoni, L.; Molteni, F.; Andreoni, G. Validation of a Wearable IMU System for Gait Analysis: Protocol and Application to a New System. Appl. Sci. 2018, 8, 1167. [Google Scholar] [CrossRef] [Green Version]
- Dahl, K.D.; Dunford, K.M.; Wilson, S.A.; Turnbull, T.L.; Tashman, S. Wearable sensor validation of sports-related movements for the lower extremity and trunk. Med. Eng. Phys. 2020, 84, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gantz, A.M.; Derrick, T.R. Kinematics and metabolic cost of running on an irregular treadmill surface. J. Sports Sci. 2018, 36, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Organero, M.; Ruiz-Blázquez, R. Detecting Steps Walking at very Low Speeds Combining Outlier Detection, Transition Matrices and Autoencoders from Acceleration Patterns. Sensors 2017, 17, 2274. [Google Scholar] [CrossRef] [Green Version]
- Rucco, R.; Sorriso, A.; Liparoti, M.; Ferraioli, G.; Sorrentino, P.; Ambrosanio, M.; Baselice, F. Type and Location of Wearable Sensors for Monitoring Falls during Static and Dynamic Tasks in Healthy Elderly: A Review. Sensors 2018, 18, 1613. [Google Scholar] [CrossRef] [Green Version]
- DeJong, A.F.; Hertel, J. Gait-training devices in the treatment of lower extremity injuries in sports medicine: Current status and future prospects. Expert Rev. Med. Devices 2018, 15, 891–909. [Google Scholar] [CrossRef]
- Greenberg, E.T.; Garcia, M.C.; Galante, J.; Werner, W.G. Acute changes in sagittal plane kinematics while wearing a novel belt device during treadmill running. Sports Biomech. 2019, 1–13. [Google Scholar] [CrossRef]
- Cheung, R.T.H.; Zhang, J.H.; Chan, Z.Y.S.; An, W.W.; Au, I.P.H.; MacPhail, A.; Davis, I.S. Shoe-mounted accelerometers should be used with caution in gait retraining. Scand. J. Med. Sci. Sports 2019, 29, 835–842. [Google Scholar] [CrossRef]
- Willy, R.W.; Meardon, S.A.; Schmidt, A.; Blaylock, N.R.; Hadding, S.A.; Willson, J.D. Changes in tibiofemoral contact forces during running in response to in-field gait retraining. J. Sports Sci. 2016, 34, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chan, Z.Y.; Au, I.P.; An, W.W.; Shull, P.B.; Cheung, R.T. Transfer Learning Effects of Biofeedback Running Retraining in Untrained Conditions. Med. Sci. Sports Exerc. 2019, 51, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Ching, E.; An, W.W.-K.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.S.; Shum, G.; Cheung, R.T.H. Impact Loading During Distracted Running Before and After Auditory Gait Retraining. Int. J. Sports Med. 2018, 39, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Clansey, A.C.; Hanlon, M.; Wallace, E.S.; Nevill, A.; Lake, M.J. Influence of tibial shock feedback training on impact loading and running economy. Med. Sci. Sports Exerc. 2014, 46, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Crowell, H.P.; Davis, I.S. Gait retraining to reduce lower extremity loading in runners. Clin. Biomech. 2011, 26, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh, O.; Steinberg, N.; Nemet, D.; Eliakim, A.; Orland, G. Visual feedback gait re-training in overweight children can reduce excessive tibial acceleration during walking and running: An experimental intervention study. Gait Posture 2019, 68, 101–105. [Google Scholar] [CrossRef]
- Crowell, H.P.; Milner, C.E.; Hamill, J.; Davis, I.S. Reducing impact loading during running with the use of real-time visual feedback. J. Orthop. Sports Phys. 2010, 40, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Creaby, M.W.; Franettovich Smith, M.M. Retraining running gait to reduce tibial loads with clinician or accelerometry guided feedback. J. Sci. Med. Sport 2016, 19, 288–292. [Google Scholar] [CrossRef]
- Wood, C.M.; Kipp, K. Use of audio biofeedback to reduce tibial impact accelerations during running. J. Biomech. 2014, 47, 1739–1741. [Google Scholar] [CrossRef]
- Cheung, R.T.H.; An, W.W.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.S.; MacPhail, A.J. Control of impact loading during distracted running before and after gait retraining in runners. J. Sports Sci. 2018, 36, 1497–1501. [Google Scholar] [CrossRef]
- Willy, R.W.; Buchenic, L.; Rogacki, K.; Ackerman, J.; Schmidt, A.; Willson, J.D. In-field gait retraining and mobile monitoring to address running biomechanics associated with tibial stress fracture. Scand. J. Med. Sci. Sports 2016, 26, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Gilgen-Ammann, R.; Wyss, T.; Troesch, S.; Heyer, L.; Taube, W. Positive Effects of Augmented Feedback to Reduce Time on Ground in Well-Trained Runners. Int. J. Sports Physiol. Perform. 2018, 13, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Busa, M.A.; Lim, J.; van Emmerik, R.E.; Hamill, J. Head and Tibial Acceleration as a Function of Stride Frequency and Visual Feedback during Running. PLoS ONE 2016, 11, e0157297. [Google Scholar] [CrossRef]
- Phanpho, C.; Rao, S.; Moffat, M. Immediate effect of visual, auditory and combined feedback on foot strike pattern. Gait Posture 2019, 74, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Halvorsen, K.A.; Gullstrand, L. Immediate effect of visual and auditory feedback to control the running mechanics of well-trained athletes. J. Sports Sci. 2011, 29, 253–262. [Google Scholar] [CrossRef]
- Sear, J.A.; Hoare, T.K.; Scanlan, A.T.; Abt, G.A.; Dascombe, B.J. The effects of whole-body compression garments on prolonged high-intensity intermittent exercise. J. Strength Cond. Res. 2010, 24, 1901–1910. [Google Scholar] [CrossRef]
- Sheerin, K.R.; Reid, D.; Besier, T.F. The measurement of tibial acceleration in runners—A review of the factors that can affect tibial acceleration during running and evidence-based guidelines for its use. Gait Posture 2019, 67, 12–24. [Google Scholar] [CrossRef]
- Heise, G.D.; Martin, P.E. Are variations in running economy in humans associated with ground reaction force characteristics? Eur. J. Appl. Physiol. 2001, 84, 438–442. [Google Scholar] [CrossRef]
- Chen, Y.T.; Tenforde, A.S.; Fredericson, M. Update on stress fractures in female athletes: Epidemiology, treatment, and prevention. Curr. Rev. Musculoskelet. Med. 2013, 6, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Brukner, P.; Bradshaw, C.; Khan, K.M.; White, S.; Crossley, K. Stress fractures: A review of 180 cases. Clin. J. Sport Med. 1996, 6, 85–89. [Google Scholar] [CrossRef]
- Van den Berghe, P.; Six, J.; Gerlo, J.; Leman, M.; De Clercq, D. Validity and reliability of peak tibial accelerations as real-time measure of impact loading during over-ground rearfoot running at different speeds. J. Biomech. 2019, 86, 238–242. [Google Scholar] [CrossRef]
- Clark, K.P.; Ryan, L.J.; Weyand, P.G. A general relationship links gait mechanics and running ground reaction forces. J. Exp. Biol. 2017, 220, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Concejero, J.; Tam, N.; Coetzee, D.R.; Oliván, J.; Noakes, T.D.; Tucker, R. Are gait characteristics and ground reaction forces related to energy cost of running in elite Kenyan runners? J. Sports Sci. 2017, 35, 531–538. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, C.J.; van Daal, S.; van Dieën, J.H. Individual optimal step frequency during outdoor running. Eur. J. Sport Sci. 2020, 20, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Aubry, R.L.; Power, G.A.; Burr, J.F. An Assessment of Running Power as a Training Metric for Elite and Recreational Runners. J. Strength Cond. Res. 2018, 32, 2258–2264. [Google Scholar] [CrossRef]
- Milner, C.E.; Ferber, R.; Pollard, C.D.; Hamill, J.; Davis, I.S. Biomechanical factors associated with tibial stress fracture in female runners. Med. Sci. Sports Exerc. 2006, 38, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Davis, I. Gait retraining: Altering the fingerprint of gait. J. Foot Ankle Res. 2011, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Hobara, H.; Hashizume, S.; Funken, J.; Willwacher, S.; Müller, R.; Grabowski, A.M.; Potthast, W. Vertical stiffness during one-legged hopping with and without using a running-specific prosthesis. J. Biomech. 2019, 86, 34–39. [Google Scholar] [CrossRef]
- Folland, J.P.; Allen, S.J.; Black, M.I.; Handsaker, J.C.; Forrester, S.E. Running Technique is an Important Component of Running Economy and Performance. Med. Sci. Sports Exerc. 2017, 49, 1412–1423. [Google Scholar] [CrossRef] [Green Version]
- Leskinen, A.; Häkkinen, K.; Virmavirta, M.; Isolehto, J.; Kyröläinen, H. Comparison of running kinematics between elite and national-standard 1500-m runners. Sports Biomech. 2009, 8, 1–9. [Google Scholar] [CrossRef]
- Trowell, D.; Phillips, E.; Saunders, P.; Bonacci, J. The relationship between performance and biomechanics in middle-distance runners. Sports Biomech. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salmoni, A.W.; Schmidt, R.A.; Walter, C.B. Knowledge of results and motor learning: A review and critical reappraisal. Psychol. Bull. 1984, 95, 355–386. [Google Scholar] [CrossRef] [PubMed]

| Terms and Strategies | Identifier |
|---|---|
| Wear * (technolog * or device * or sensor *) or “real-time sensor” | 1 |
| Inertial sensor” or “inertial measurement unit” or gyroscope * or magnetometer * or acceleromet * or “cell phone” or “smart phone *” | 2 |
| Run * or running or “runner” or “running injuries” | 3 |
| Feedback * or biofeedback * or augment * or “real time” | 4 |
| Visual (signal * or feedback * or cue or biofeed * or augment *) or (visual (train * or retrain *)) | 5 |
| Auditory (signal * or feedback * or cue * or biofeed * or augment *) or (auditory (train * or retrain *)) | 6 |
| Haptic (signal * or feedback * or cue * or biofeed * or augment *)) or (vibrat * (signal * or feedback * or cue or biofeed * or augment *)) or (haptic (train * or retrain *)) or (vibrat * (train * or retrain *)) | 7 |
| Mechanic * or load * or performance or postural * or “ambulatory monitoring” | 8 |
| “Ground reaction” or force or kinematic * or biomech * or acceleration * or cadence or “step length” or “step width” or “step time” or “stride length” or “stride time” or “stance phase” or “swing phase” or “stance time” or “swing time” or “single support” or “double support” or “ground contact” or “gait speed” or “walking speed” or “running speed” or “heel-strike” or “toe off” or “speed” or “center of mass” or “center of mass” or “center of gravity (CG)” or “center of gravity” | 9 |
| Patient or stroke or Parkinson’s | 10 |
| Citation | Year | Sample | Participants Conditions | Age (yrs) |
|---|---|---|---|---|
| [75] | 2019 | 15:11 F; 4 M | Healthy recreational runners. Run: at least twice a week for at least 30 min per session. Rearfoot striker pattern. | 25.67 ± 3.99 |
| [60] | 2019 | 10:7 F; 3 M | Healthy runners. Run: at least 10 miles per week. No injuries in the last 3 months, at least 2 factors of high-risk running technique. | 28.3 ± 2.8 |
| [63] | 2019 | 13:3 F; 10 M | Healthy recreational runners. No injuries. Run: weekly running distance of 30.7 ± 22.2 km. Running experience: min 2 years (6.8 ± 4.4 yr). PPA tibia > 8 g. | 41 ± 6.9 |
| [67] | 2019 | 37 | 25 over-weight children (11 CG, 14 IG). 12 non weight children (CG) | 9.05 ± 1.64 |
| [61] | 2019 | 14:7 F; 7 M | Healthy recreational runners. Run: more than 12 km per week. No injuries in the last 12 months Rearfoot strike pattern. Foot strike angle > 8°. | 26 ± 11.2 |
| [56] | 2018 | 16:9 F; 7 M | Runners. Run: at least 10 miles per week. No injuries in the last 6 months. | 22 ± 2.4 F; 24 ± 4.5 M |
| [64] | 2018 | 16:9 F; 7 M | Healthy recreational runners. Run: At least 12 km per week for at min. one year. Experience running 3.2 ± 0.9 years. No injuries in the last year, PPA> 8 g. | 25 ± 7.9 |
| [73] | 2018 | 30:12 F; 17 M CG: 20, IG: 10 | Healthy runners (late preparation phase for a 5–15 km race). Run: 409 ± 182 min weekly. 11.3 ± 7.5 years of running experience. | 31.0 ± 7.5 |
| [71] | 2018 | 16:5 F; 11 M | Healthy recreational runners. Run: at least 5 km per week for about a year. No injuries. | 28 ± 6.2 |
| [74] | 2016 | 12:4 F; 8 M | Healthy recreational runners. Run: Min preferred treadmill running speed of 2.3 m/s at least twice a week. No injuries in the last year. | 29.67 ± 4.4 |
| [69] | 2016 | 22 M; CG: 11; G: 11 | Healthy runners. Run: more than 10 km per week. | 18–45 |
| [72] | 2016 | 30:16 F; 14 M 14/16 IG | Healthy runners. Run: at least 11.3 km/week, at-risk runners who exhibited high-impact forces No injuries in the last 90 days. High-impact runners with VILR ≥85 body weight/s in either limb. | 22.1 ± 10 |
| [62] | 2016 | 30:14 F; 16 M; 15 IG | Healthy runners, Run: at least 10 km per week, No injuries in the last 6 months, Vertical GRF ≥ 85 body weight/s in either limb. | 18–35 |
| [65] | 2014 | 22 M; 12 IG | Healthy runners. Run: at least 30 km per week. No injuries in the last 6 months. PPA >9 g. | CG 33.3 ± 9.0 IG 30 ± 11 |
| [70] | 2014 | 9:6 F; 3 M | Healthy runners. Run: at least 10 miles per week, 2 times per week. Heel-strike footfall pattern. | 20 ± 1.5 |
| [66] | 2011 | 10:6 F; 4 M | Healthy runners, PPA tibia > 8 g, Run: 16 km per week. Rearfoot strike running pattern. | 26 ± 7 |
| [76] | 2011 | 18:7 F; 11 M | Healthy runners: 16 runners oriented at a national level, 2 recently retired competing at international level. Run: Comfortable running at 16 km/h. | 28.4 ± 6.4 |
| [68] | 2010 | 5 F | Healthy runners. Experienced running on a treadmill. Run: at least 32 km per week for at least 3 months. | 26 ± 2 |
| [77] | 2010 | 8 | Amateur team-sport athletes. Prolonged High-Intensity Intermittent Exercise training time: 2 h, 3 times per week + 1 weekly match. Competed at least during the last 3 years prior to the experiment. | 20.6 ± 1.2 |
| ID | Sensors and Equipment | Tracking Characteristics | Body Place of Sensors | Type of Feedback and Instruction | Feedback Body Placement and Form |
|---|---|---|---|---|---|
| [62] | 3D accelerometer and running computer (Forerunner 70™ Garmin) AE: CMCY and an instrumented treadmill | Step rate transmitted to wrist computer | Right shoelace of the shoe | Visual—maintain step rate increment of 75% | Wrist: numerical |
| [71] | 3D accelerometer (Maestro WB, 01DB-Stell) AE: instrumented treadmill | Device tracks tibia acceleration | Posterior heel counter of the shoe | Visual—land softer aiming for a target with a second task | Monitor: colored circles representing target accuracy |
| [66] | Accelerometer #axis: not mentioned (PCB Piezotronics—Model 356A32) AE: CMCY | Device tracks tibia acceleration | Left anteromedial distal aspect of tibia | Visual—run softer and keep acceleration peaks below the threshold (line) | Monitor: acceleration graph |
| [68] | Uniaxial accelerometer (PCB Piezotron-ics, Inc) AE: force transducers | Device tracks tibia acceleration | Right anteromedial distal aspect of tibia | Visual—run softer and keep acceleration peaks below the threshold (line) | Monitor: acceleration graph |
| [69] | 3D accelerometer (PCB Piezotronics- Model: PCB356A32/NC) AE: CMCY | Device tracks tibia acceleration | Right anteromedial distal aspect of tibia | Visual/auditory Run softer and keep inside the threshold area (Line?) | Monitor: acceleration graph and clinicians verbal instructions |
| [61] | 2D accelerometer (ADXL278) and MEMS AE: instrumented treadmill | Device tracks acceleration from the shoe and the tibia; Both values represent tibia acceleration | Right anteromedial distal aspect of tibia and posterior heel counter of the shoe | Visual—run softer and keep acceleration peaks below the threshold (green light) | Monitor: dichotomic signal Red and green circle light signals |
| [70] | 3D accelerometer: (G-Link -LXRS: Lord Micro strain) AE: non-instrumented treadmill | Device tracks tibia acceleration | Left anteromedial aspect of the distal end of the tibia | Auditory—avoid sounds | Beep with different pitch levels |
| [67] | IMU with 3D accelerometer: (YEI 3-space sensor, YEI Corporation AE: unspecified treadmill | Device tracks tibia acceleration | Anteromedial aspect of the distal end of the tibia (unknown leg) | Visual—run softer and keep acceleration peaks below the threshold (line) | Monitor: acceleration graph |
| [76] | Position transducer (Mod 1850-050, HIS-Houston Scientific International Inc) | Waist strap on the back connected to position transducer Tracking runner center of vertical mass displacement | Waist | Auditory—pre-recorded verbal instructions Visual—reach VD and speed target | Wireless headset: volume proportional to the error Monitor: 3 vertical bars |
| [75] | Sock embedded pressure sensors (Sensoria®, Sensoria Inc) and heel FRS AE: Flexible insole with 99 capacitive sensors (Pedar®-X; Novel Inc), treadmill and tablet | Device tracks cadence and identifies if the heel is loaded | FRS placed at the heel of each foot pressure sensors at heel and metatarsals | Visual/auditory—increase cadence, avoid landing on the heel and sound (Red circle?) | Tablet: graphical foot strike Beep (not from iPad) at heel strike |
| [64] | 3D accelerometer (Maestro WB, 01DB-Stell) AE: instrumented treadmill | Device tracks acceleration from the back of the shoe Values represent tibia acceleration | Right anteromedial distal aspect of tibia and posterior heel counter of the shoe | Auditory—run softer and avoid sound Simultaneous cognitive task | High pitched sound representing error intensity proportional to error |
| [72] | 3D accelerometer and wrist computer (Garmin FR70™ and footpad) AE: CMCY and instrumented treadmill | The footpod calculates step rate that is sent to the wrist computer | Right shoelace of the shoe | Visual—maintain step rate increment of 75% | Wrist: numerical Participant free to decide when to check their data |
| [56] | Accelerometers (model 353B17, PCB Piezotronics accelerometers) # axis not defined, AE: CMCY and mouthpiece Vo2 | Device tracks tibia acceleration, and results were displayed as leg and head power spectral densities (PSD) | Right anteromedial distal aspect of tibia and head frontal bone | Visual—maintain a target eversion angle Reference angle from an irregular terrain | Monitor: gray area represents target angle |
| [73] | 3D inertial measurement unit (Axiamote, motion Tracking™ device) AE: HR chest strap, instrumented treadmill, capillary blood sampling | Device tracks step frequency and CGT | Right shoelace of the shoe | Visual—reduce CGT while running with highest possible self-paced | Tablet screen: CGT bar displayed during resting times |
| [63] | 2D accelerometers AE: instrumented treadmill | Device tracks tibia acceleration | Bilateral anteromedial distal aspect of tibia | Visual—land softer | Monitor: acceleration graph |
| [60] | Not wearable sensors AE: CMCY | Lower body kinematics and COM data were collected with no wearable devices | None | Haptic—run normally while maintaining a preset target speed | Permanent resistance in lower limbs |
| [74] | Two 3D accelerometers (Trigno; Delsys, Inc) AE: CMCY | Device tracks tibial and head acceleration | Right anteromedial distal aspect of tibia and head frontal bone | Visual—match footfalls to metronome and maintain head in target area (dot line and square box) | 12 × 16 m Screen: head trajectory graph and target gaze area |
| [77] | No wearable sensors AE: nonmotorized treadmill, reflective object sensor and capillary blood sampling | Device tracks running velocity | None | Haptic, visual and audio Keep target speeds and cover maximum possible distance | Compression by a full bodysuit Monitor: numerical speed Audio: tone and verbal command before each run |
| [65] | 3D accelerometer (2400 T G2, Noraxon) AE: infrared CMCY | Device tracks tibia acceleration | Right anteromedial distal aspect of tibia | Visual and auditory Remain under shock threshold with a self-selected running strategy | Large 5 m screen: colored circles representing target accuracy Sound: laboratory speakers |
| ID | R | Protocol | Follow-up | Trigger |
|---|---|---|---|---|
| [65] | Yes | 6 sessions 20 min each. Feedback every 5 gait cycles all sessions. | 1 month | 50% from BS PPA. Displayed 50–75% and over 75% |
| [66] | No | 8 sessions in a 2-week period. Increasing 15 to 30 min. Feedback was reduced gradually during the last 4 sessions. | 1 month | 50% of BS of PPA |
| [68] | No | Single session of 30 min. 10 min No feedback followed by 10 min feedback. | No | 50% of BS of PPA |
| [69] | No | 2 sessions. 30 min. 10 min No feedback followed by 10 min feedback. | 1 week | 50% of BS of PPA |
| [71] | No | 8 sessions in a 2-week period. Increasing 15 to 30 min. Feedback was reduced gradually during the last 4 sessions. Each condition included a numeral cognitive task. Feedback was randomized. | No | 80% of BS of PPA |
| [61] | No | 8 sessions in a 2-week period. Increasing 15 to 30 min. Feedback was reduced gradually during the last 4 sessions. | No | 80% of BS of PPA |
| [70] | No | Single session. 20 min with intermittent feedback every 5 min. | No | 75–80% of BS of PPA |
| [64] | No | 8 sessions in a 2-week period. Increasing 15 to 30 min. Feedback was reduced gradually during the last 4 sessions. Each condition included a numerical cognitive task. | No | 80% of BS of PPA with a constant speed and cognitive and verbal task |
| [67] | Yes | 9 sessions. 6 min of feedback during the first 8 sessions. Participants had 1-hour endurance training before the feedback. Session #9 was a 1-month follow-up. | 1 month | 100%, 120% Speed and maintaining PPA under 100% of BS |
| [63] | No | 8 sessions in a 2-week period. Increasing 15 to 30 min. Feedback was reduced gradually during the last 4 sessions. | No | 90% and 110% BS speed and 80% of BS of PPA |
| [73] | Yes | 8 outdoor sessions in a 4-week period. Training sessions with a structure mixing 400 m to 1000 m runs. Data collection was measured at 80% of the max speed with 1% of treadmill inclination. Feedback was presented during resting times (approx. 3 min). | 1 week | Decrease GCT from previous run with a structured distance protocol aiming 80% of maximal velocity |
| [72] | Yes | 8 outdoor sessions in a 4-week period. Constant feedback during the session. Self-controlled. Run time: free. | 1 month | 7.5% increment of SF |
| [62] | Yes | 8 sessions (indoor or outdoor) in a 4-week period. Feedback on sessions #1–3, 5 and 7. Constant feedback during the session. Self-controlled. Run time: free. Groups were blinded. | 1 month | 7.5% increment of SF |
| [74] | No | “Series of short runs”. Protocol does not specify the frequency or intensity. Feedback session had continuously head trajectory. | No | 80%, 90%, 110% speed; 80% to 120% SF |
| [75] | No | Single run following a metronome. 15 min of feedback. 5 min for each condition. Visual, auditory and combined. | No | Incremented 10% BS of cadence and if heel strike load was sensed |
| [76] | No | Single session. Running or feedback time not specified. 11 running trials combining 2 control variables and feedback: Vertical displacement and step frequency. | No | 80%,90% and 100% VD; 80% and 90% of BS SF |
| [56] | Yes | Single session. 30 min session. 10 min control, 10 min irregular surface, 10 min with feedback. | No | Matching FA of irregular surface run ±1 degree |
| [77] | Yes | Single session. 45 min high-intensity session. 3 runs of 15 min with 6 different running speeds and last 2 min with self-selected speed covering as much distance as they can. Continuous feedback. | No | 0%, 20%, 35%, 50%, 70% and 100% of BS sprint. 50% considered running |
| [60] | No | Single session. 6 min control and 6 min feedback with 10 min rest. 6 min counted from reaching self-selected speed with typical moderate intensity. | No | 5 kg resistance in lower limbs from a wearable and portable belt |
| ID | Parameter of Change | PPA | VALR | VILR | VIP | Ankle Angle | Comments |
|---|---|---|---|---|---|---|---|
| [65] | PPA 50% | 31% R: 22% | 18% R: 6% | 19% R: 9% | ↓ 200%; ↓ FStrike angle 44% | R: 1 month | |
| [66] | PPA 50% | 48% R: 44% | 32% R: 27% | 34% R: 30% | R: 1 month | ||
| [68] | PPA 50% | 17–60% | 16–39% | 15–39% | 10–30% | Results were given as a tendency. Values were analyzed in each participant. | |
| [69] | PPA 50% | 18.9%; R: 21.2% | R: 1 week | ||||
| [71] | PPA 20% (cognitive load) | 41% | 24% | 18% | |||
| [61] | PPA 20% | 26.4%* | 16.40% | 17.30% | * Value from the tibia. PPA in the shoe: 40.9%. However, PPA in the shoe was not significantly correlated with VALR and VILR. The measurements registered in the shoe were 4 times higher than in the tibia. | ||
| [70] | PPA 15–20% | 11%–8% | Values changed across checking points over time—starting in 11% ending in 8%. | ||||
| [64] | PPA 20% (cognitive load) | 33.8%* | 25% | 22% | * Values from the shoe. PPA in the tibia: 10%; post feedback: 21.5%, but there was no significant correlation with VALR and VILR. | ||
| [67] | ↑Speed 120% and free ↓ PPA | 16%; R: 16% | R: 1 month. Significant changes observed from the 5th session. Complementary tables are not given. Retention: 1 month. | ||||
| [63] | ↑&↓Speed 10% and ↓ PPA 20% | R: 35–37% | R: 1 week. Changes observed across all speeds. There was no significant difference between limbs. Training effect did not interact with speed. |
| ID | Parameter of Change | SF | VD- COP | Ankle-Foot Changes | Knee Changes | Hip Changes | Spatiotemporal Changes | Comments |
|---|---|---|---|---|---|---|---|---|
| [62] | SF ↑ 7.5% | ↑ 8.6%; R: 8.5% | ↓: 9% *; R: 6.2% | kinematics: ↓Foot at FStrike: 22%, R: 19% ↓ Tibia at Fstrike: 31%; R 35% | kinematics: ↑ Flex at Fstrike: 25%; R 32%. kinetics: CKF: ↓ 7.6–10.61% R: 4.5–8.24%. | Step length: ↓ 8.36%; R: 8.2% | * COP distance from body COM at FStrike | |
| [72] | SF ↑ 7.5% | ↑ 8.6%; R ↑ 8.5% | kinetics: VALR; 19%; R: 20%; VILR: 18%; R: 17.5% | kinetics: Abs↓ 27.27% R: ↓ 21.21%; Abs/km ↓ 21.1%, R: ↓ 14% | kinematics: Add ↓ 27%; R: ↓ 21% | |||
| [75] | SF ↑ 10% and loading FS | ↑ 10% | COP foot position: Moved forward SF: 6%; SF and Visual FB: 37.31%; SF and Auditory FB: 45.35% Combined: 45.51% | kinetics: Area loaded: midfoot ↓ 40%, heel ↓ 80%, forefoot ↑ 10–36%; great toe ↑ 30% | ||||
| [74] | SF (↑ 10%, 20%, ↓ 10%, ↓ 20) & head trajectory | ↑ 10–20% | kinetics: Tibia shock attenuation: SF + 20 = ↑ 12%; SF + 10 = ↑ 5% peak impact acceleration ↓ 1.9% | Significant changes across all SF conditions. Larger impact on Tibia with SF-20. Changes reported in this table: +20, +10. Feedback was not significantly correlated with variables. | ||||
| [76] | SF ↓ 0–10%; VD: ↓ 10–20% and P ↓ 10–20% | ↑ up 7% and ↓ 10% * | ↓ 20% and ↑ 5% * | Trend value. Not significant |
| ID | Parameter of Change | SF | Ankle–Foot Changes | Knee Changes | Spatiotemporal Changes | Metabolic | Comments |
|---|---|---|---|---|---|---|---|
| [73] | CGT ↓ defined by the user | 1.5↓ | ↑ swing time: 2.32%; ↓ run time (400 m):1.5%; ↓ TOGC: 1.9% | ↓ HR: 2.5% | |||
| [56] | ↓ Inv to BS irregular surface | kinematic: Inv ↓ 20% (↓ 14.4) * kinetic: PSD: ↑ 35% * | Flex at Fstrike: ↓ 20.7% (↑ 17%) * | ↓ VO2:10%. | * Inv and knee flex ↓, but it was tested on a different surface. On the same surface, they ↓ 14.4% and ↑ 17%, respectively, but with no significant differences. * SPD ↑, but shock attenuation was the same (−9.8). | ||
| [77] | 50% of BS sprint speed | ↑ Distance coved: 7.1%; ↑ Speed: 8.5%; | ↑ HR 1–2%; 4% ↑ TOI; | Lactate was not significant | |||
| [60] | Device resistance (5 k) | VD ↓ 12.7% | kinematic: Angle of tibia at Fstrike: ↓ 34.7%, ↓ Exc: 14.9%; ↑ Flex: 6% | Step length: no significant changes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Pedroza, A.; Lee, W.C.-C.; Lam, W.-K.; Coman, R.; Alici, G. Effects of Wearable Devices with Biofeedback on Biomechanical Performance of Running—A Systematic Review. Sensors 2020, 20, 6637. https://doi.org/10.3390/s20226637
Giraldo-Pedroza A, Lee WC-C, Lam W-K, Coman R, Alici G. Effects of Wearable Devices with Biofeedback on Biomechanical Performance of Running—A Systematic Review. Sensors. 2020; 20(22):6637. https://doi.org/10.3390/s20226637
Chicago/Turabian StyleGiraldo-Pedroza, Alexandra, Winson Chiu-Chun Lee, Wing-Kai Lam, Robyn Coman, and Gursel Alici. 2020. "Effects of Wearable Devices with Biofeedback on Biomechanical Performance of Running—A Systematic Review" Sensors 20, no. 22: 6637. https://doi.org/10.3390/s20226637
APA StyleGiraldo-Pedroza, A., Lee, W. C.-C., Lam, W.-K., Coman, R., & Alici, G. (2020). Effects of Wearable Devices with Biofeedback on Biomechanical Performance of Running—A Systematic Review. Sensors, 20(22), 6637. https://doi.org/10.3390/s20226637







