Behavioral, Physiological and EEG Activities Associated with Conditioned Fear as Sensors for Fear and Anxiety †
Abstract
:1. Introduction
2. Methods and Rationales
3. Methods: Behavioral Protocol and Metrics
3.1. Methods: Laser, SCR, and EEG
3.2. Results and Interpretation
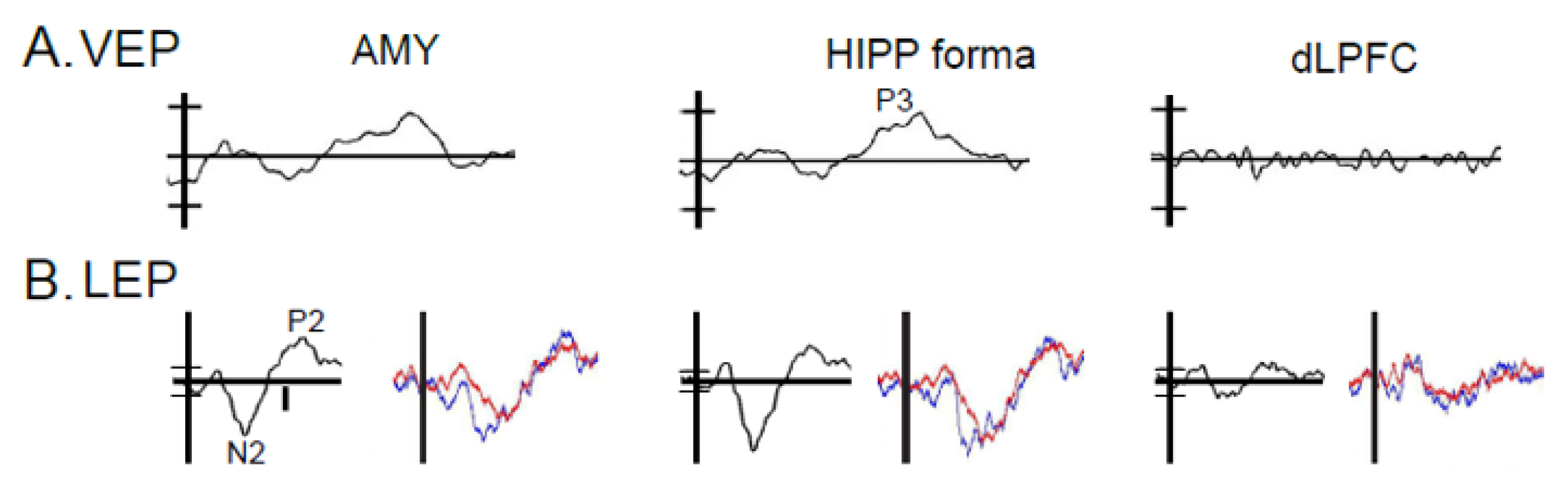
3.3. Results: Anatomic Convergence of VEPs and LEPs by Electrode
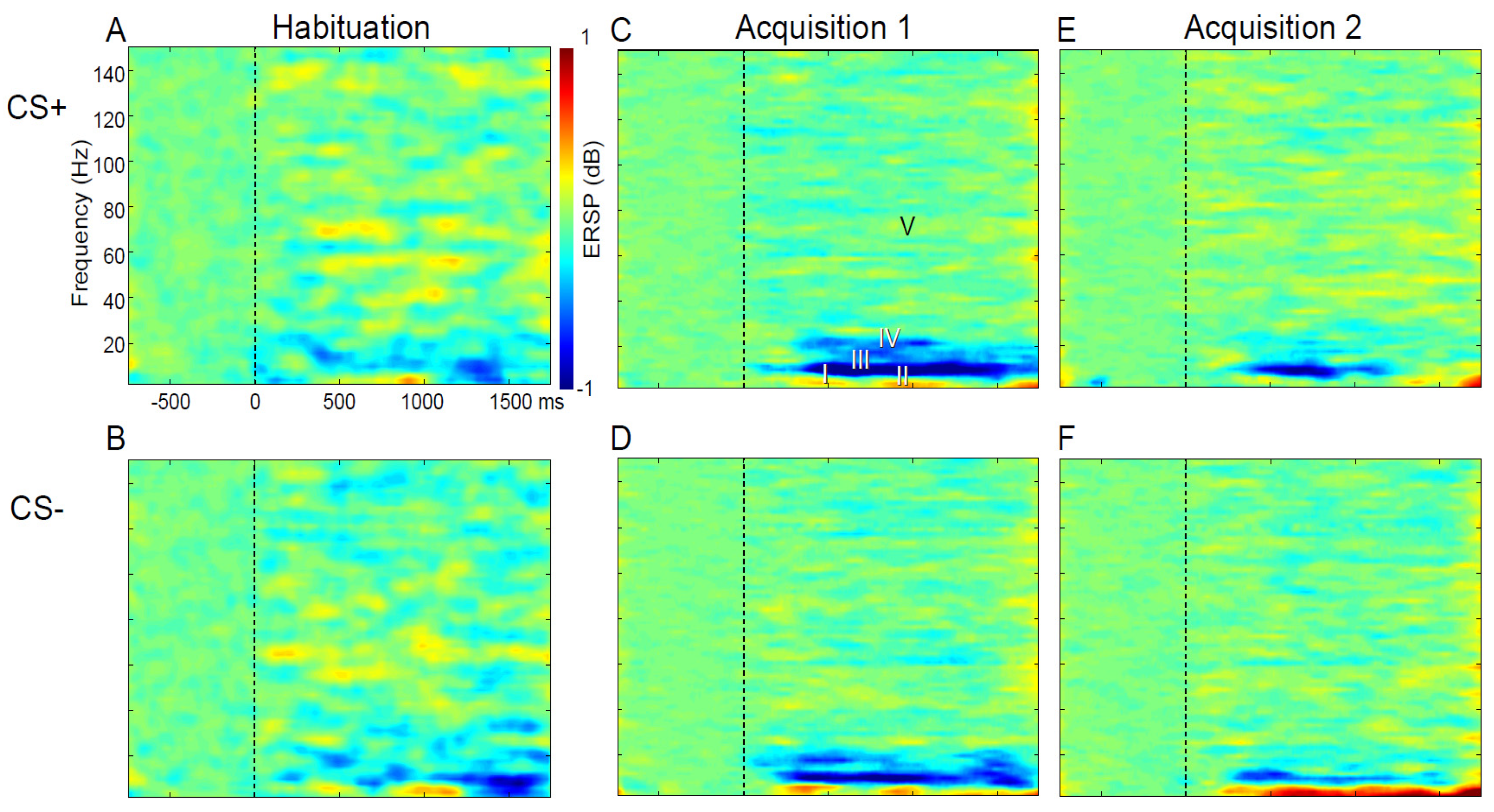
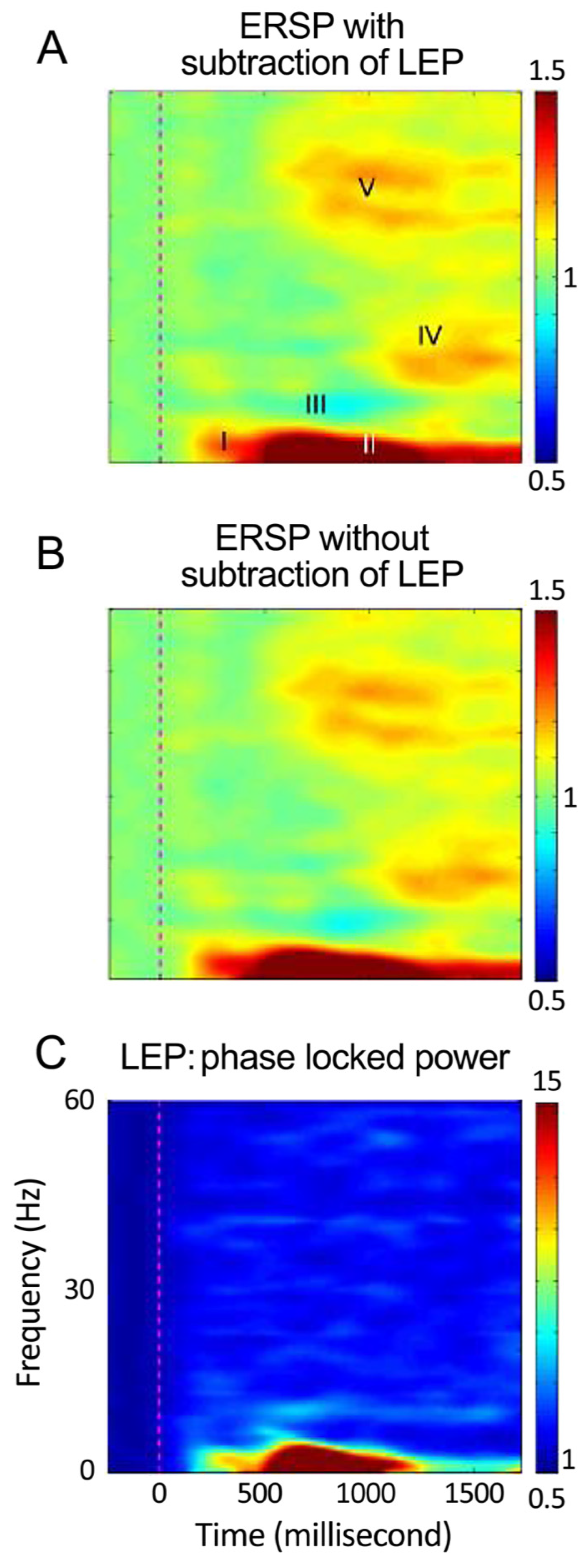
3.4. Results: ERSP and CRs
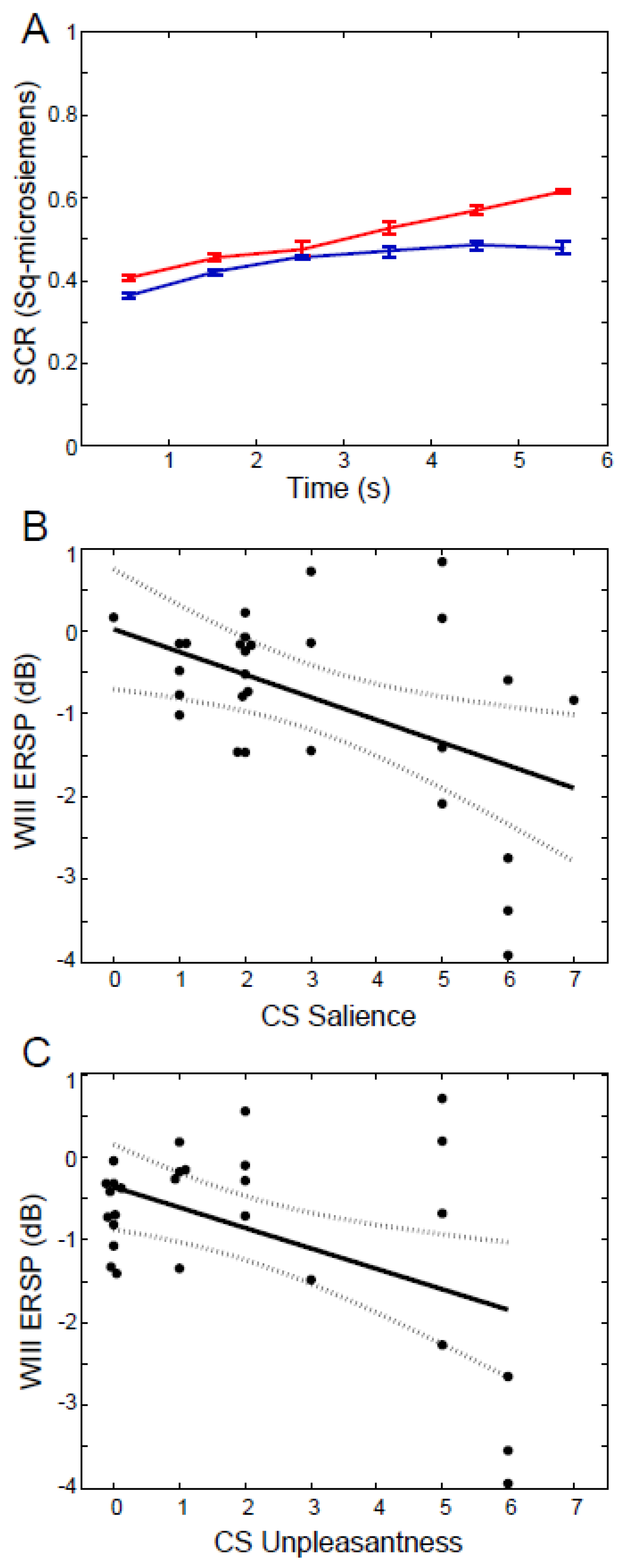
3.5. Results: ERSP Correlated with Behavior
3.6. Results: ERSP Correlated with Painful Laser
3.7. Results: ERSP Correlated with CRs
4. Sensors for Fear and Anxiety
Author Contributions
Funding
Conflicts of Interest
References
- Demyttenaere, K.; Bruffaerts, R.; Posada-Villa, J.; Gasquet, I.; Kovess, V.; Lepine, J.P.; Angermeyer, M.C.; Bernert, S.; de Girolamo, G.; Morosini, P.; et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 2004, 291, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Avenevoli, S.; Costello, E.J.; Georgiades, K.; Green, J.G.; Gruber, M.J.; He, J.P.; Koretz, D.; McLaughlin, K.A.; Petukhova, M.; et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch. Gen. Psychiatry 2012, 69, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Lane, M.C.; Shahly, V.; Stang, P.E. Accounting for comorbidity in assessing the burden of epilepsy among US adults: Results from the National Comorbidity Survey Replication (NCS−R). Mol. Psychiatry 2011, 17, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, F.; Cuijpers, P.; Oostenbrink, J.; Batelaan, N.; de Graaf, R.; Beekman, A. Costs of nine common mental disorders: Implications for curative and preventive psychiatry. J. Ment. Health Policy Econ. 2006, 9, 193–200. [Google Scholar]
- Greenberg, P.E.; Sisitsky, T.; Kessler, R.C.; Finkelstein, S.N.; Berndt, E.R.; Davidson, J.R.; Ballenger, J.C.; Fyer, A.J. The economic burden of anxiety disorders in the 1990s. J. Clin. Psychiatry 1999, 60, 427–435. [Google Scholar] [CrossRef]
- Katon, W.; Lin, E.H.; Kroenke, K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen. Hosp. Psychiatry 2007, 29, 147–155. [Google Scholar] [CrossRef]
- Boulanger, L.; Zhao, Y.; Foster, T.S.; Fraser, K.; Bledsoe, S.L.; Russell, M.W. Impact of comorbid depression or anxiety on patterns of treatment and economic outcomes among patients with diabetic peripheral neuropathic pain. Curr. Med. Res. Opin. 2009, 25, 1763–1773. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Chiu, W.T.; Demler, O.; Merikangas, K.R.; Walters, E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Hengartner, M.P. The Detrimental Impact of Maladaptive Personality on Public Mental Health: A Challenge for Psychiatric Practice. Front. Psychiatry 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, J.H.; Colloca, L.; Korzeniewska, A.; Campbell, C.M.; Hillis, A.E.; Lenz, F.A. Oscillatory EEG Activity induced by Conditioning Stimuli during Fear Conditioning reflects Salience and Valence of these Stimuli more than Expectancy. Neuroscience 2017, 346, 81–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muris, P.; Vlaeyen, J.; Meesters, C. The relationship between anxiety sensitivity and fear of pain in healthy adolescents. Behav. Res. Ther. 2001, 39, 1357–1368. [Google Scholar] [CrossRef]
- Reiss, S.; Peterson, R.A.; Gursky, D.M.; McNally, R.J. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav. Res. Ther. 1986, 24, 1–8. [Google Scholar] [CrossRef]
- Spielberger, S. Manual for the State and Trait Anxiety Inventory (Form Y); Mind Garden: Palo Alto, CA, USA, 1983. [Google Scholar]
- Breeman, S.; Cotton, S.; Fielding, S.; Jones, G.T. Normative data for the Hospital Anxiety and Depression Scale. Qual. Life Res. 2015, 24, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Shear, M.K.; Vander, B.J.; Rucci, P.; Endicott, J.; Lydiard, B.; Otto, M.W.; Pollack, M.H.; Chandler, L.; Williams, J.; Ali, A.; et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress. Anxiety 2001, 13, 166–178. [Google Scholar] [CrossRef]
- McNeil, D.W.; Rainwater, A.J., III. Development of the Fear of Pain Questionnaire--III. J. Behav. Med. 1998, 21, 389–410. [Google Scholar] [CrossRef]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear- avoidance beliefs in chronic low back pain and disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef]
- Waddell, G.; McCulloch, J.A.; Kummel, E.; Venner, R.M. Nonorganic physical signs in low-back pain. Spine 1980, 5, 117–125. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- McCracken, L.M.; Zayfert, C.; Gross, R.T. The Pain Anxiety Symptoms Scale: Development and validation of a scale to measure fear of pain. Pain 1992, 50, 67–73. [Google Scholar] [CrossRef]
- McCracken, L.M.; Gross, R.T.; Aikens, J.; Carnrike, C.L., Jr. The assessment of anxiety and fear in persons with chronic pain: A comparison of instruments. Behav. Res. Ther. 1996, 34, 927–933. [Google Scholar] [CrossRef]
- Linnman, C.; Rougemont-Bucking, A.; Beucke, J.C.; Zeffiro, T.A.; Milad, M.R. Unconditioned responses and functional fear networks in human classical conditioning. Behav. Brain Res. 2011, 221, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blechert, J.; Michael, T.; Vriends, N.; Margraf, J.; Wilhelm, F.H. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res. Ther. 2007, 45, 2019–2033. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Milad, M.R.; Rauch, S.L.; Pitman, R.K.; Quirk, G.J. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006, 73, 61–71. [Google Scholar] [CrossRef]
- Palazzo, E.; Fu, Y.; Ji, G.; Maione, S.; Neugebauer, V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology 2008, 55, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Crone, N.E.; Franaszczuk, P.J.; Cheng, D.; Schretlen, D.S.; Lenz, F.A. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience 2011, 189, 359–369. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, J.E. Coming to terms with fear. Proc. Natl. Acad. Sci. USA 2014, 111, 2871–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baas, J.M.; van Ooijen, L.; Goudriaan, A.; Kenemans, J.L. Failure to condition to a cue is associated with sustained contextual fear. Acta Psychol. (Amst.) 2008, 127, 581–592. [Google Scholar] [CrossRef]
- Davey, H.M.; Barratt, A.L.; Butow, P.N.; Deeks, J.J. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. J. Clin. Epidemiol. 2007, 60, 356–360. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.; Vangronsveld, K.; Peters, M.L.; Goossens, M.E.; Onghena, P.; Bulte, I.; Vlaeyen, J.W. Reduction of pain-related fear and disability in post-traumatic neck pain: A replicated single-case experimental study of exposure in vivo. J. Pain 2008, 9, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Sandberg, K.; Timmermans, B.; Overgaard, M.; Cleeremans, A. Measuring consciousness: Is one measure better than the other? Conscious. Cogn. 2010, 19, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Dienes, Z.; Cleeremans, A.; Overgaard, M.; Pessoa, L. Measuring consciousness: Relating behavioural and neurophysiological approaches. Trends Cogn. Sci. 2008, 12, 314–321. [Google Scholar] [CrossRef]
- Clark, C.R.; Galletly, C.A.; Ash, D.J.; Moores, K.A.; Penrose, R.A.; McFarlane, A.C. Evidence-based medicine evaluation of electrophysiological studies of the anxiety disorders. Clin. EEG Neurosci. 2009, 40, 84–112. [Google Scholar] [CrossRef]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Review of EEG, ERP, and Brain Connectivity Estimators as Predictive Biomarkers of Social Anxiety Disorder. Front. Psychol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef]
- Falconer, E.M.; Felmingham, K.L.; Allen, A.; Clark, C.R.; McFarlane, A.C.; Williams, L.M.; Bryant, R.A. Developing an integrated brain, behavior and biological response profile in posttraumatic stress disorder (PTSD). J. Integr. Neurosci. 2008, 7, 439–456. [Google Scholar] [CrossRef]
- Putman, P.; van Peer, J.; Maimari, I.; van der Werff, S. EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol. Psychol. 2010, 83, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Basar, E.; Guntekin, B. Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. Suppl. Clin. Neurophysiol. 2013, 62, 303–341. [Google Scholar] [PubMed]
- Andersen, S.B.; Moore, R.A.; Venables, L.; Corr, P.J. Electrophysiological correlates of anxious rumination. Int. J. Psychophysiol. 2009, 71, 156–169. [Google Scholar] [CrossRef]
- Wessa, M.; Flor, H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. Am. J. Psychiatry 2007, 164, 1684–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picton, T. The P300 Wave of the Human Event-Related Potential. J. Clin. Neurophysiol. 1992, 9, 456–479. [Google Scholar] [CrossRef]
- Chen, C.; Hu, C.H.; Cheng, Y. Mismatch negativity (MMN) stands at the crossroads between explicit and implicit emotional processing. Hum. Brain Mapp. 2017, 38, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.M.; Panitz, C.; Hermann, C.; Pizzagalli, D.A. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J. Neurosci. 2014, 34, 7059–7066. [Google Scholar] [CrossRef]
- Caliskan, G.; Stork, O. Hippocampal network oscillations at the interplay between innate anxiety and learned fear. Psychopharmacology 2019, 236, 321–338. [Google Scholar] [CrossRef]
- Luo, Q.; Mitchell, D.; Cheng, X.; Mondillo, K.; McCaffrey, D.; Holroyd, T.; Carver, F.; Coppola, R.; Blair, J. Visual awareness, emotion, and gamma band synchronization. Cereb. Cortex 2009, 19, 1896–1904. [Google Scholar] [CrossRef] [Green Version]
- Klahn, A.L.; Klinkenberg, I.A.; Lueken, U.; Notzon, S.; Arolt, V.; Pantev, C.; Zwanzger, P.; Junghoefer, M. Commonalities and differences in the neural substrates of threat predictability in panic disorder and specific phobia. Neuroimage Clin. 2017, 14, 530–537. [Google Scholar] [CrossRef]
- Boubela, R.N.; Kalcher, K.; Huf, W.; Seidel, E.M.; Derntl, B.; Pezawas, L.; Nasel, C.; Moser, E. fMRI measurements of amygdala activation are confounded by stimulus correlated signal fluctuation in nearby veins draining distant brain regions. Sci. Rep. 2015, 5, 10499. [Google Scholar] [CrossRef] [PubMed]
- Lenz, F.A.; Casey, K.L.; Jones, E.G.; Willis, W.D.J. The Human Pain System: Experimental and Clinical Perspectives, 1st ed.; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Friston, K.J.; Bastos, A.M.; Pinotsis, D.; Litvak, V. LFP and oscillations-what do they tell us? Curr. Opin. Neurobiol. 2015, 31, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, A.; Andersson, M.; Josephson, C.; Johannesson, M.; Knutsson, H. Does parametric fMRI analysis with SPM yield valid results? An empirical study of 1484 rest datasets. Neuroimage 2012, 61, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Olman, C.A.; Davachi, L.; Inati, S. Distortion and signal loss in medial temporal lobe. PLoS ONE 2009, 4, e8160. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, T.D.; Suzuki, S.; Grabowecky, M.; Paller, K.A. Detecting and categorizing fleeting emotions in faces. Emotion 2013, 13, 76–91. [Google Scholar] [CrossRef]
- Fan, Y.T.; Chen, C.; Cheng, Y. The Neural Mechanisms of Social Learning from Fleeting Experience with Pain. Front. Behav. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Esslen, M.; Pascual-Marqui, R.D.; Hell, D.; Kochi, K.; Lehmann, D. Brain areas and time course of emotional processing. NeuroImage 2004, 21, 1189–1203. [Google Scholar] [CrossRef]
- Fredrikson, M.; Faria, V. Neuroimaging in anxiety disorders. Mod. Trends Pharmacopsychiatry 2013, 29, 47–66. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef] [Green Version]
- Amunts, K.; Zilles, K. Architectonic Mapping of the Human Brain beyond Brodmann. Neuron 2015, 88, 1086–1107. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.A.; Berger, G.R.; Derbyshire, S.W. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 2003, 18, 3134–3144. [Google Scholar] [CrossRef] [Green Version]
- Nitschke, J.B.; Sarinopoulos, I.; Oathes, D.J.; Johnstone, T.; Whalen, P.J.; Davidson, R.J.; Kalin, N.H. Anticipatory Activation in the Amygdala and Anterior Cingulate in Generalized Anxiety Disorder and Prediction of Treatment Response. Am. J. Psychiatry 2009, 34, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Whalen, P.J. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J. Neurosci. 2009, 29, 11614–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.P.; Hilgetag, C.C.; Scannell, J.W. On imputing function to structure from the behavioural effects of brain lesions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Altamura, C.; Dell’Osso, B.; Domschke, K.; Fineberg, N.A.; Grünblatt, E.; Jarema, M.; Maron, E.; et al. Biological markers for anxiety disorders, OCD and PTSD—A consensus statement. Part I: Neuroimaging and genetics. World J. Biol. Psychiatry 2016, 17, 321–365. [Google Scholar] [CrossRef]
- Wanigasekera, V.; Mezue, M.; Andersson, J.; Kong, Y.; Tracey, I. Disambiguating Pharmacodynamic Efficacy from Behavior with Neuroimaging: Implications for Analgesic Drug Development. Anesthesiology 2016, 124, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, A.; Matthews, S.C.; Paulus, M.P.; Stein, M.B. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci. Lett. 2008, 430, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Schiller, D.; Levy, I.; Niv, Y.; LeDoux, J.E.; Phelps, E.A. From fear to safety and back: Reversal of fear in the human brain. J. Neurosci. 2008, 28, 11517–11525. [Google Scholar] [CrossRef]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef]
- Sehlmeyer, C.; Schoning, S.; Zwitserlood, P.; Pfleiderer, B.; Kircher, T.; Arolt, V.; Konrad, C. Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE 2009, 4, e5865. [Google Scholar] [CrossRef]
- Palermo, S.; Benedetti, F.; Costa, T.; Amanzio, M. Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Hum. Brain Mapp. 2015, 36, 1648–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Benedetti, F. Placebo and nocebo effects: An introduction to psychological and biological mechanisms. Handb. Exp. Pharmacol. 2014, 225, 3–15. [Google Scholar] [PubMed]
- Cheng, D.T.; Richards, J.; Helmstetter, F.J. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn. Mem. 2007, 14, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.T.; Knight, D.C.; Smith, C.N.; Helmstetter, F.J. Human amygdala activity during the expression of fear responses. Behav. Neurosci. 2006, 120, 1187–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, D.C.; Smith, C.N.; Cheng, D.T.; Stein, E.A.; Helmstetter, F.J. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn. Affect. Behav. Neurosci. 2004, 4, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Carter, R.M.; O’Doherty, J.P.; Seymour, B.; Koch, C.; Dolan, R.J. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage 2006, 29, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Knight, D.C.; Cheng, D.T.; Smith, C.N.; Stein, E.A.; Helmstetter, F.J. Neural substrates mediating human delay and trace fear conditioning. J. Neurosci. 2004, 24, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Brancucci, A.; Del Percio, C.; Capotosto, P.; Arendt-Nielsen, L.; Chen, A.C.N.; Rossini, P.M. Anticipatory Electroencephalography Alpha Rhythm Predicts Subjective Perception of Pain Intensity. J. Pain J. Am. Pain Soc. 2006, 7, 709–717. [Google Scholar] [CrossRef]
- Seifert, F.; Schuberth, N.; De, C.R.; Peltz, E.; Nickel, F.T.; Maihofner, C. Brain activity during sympathetic response in anticipation and experience of pain. Hum. Brain Mapp. 2013, 34, 1768–1782. [Google Scholar] [CrossRef]
- Ohara, S.; Crone, N.E.; Weiss, N.; Lenz, F.A. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin. Neurophysiol. 2004, 115, 1641–1652. [Google Scholar] [CrossRef]
- Liu, C.C.; Ohara, S.; Franaszczuk, P.J.; Zagzoog, N.; Gallagher, M.; Lenz, F.A. Painful stimuli evoke potentials recorded from the medial temporal lobe in humans. Neuroscience 2010, 165, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Colloca, L.; Finniss, D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA 2012, 307, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Meyer, R.A.; Lesser, R.P. Theshold temperatures for first pain sensation, laser-evoked potentials and nociceptor act. Prog. Pain. Res. Manag. 1994, 2, 857–865. [Google Scholar]
- Kenton, B.; Coger, R.; Crue, B.; Pinsky, J.; Friedman, Y.; Carmon, A. Peripheral fiber correlates to noxious thermal stimulation in humans. Neurosci. Lett. 1980, 17, 301–306. [Google Scholar] [CrossRef]
- Markman, T.M.; Liu, C.C.; Chien, J.H.; Crone, N.E.; Zhang, J.; Lenz, F.A. ERC analysis of scalp EEG reveals widespread directed functional interactions related to a painful cutaneous laser stimulus. J. Neurophysiol. 2013, 110, 2440–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, J.H.; Liu, C.C.; Kim, J.H.; Markman, T.M.; Lenz, F.A. Painful cutaneous laser stimuli induce event-related oscillatory EEG activities that are different from those induced by nonpainful electrical stimuli. J. Neurophysiol. 2014, 112, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Kucyi, A.; Salomons, T.V.; Davis, K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl. Acad. Sci. USA 2013, 110, 18692–18697. [Google Scholar] [CrossRef] [Green Version]
- Lipp, O.V.; Purkis, H.M. The effects of assessment type on verbal ratings of conditional stimulus valence and contingency judgments: Implications for the extinction of evaluative learning. J Exp. Psychol. Anim. Behav. Process 2006, 32, 431–440. [Google Scholar] [CrossRef]
- Blechert, J.; Michael, T.; Williams, L.S.; Purkis, H.M.; Wilhelm, F.H. When two paradigms meet: Does evaluative learning extinguish in differential fear conditioning? Learn. Motiv. 2006, 32, 431–440. [Google Scholar] [CrossRef]
- Miro, E.; Lupianez, J.; Hita, E.; Martinez, M.P.; Sanchez, A.I.; Buela-Casal, G. Attentional deficits in fibromyalgia and its relationships with pain, emotional distress and sleep dysfunction complaints. Psychol. Health 2011, 26, 765–780. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Vlaeyen, J.W.; Crombez, G. Understanding and Treating the Fear of Pain; Oford University Press: Oxford, NY, USA, 2004. [Google Scholar]
- Gracely, R.H.; Geisser, M.E.; Giesecke, T.; Grant, M.A.; Petzke, F.; Williams, D.A.; Clauw, D.J. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004, 127, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.A.; McNaughton, N.J. The Neuropsychology of Anxiety; Oford University Press: Oxford, NY, USA, 2010. [Google Scholar]
- Higgins, S.T.; Morris, E.K. Generality of free-operant avoidance conditioning to human behavior. Psychol. Bull. 1984, 96, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Nader, K.; Schafe, G.E.; LeDoux, J.E. The labile nature of consolidation theory. Nat. Rev. Neurosci. 2000, 1, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Nearing, K.I.; LeDoux, J.E.; Phelps, E.A. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 2008, 59, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracely, R.H.; Kwilosz, D.M. The descriptor differential scale: Applying psychophysical principles to clinical pain assesment. Pain 1988, 35, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Gracely, R.H.; McGrath, P.; Dubner, R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: Manipulation of affect by Diazepam. Pain 1978, 5, 19–29. [Google Scholar] [CrossRef]
- Duncan, J.S. Imaging and epilepsy. Brain J. Neurol. 1997, 120 Pt 2, 339–377. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, A.; Xia, S.; Branch, C.; Li, X. A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front. Hum. Neurosci. 2013, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Jasper, H.H. The Ten-Twenty Electrode System of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Vilkki, J. Effects of pulvinotomy and ventrolateral thalamotomy on some cognitive functions. In Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy; Sweet, W.H., Obrador, S., Martinez-Rodriguez, J.G., Eds.; University Park Press: Baltimore, MD, USA, 1977; pp. 673–677. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Buchel, C.; Dolan, R.J.; Armony, J.L.; Friston, K.J. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J. Neurosci. 1999, 19, 10869–10876. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.A.; Dolan, R.J. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat. Neurosci 2004, 7, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, J.B.; Sarinopoulos, I.; Mackiewicz, K.L.; Schaefer, H.S.; Davidson, R.J. Functional neuroanatomy of aversion and its anticipation. NeuroImage 2006, 29, 106–116. [Google Scholar] [CrossRef]
- Klucken, T.; Schweckendiek, J.; Koppe, G.; Merz, C.J.; Kagerer, S.; Walter, B.; Sammer, G.; Vaitl, D.; Stark, R. Neural correlates of disgust- and fear-conditioned responses. Neuroscience 2012, 201, 209–218. [Google Scholar] [CrossRef]
- Ploghaus, A.; Tracey, I.; Gati, J.S.; Clare, S.; Menon, R.S.; Matthews, P.M.; Rawlins, J.N. Dissociating pain from its anticipation in the human brain. Science 1999, 284, 1979–1981. [Google Scholar] [CrossRef] [Green Version]
- Milad, M.R.; Orr, S.P.; Pitman, R.K.; Rauch, S.L. Context modulation of memory for fear extinction in humans. Psychophysiology 2005, 42, 456–464. [Google Scholar] [CrossRef]
- Marschner, A.; Kalisch, R.; Vervliet, B.; Vansteenwegen, D.; Buchel, C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J. Neurosci. 2008, 28, 9030–9036. [Google Scholar] [CrossRef] [Green Version]
- Chien, J.H.; Lenz, F.A.; Schmid, A.C.; Kim, J.H.; Cheng, D.T.; Anderson, W.; Liu, C. Human contextual fear conditioning using painful laser. Soc. Neurosci. Abstr. 2015, 12, 76–85. [Google Scholar]
- Trost, Z.; France, C.R.; Thomas, J.S. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain 2011, 152, 1540–1547. [Google Scholar] [CrossRef]
- Eifert, G.H.; Heffner, M. The effects of acceptance versus control contexts on avoidance of panic-related symptoms. J. Behav. Ther. Exp. Psychiatry 2003, 34, 293–312. [Google Scholar] [CrossRef]
- Wiech, K.; Tracey, I. Pain, decisions, and actions: A motivational perspective. Front. Neurosci. 2013, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Bienvenu, O.J.; Samuels, J.F.; Costa, P.T.; Reti, I.M.; Eaton, W.W.; Nestadt, G. Anxiety and depressive disorders and the five-factor model of personality: A higher- and lower-order personality trait investigation in a community sample. Depress. Anxiety 2004, 20, 92–97. [Google Scholar] [CrossRef]
- Crombez, G.; Vervaet, L.; Lysens, R.; Eelen, P.; Baeyens, F. Avoidance and confrontation of painful back straining movements in chronic back pain patients. Behav. Modif. 1998, 22, 67–72. [Google Scholar] [CrossRef]
- van Middendorp, H.; Lumley, M.A.; Jacobs, J.W.; van Doornen, L.J.; Bijlsma, J.W.; Geenen, R. Emotions and emotional approach and avoidance strategies in fibromyalgia. J. Psychosom. Res. 2008, 64, 159–167. [Google Scholar]
- Wiech, K.; Tracey, I. The influence of negative emotions on pain: Behavioral effects and neural mechanisms. Neuroimage 2009, 47, 987–994. [Google Scholar] [CrossRef]
- Alschuler, K.N.; Molton, I.R.; Jensen, M.P.; Riddle, D.L. Prognostic value of coping strategies in a community-based sample of persons with chronic symptomatic knee osteoarthritis. Pain 2013, 154, 2775–2781. [Google Scholar] [CrossRef] [Green Version]
- Heuts, P.H.; Vlaeyen, J.W.; Roelofs, J.; de Bie, R.A.; Aretz, K.; van Weel, C.; van Schayck, O.C. Pain-related fear and daily functioning in patients with osteoarthritis. Pain 2004, 110, 228–235. [Google Scholar] [CrossRef]
- Martin, M.Y.; Bradley, L.A.; Alexander, R.W.; Alarcon, G.S.; Triana-Alexander, M.; Aaron, L.A.; Alberts, K.R. Coping strategies predict disability in patients with primary fibromyalgia. Pain 1996, 68, 45–53. [Google Scholar] [CrossRef]
- de Gier, M.; Peters, M.L.; Vlaeyen, J.W. Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain 2003, 104, 121–130. [Google Scholar] [CrossRef]
- Pare, D.; Quirk, G.J.; LeDoux, J.E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004, 92, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef]
- Sotres-Bayon, F.; Cain, C.K.; LeDoux, J.E. Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry 2006, 60, 329–336. [Google Scholar] [CrossRef]
- Rauch, S.L.; Shin, L.M.; Phelps, E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research--past, present, and future. Biol. Psychiatry 2006, 60, 376–382. [Google Scholar] [CrossRef]
- Liu, C.C.; Shi, C.Q.; Franaszczuk, P.J.; Crone, N.E.; Schretlen, D.; Ohara, S.; Lenz, F.A. Painful laser stimuli induce directed functional interactions within and between the human amygdala and hippocampus. Neuroscience 2011, 178, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Ohara, S.; Franaszczuk, P.J.; Lenz, F.A. Attention to painful cutaneous laser stimuli evokes directed functional connectivity between activity recorded directly from human pain-related cortical structures. Pain 2011, 152, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Chien, J.H.; Kim, J.H.; Chang, Y.F.; Anderson, W.S.; Lenz, F.A. Functional role of induced gamma oscillatory responses upon processing noxious and innocuous sensory events in humans. Neuroscience 2015, 303, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.A.; Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 2012. [Google Scholar] [CrossRef]
- McDonald, A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998, 55, 257–332. [Google Scholar] [CrossRef]
- Barbas, H.; Saha, S.; Rempel-Clower, N.; Ghashghaei, T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Pape, H.C.; Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010, 90, 419–463. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Chien, J.H.; Kim, J.H.; Chuang, Y.F.; Cheng, D.T.; Lenz, F.A. Cross-frequency coupling in deep brain structures upon processing the painful sensory inputs. Neuroscience 2015, 303, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Lenz, F.A.; Rios, M.; Zirh, A.; Chau, D.; Krauss, G.; Lesser, R.P. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J. Neurophysiol. 1998, 79, 2231–2234. [Google Scholar] [CrossRef]
- Ohara, S.; Crone, N.E.; Weiss, N.; Treede, R.D.; Lenz, F.A. Cutaneous painful laser stimuli evoke responses recorded directly from primary somatosensory cortex in awake humans. J. Neurophysiol. 2004, 91, 2734–2746. [Google Scholar] [CrossRef]
- Brown, L.T.; Mikell, C.B.; Youngerman, B.E.; Zhang, Y.; McKhann, G.M., 2nd; Sheth, S.A. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: A systematic review of observational studies. J. Neurosurg. 2016, 124, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Barbanoj, M.J.; Perez, V.; Sacristan, M. Transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder. Cochrane Database Syst. Rev. 2003, CD003387. [Google Scholar] [CrossRef]
- Milad, M.R.; Quirk, G.J.; Pitman, R.K.; Orr, S.P.; Fischl, B.; Rauch, S.L. A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry 2007, 62, 1191–1194. [Google Scholar] [CrossRef]
- Anderson, A.K.; Phelps, E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 2001, 411, 305–309. [Google Scholar] [CrossRef]
- Frankenstein, U.N.; Richter, W.; McIntyre, M.C.; Remy, F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. NeuroImage 2001, 14, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Longe, S.E.; Wise, R.; Bantick, S.; Lloyd, D.; Johansen-Berg, H.; McGlone, F.; Tracey, I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: An fMRI study. Neuroreport 2001, 12, 2021–2025. [Google Scholar] [CrossRef] [Green Version]
- Sander, D.; Grafman, J.; Zalla, T. The human amygdala: An evolved system for relevance detection. Rev. Neurosci. 2003, 14, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 2002, 87, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchel, C.; Morris, J.; Dolan, R.J.; Friston, K.J. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron 1998, 20, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Clancy, K.; Ding, M.; Bernat, E.; Schmidt, N.B.; Li, W. Restless ‘rest’: Intrinsic sensory hyperactivity and disinhibition in post-traumatic stress disorder. Brain 2017, 140, 2041–2050. [Google Scholar] [CrossRef]
- Taylor, S. The structure of fundamental fears. J. Behav. Ther. Exp. Psychiatry 1993, 24, 289–299. [Google Scholar] [CrossRef]
- Mobbs, D.; Yu, R.; Rowe, J.B.; Eich, H.; FeldmanHall, O.; Dalgleish, T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. USA 2010, 107, 20582–20586. [Google Scholar] [CrossRef] [Green Version]
- Marek, R.; Sun, Y.; Sah, P. Neural circuits for a top-down control of fear and extinction. Psychopharmacology 2019, 236, 313–320. [Google Scholar] [CrossRef]
- Wiech, K.; Edwards, R.; Moseley, G.L.; Berna, C.; Ploner, M.; Tracey, I. Dissociable neural mechanisms underlying the modulation of pain and anxiety? An FMRI pilot study. PLoS ONE 2014, 9, e110654. [Google Scholar] [CrossRef] [Green Version]
- Neuper, C.; Wortz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [PubMed]
- Bardouille, T.; Picton, T.W.; Ross, B. Attention modulates beta oscillations during prolonged tactile stimulation. Eur. J. Neurosci. 2010, 31, 761–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachaux, J.P.; Axmacher, N.; Mormann, F.; Halgren, E.; Crone, N.E. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog. Neurobiol. 2012, 98, 279–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basar, E.; Golbasi, B.T. Event related desynchronization: Use as a neurophysiologic marker is restricted. Cogn. Neurodyn. 2014, 8, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Lezak, M.D. Neuropsychological Assessment, 3rd ed.; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Frewen, P.A.; Dozois, D.J.; Joanisse, M.F.; Neufeld, R.W. Selective attention to threat versus reward: Meta-analysis and neural-network modeling of the dot-probe task. Clin. Psychol. Rev. 2008, 28, 307–337. [Google Scholar] [CrossRef] [Green Version]
- Melzack, R.; Wall, P. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef] [Green Version]
- Kucyi, A.; Davis, K.D. The dynamic pain connectome. Trends Neurosci. 2015, 38, 86–95. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Peyron, R. Pain matrices and neuropathic pain matrices: A review. Pain 2013, 154 (Suppl. 1), S29–S43. [Google Scholar] [CrossRef] [Green Version]
- Jordan, M.I.; Jacobs, R.A. Modular and hierarchical learning systems. In The Handbook of Brain Theory and Neural Networks, 2nd ed.; Arbib, M.A., Ed.; The MIT Press: Cambridge, MA, USA, 2002; pp. 669–672. [Google Scholar]
- Bullinaria, J.A. Lesioned networks as models of neuropsychological deficits. In The Handbook of Brain Theory and Neural Networks, 2nd ed.; Arbib, M.A., Ed.; The MIT Press: Cambridge, MA, USA, 2002; pp. 635–638. [Google Scholar]
- Churchland, P.S.; Sejnowski, T.J. The Computational Brain; Sejnowski, T.J., Poggio, T.A., Eds.; MIT Press: Cambridge, UK, 1992. [Google Scholar]
- Hu, L.; Peng, W.; Valentini, E.; Zhang, Z.; Hu, Y. Functional features of nociceptive-induced suppression of alpha band electroencephalographic oscillations. J. Pain 2013, 14, 89–99. [Google Scholar] [CrossRef]
- Mouraux, A.; Guerit, J.M.; Plaghki, L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial partial differential- and C-fibre afferent volleys. Clin. Neurophysiol. 2003, 114, 710–722. [Google Scholar] [CrossRef]
- Hauck, M.; Lorenz, J.; Engel, A.K. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J. Neurosci. 2007, 27, 9270–9277. [Google Scholar] [CrossRef]
- Chien, J.H.; Korzeniewska, A.; Hillis, A.E.; Kim, J.H.; Emerson, N.; Greenspan, J.D.; Campbell, C.M.; Meeker, T.J.; Markman, T.M.; Lenz, F.A. ‘Vigilance Behaviors and EEG Activity in Sustained Attention may Affect Acute Pain. J. Syst. Integr. Neurosci. 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downar, J.; Mikulis, D.J.; Davis, K.D. Neural correlates of the prolonged salience of painful stimulation. NeuroImage 2003, 20, 1540–1551. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Hadjistavropoulos, H.D. Is high fear of pain associated with attentional biases for pain-related or general threat? A categorical reanalysis. J. Pain 2007, 8, 11–18. [Google Scholar] [CrossRef]
- Mouraux, A.; Iannetti, G.D. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J. Neurophysiol. 2009, 101, 3258–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yordanova, J.; Kolev, V. Event-related alpha oscillations are functionally associated with P300 during information processing. Neuroreport 1998, 9, 3159–3164. [Google Scholar] [CrossRef] [PubMed]
- Gurtubay, I.G.; Alegre, M.; Labarga, A.; Malanda, A.; Artieda, J. Gamma band responses to target and non-target auditory stimuli in humans. Neurosci. Lett. 2004, 367, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W.; Hillyard, S.A. Endogenous event-related potentials. In Human Event-Related Potentials; Picton, T.W., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 1, pp. 361–416. [Google Scholar]
- Harris, J.B. Differential conditioning of alpha amplitude: A fresh look at an old phenomenon. Clin. Neurophysiol. 2005, 116, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.S.; Shevrin, H.; Williams, W.J. Conscious and nonconscious processes: An ERP index of an anticipatory response in a conditioning paradigm using visually masked stimuli. Psychophysiology 1994, 31, 87–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald-Zuberbier, E.; Grunewald, G.; Rasche, A.; Netz, J. Contingent negative variation and alpha attenuation responses in children with different abilities to concentrate. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 37–47. [Google Scholar] [CrossRef]
- Filipovic, S.R.; Jahanshahi, M.; Rothwell, J.C. Uncoupling of contingent negative variation and alpha band event-related desynchronization in a go/no-go task. Clin. Neurophysiol. 2001, 112, 1307–1315. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Induced oscillations in the alpha band: Functional meaning. Epilepsia 2003, 44 (Suppl. 12), 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Hu, L.; Zhang, Z.; Hu, Y. Causality in the association between P300 and alpha event-related desynchronization. PLoS ONE 2012, 7, e34163. [Google Scholar] [CrossRef] [Green Version]
- Ohara, S.; Crone, N.E.; Weiss, N.; Vogel, H.; Treede, R.D.; Lenz, F.A. Attention to pain is processed at multiple cortical sites in man. Exp. Brain Res. 2004, 156, 513–517. [Google Scholar] [CrossRef]
- Qu, M.; Mittmann, T.; Luhmann, H.J.; Schleicher, A.; Zilles, K. Long-term changes of ionotropic glutamate and GABA receptors after unilateral permanent focal cerebral ischemia in the mouse brain. Neuroscience 1998, 85, 29–43. [Google Scholar] [CrossRef]
- Lueken, U.; Straube, B.; Konrad, C.; Wittchen, H.U.; Strohle, A.; Wittmann, A.; Pfleiderer, B.; Uhlmann, C.; Arolt, V.; Jansen, A.; et al. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am. J. Psychiatry 2013, 170, 1345–1355. [Google Scholar] [CrossRef]
- Michael, T.; Blechert, J.; Vriends, N.; Margraf, J.; Wilhelm, F.H. Fear conditioning in panic disorder: Enhanced resistance to extinction. J. Abnorm. Psychol. 2007, 116, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Hunt, M.G.; Momjian, A.J.; Wong, K.K. Effects of diurnal variation and caffeine consumption on Test of Variables of Attention (TOVA) performance in healthy young adults. Psychol. Assess. 2011, 23, 226–233. [Google Scholar] [CrossRef]
- Dillard, M.B.; Warm, J.S.; Funke, G.J.; Funke, M.E.; Finomore, V.S., Jr.; Matthews, G.; Shaw, T.H.; Parasuraman, R. The sustained attention to response task (SART) does not promote mindlessness during vigilance performance. Hum. Factors 2014, 56, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Sauter, C.; Danker-Hopfe, H.; Loretz, E.; Zeitlhofer, J.; Geisler, P.; Popp, R. The assessment of vigilance: Normative data on the Siesta sustained attention test. Sleep Med. 2013, 14, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lin, G.H.; Chen, M.H.; Hsueh, I.P.; Hsieh, C.L. Development of a computerized Digit Vigilance Test and validation in patients with stroke. J. Rehabil. Med. 2015, 47, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiech, K.; Ploner, M.; Tracey, I. Neurocognitive aspects of pain perception. Trends Cogn. Sci. 2008, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lenz, F.A.; Dougherty, P.M. Pain processing in the human thalamus. In Thalamus: Volume II, 1st ed.; Steriade, M., Jones, E.G., McCormick, D.A., Eds.; Elsevier: Oxford, UK, 1997; pp. 617–651. [Google Scholar]
- Schoffelen, J.M.; Gross, J. Source connectivity analysis with MEG and EEG. Hum. Brain Mapp. 2009, 30, 1857–1865. [Google Scholar] [CrossRef]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, R.L. Psychiatric diagnosis: Are clinicians still necessary? Compr. Psychiatry 1983, 24, 399–411. [Google Scholar] [CrossRef]
- Hall, R.C.; Hall, R.C. Detection of malingered PTSD: An overview of clinical, psychometric, and physiological assessment: Where do we stand? J. Forensic Sci. 2007, 52, 717–725. [Google Scholar] [CrossRef]
- Knoll, J.; Resnick, P.J. The detection of malingered post-traumatic stress disorder. Psychiatr. Clin. N. Am. 2006, 29, 629–647. [Google Scholar] [CrossRef]
- Heron-Delaney, M.; Kenardy, J.; Charlton, E.; Matsuoka, Y. A systematic review of predictors of posttraumatic stress disorder (PTSD) for adult road traffic crash survivors. Injury 2013, 44, 1413–1422. [Google Scholar] [CrossRef]
- Constans, J.I.; McCloskey, M.S.; Vasterling, J.J.; Brailey, K.; Mathews, A. Suppression of attentional bias in PTSD. J. Abnorm. Psychol. 2004, 113, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Waitzkin, H.; Cruz, M.; Shuey, B.; Smithers, D.; Muncy, L.; Noble, M. Military Personnel Who Seek Health and Mental Health Services Outside the Military. Mil. Med. 2018, 183, e232–e240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilek, D.; Carriere, J.S.; Cheyne, J.A. Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia 2010, 48, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Manly, T.; Robertson, I.H.; Galloway, M.; Hawkins, K. The absent mind: Further investigations of sustained attention to response. Neuropsychologia 1999, 37, 661–670. [Google Scholar] [CrossRef]

| ERSP Window I (WI), Delta/Theta | ERSP Window II (WII), Delta/Theta | Window III (WIII), Alpha | Window IV (WIV), Beta | Window V (WV) Gamma | |
|---|---|---|---|---|---|
| Attend Laser Protocol: Latency is Time after the Laser Pulse | |||||
| ERSP induced by Painful Laser US | ERS | ERS | ERD | ERS | ERS |
| Latency | 200–400 ms | 600–1400 ms | 500–900 ms | 1200–1600 ms | 800–1200 ms |
| Frequency | 0–8 Hz | 0–8 Hz | 8–10 Hz | 15–25 Hz | 40–50 Hz |
| Fear Conditioning protocol: Latency is Time after the CS | |||||
| Conditioning stimuli—CS ERS/ERD | ERS | ERS | ERD | ERD | ERD/ERS |
| Latency | 190–500 ms | 600–1400 ms | 200–1400 ms | 200–1600 ms | 0–1200 ms |
| Frequency Range | 0–8 Hz | 0–8 Hz | 8–14 Hz | 16–25 Hz | 30–120 Hz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, J.-H.; Colloca, L.; Korzeniewska, A.; Meeker, T.J.; Bienvenu, O.J.; Saffer, M.I.; Lenz, F.A. Behavioral, Physiological and EEG Activities Associated with Conditioned Fear as Sensors for Fear and Anxiety. Sensors 2020, 20, 6751. https://doi.org/10.3390/s20236751
Chien J-H, Colloca L, Korzeniewska A, Meeker TJ, Bienvenu OJ, Saffer MI, Lenz FA. Behavioral, Physiological and EEG Activities Associated with Conditioned Fear as Sensors for Fear and Anxiety. Sensors. 2020; 20(23):6751. https://doi.org/10.3390/s20236751
Chicago/Turabian StyleChien, Jui-Hong, Luana Colloca, Anna Korzeniewska, Timothy J. Meeker, O. Joe Bienvenu, Mark I. Saffer, and Fred A. Lenz. 2020. "Behavioral, Physiological and EEG Activities Associated with Conditioned Fear as Sensors for Fear and Anxiety" Sensors 20, no. 23: 6751. https://doi.org/10.3390/s20236751
APA StyleChien, J.-H., Colloca, L., Korzeniewska, A., Meeker, T. J., Bienvenu, O. J., Saffer, M. I., & Lenz, F. A. (2020). Behavioral, Physiological and EEG Activities Associated with Conditioned Fear as Sensors for Fear and Anxiety. Sensors, 20(23), 6751. https://doi.org/10.3390/s20236751





