Domiciliary Hospitalization through Wearable Biomonitoring Patches: Recent Advances, Technical Challenges, and the Relation to Covid-19
Abstract
:1. Introduction
1.1. Background
1.2. The Relationship to the Global Epidemic
1.3. Domiciliary Hospitalization through Wearable Biomonitoring Patches: Article Overview
2. Wearable Bioelectronics for an IoMT System
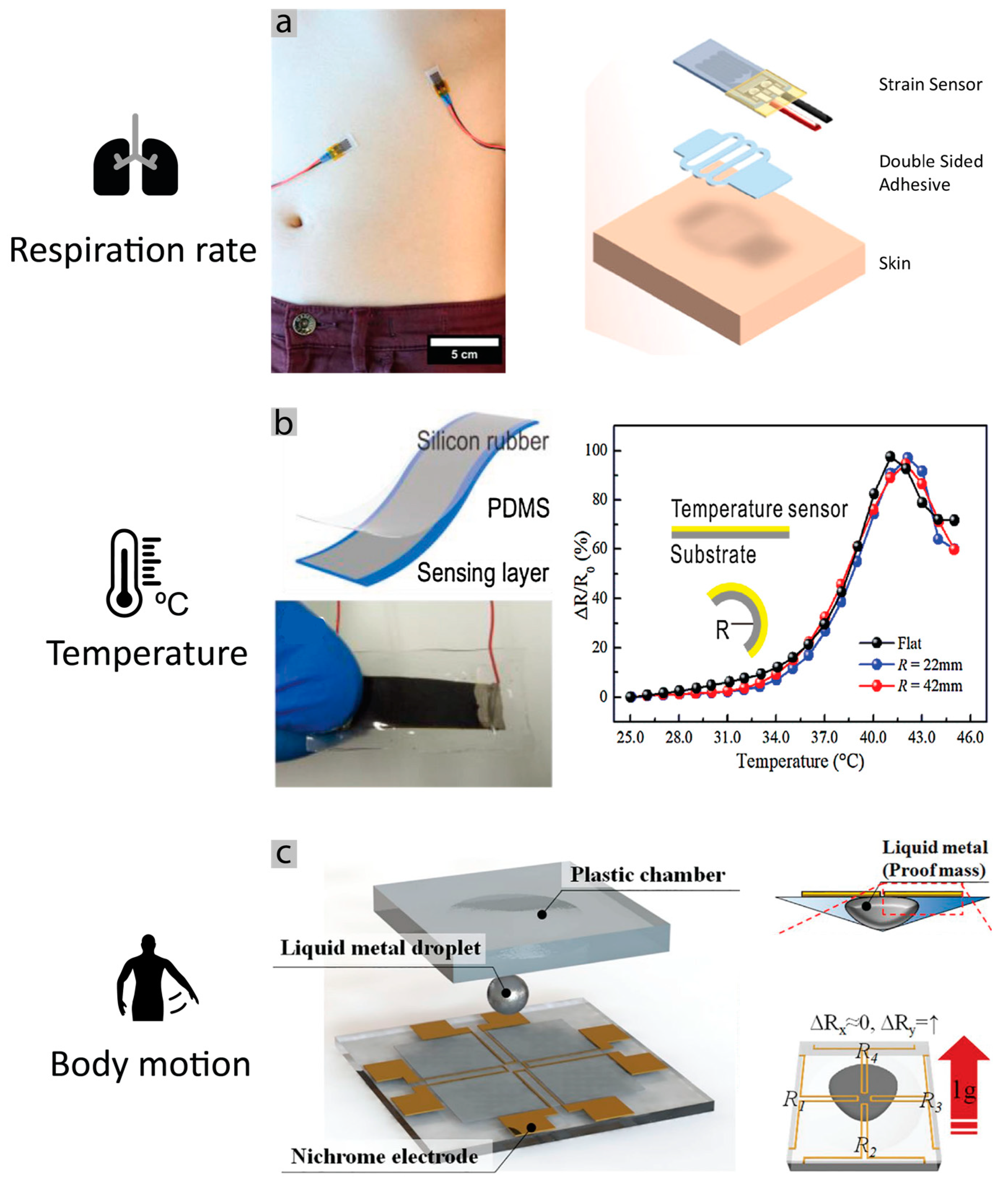
2.1. Skin Interfacing Electrophysiological Sensing: What Can Be Measured?
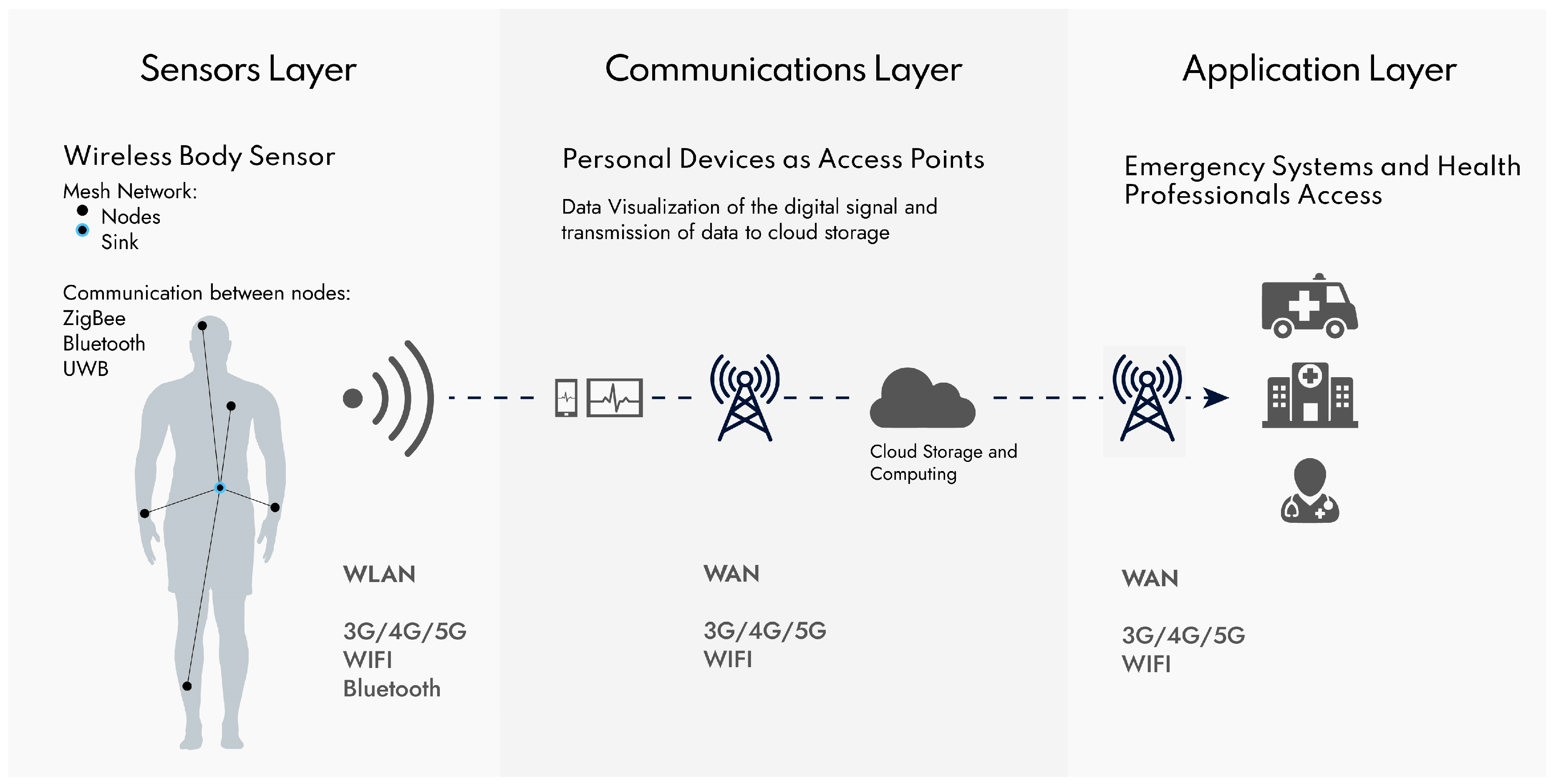
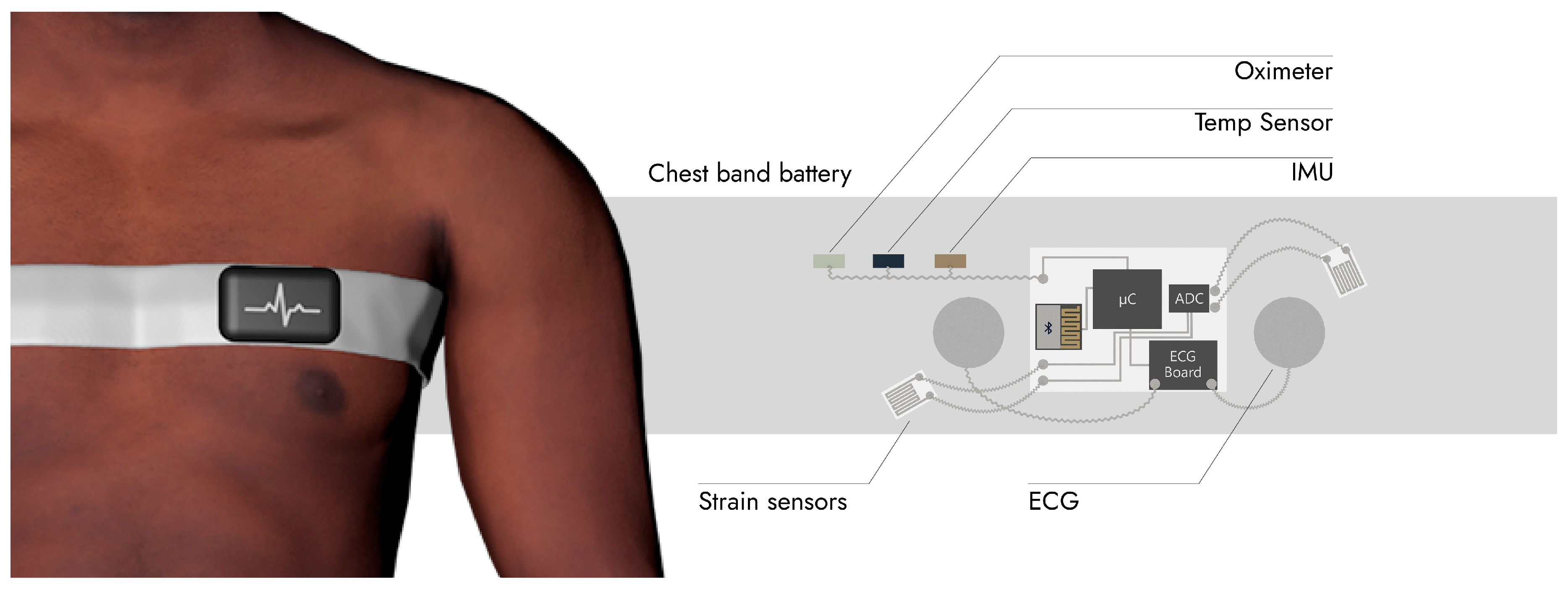
2.2. IoMT System for Patient Monitoring and Domiciliary Hospitalization
Technologies for Wireless Communications
| Recent Version | Range (m) | Data Rate | Frequency | Band | Standard | Energy Consumption | |
|---|---|---|---|---|---|---|---|
| Bluetooth [63] | 5.2 (2020) | <10–500+ | 1–3 Mbit/s | 2.402–2.480 GHz | ISM | IEEE 802.15.1 | <30 mA |
| Bluetooth Low energy | - | 500+ | 125 kbit/s-2 Mbit/s | 2.400–2.4835 GHz | ISM | - | <15 mA |
| ZigBee [73] | 2015 | 10–300+ | 250 Kbit/s | 2.40 GHz | ISM | IEEE 802.15.4 | <16 mA |
| UWB [74] | - | short | 675 Mbit/s | 3.1–10.6 GHz (500 MHz channels) | - | IEEE 802.15.6-2012 | |
| ANT [75] | ANT+ | 30 | 60 Kbit/s | 2.4 GHz | ISM | - | <60µA |
| RuBee [76] | - | 20 | 1200 kB/s | 131 kHz | IEEE 1902.1 | ||
| Sensium [77] (HR monitor) | >3 | 160 kb/s | 900 MHz | <3 mA | |||
| Zarlink [78] (implants) | ZL70101 ZL70081 ZL70250 | <2 | <800 kb/s | 402–405 MHz; | MICS/ ISM | <6 mA | |
| Z-Wave [79,80] (Homecare) | Z-Wave Plus V2 | 10–100 | 100 Kbit/s | 2.4 GHz and 900 MHz | ISM | IEEE 802.15.4 | <38.8 mA (13 dB) <12.9 mA (0 dB) |
| NFC [81] | - | 0.1 | 424 Kbit/s | 13.56 MHz | ISM | ISO/IEC 18000-3 | - |
| RFID [82] | - | <12 (100) | - | 120–150 kHz; 13.56–928 MHz; 2.45–5.8 GHz | ISM | ISO/IEC 18000 | - |
| Mobile technology [83] | 5G | - | 100–900 Mbit/s | 600–700 MHz | - | - | - |
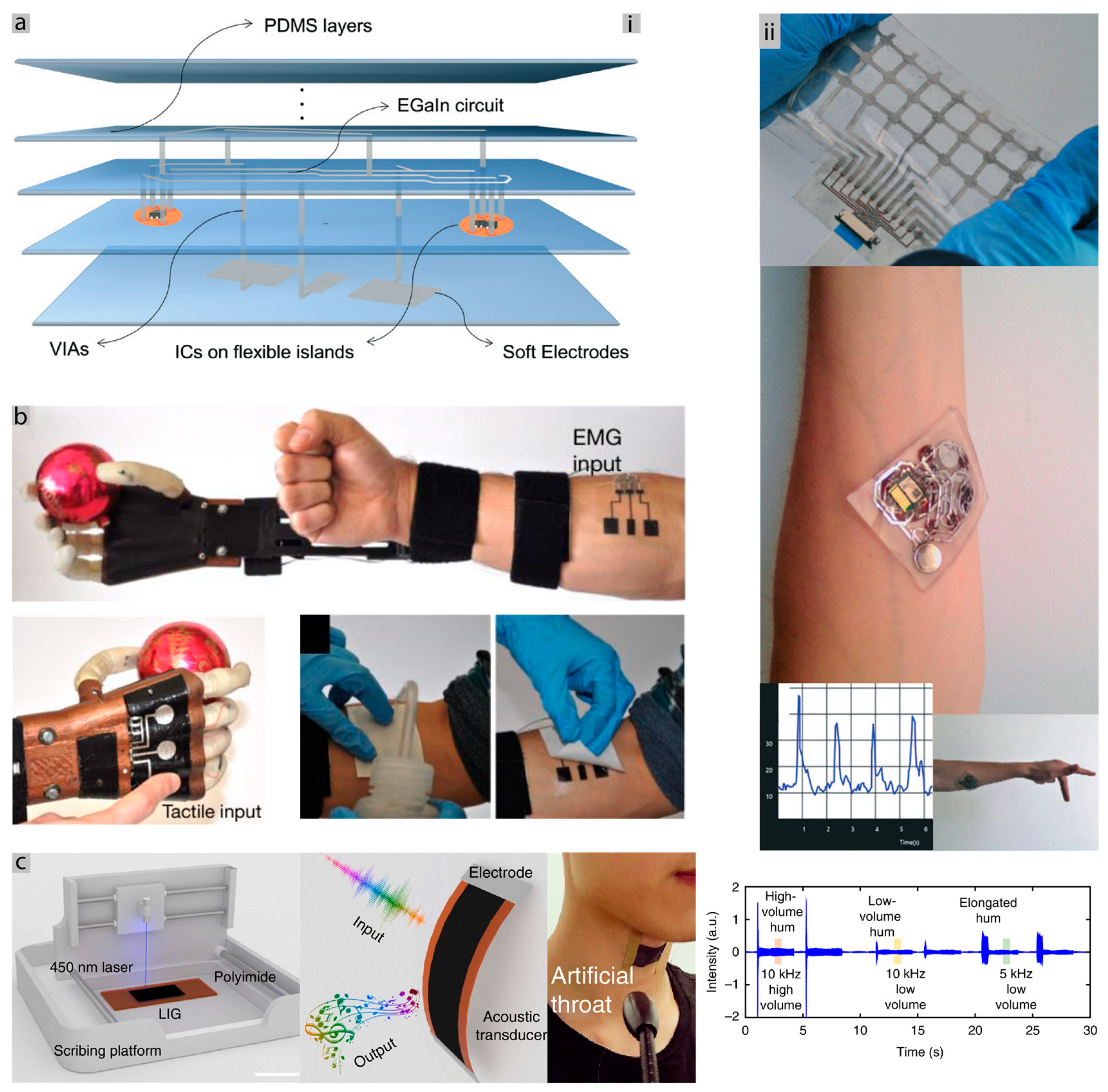
3. Novel Forms of Conformal Bioelectronics
3.1. Materials and Fabrication, Challenges and Methods for Bioelectronics Circuits
3.1.1. Fabrication Challenge
3.1.2. Long Term Use Challenge

3.1.3. Interfacing Challenge
3.2. Energy Supply for Wearable Biomonitoring
Energy Consumption
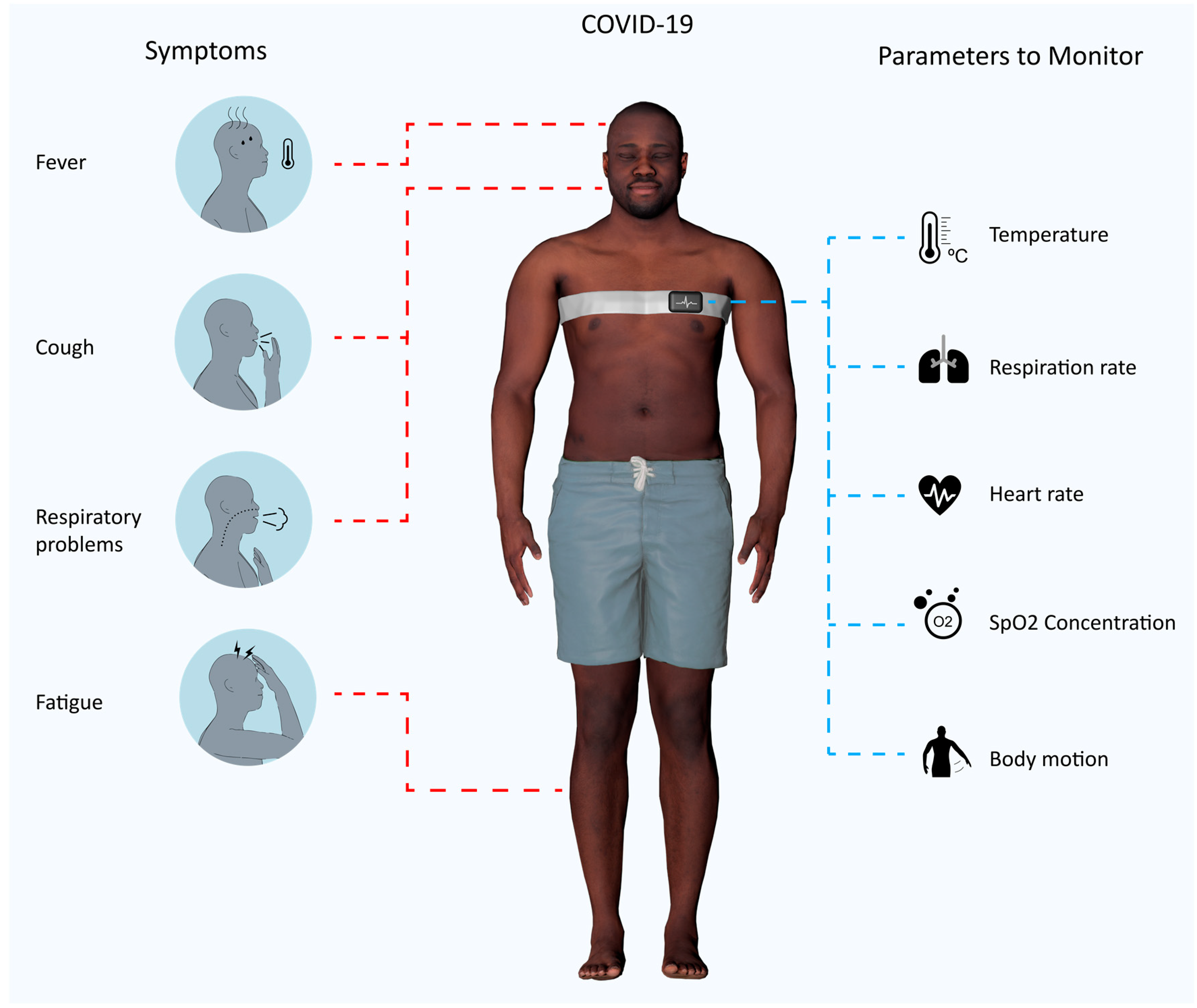
4. Sensing Architecture for Covid-19 Patients
4.1. How to Monitor a Covid-19 Patient
4.2. An IoMT System Dedicated to Covid-19 Monitoring
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Uptake of Digital Solutions in the Healthcare Industry. No. January. 2017. Available online: https://ec.europa.eu/growth/tools-databases/dem/monitor/sites/default/files/DTM_Uptake of digital solutions v1.pdf (accessed on 24 November 2020).
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Ahmadi, H.; Arji, G.; Shahmoradi, L.; Safdari, R.; Nilashi, M.; Alizadeh, M. The application of internet of things in healthcare: A systematic literature review and classification. Univers. Access Inf. Soc. 2019, 18, 837–869. [Google Scholar] [CrossRef]
- Campbell, P. Population Projections: States, 1995–2025; Census Bureau: Suitland-Silver Hill, MD, USA, 1997; pp. 1–6. [Google Scholar]
- Gouda, K.; Okamoto, R. Current status of and factors associated with social isolation in the elderly living in a rapidly aging housing estate community. Environ. Health Prev. Med. 2012, 17, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Megari, K. Quality of Life in Chronic Disease Patients. Health Psychol. Res. 2013, 1, 27. [Google Scholar] [CrossRef]
- Keehan, S.P.; Cuckler, G.A.; Sisko, A.M.; Madison, A.J.; Smith, S.D.; Stone, D.A.; Poisal, J.A.; Wolfe, C.J.; Lizonitz, J.M. National Health Expenditure Projections, 2014–2024: Spending Growth Faster Than Recent Trends. Health Aff. 2015, 34, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Jost, T.S. Our Broken Health Care System and How to Fix It: An Essay on Health Law and Policy. Wake For. L. Rev. 2006, 41, 537. Available online: https://heinonline.org/HOL/Page?handle=hein.journals/wflr41&id=547&div=&collection= (accessed on 31 May 2020).
- Internet of Things (IoT) in Healthcare Market is Expected to Grow at a CAGR of 29.9% to Reach $322.2 billion by 2025: Meticulous Research®. Available online: https://www.globenewswire.com/news-release/2020/03/19/2003195/0/en/Internet-of-Things-IoT-in-Healthcare-Market-is-Expected-to-Grow-at-a-CAGR-of-29-9-to-Reach-322-2-billion-by-2025-Meticulous-Research.html (accessed on 31 May 2020).
- Onag, G. IoT Developers to Focus more on Smart Healthcare Post-COVID-19. FutureIOT. 2020. Available online: https://futureiot.tech/iot-developers-to-focus-more-smart-healthcare-post-covid-19/ (accessed on 20 April 2020).
- That ‘Internet of Things’ Thing—RFID Journal. Available online: https://www.rfidjournal.com/that-internet-of-things-thing (accessed on 31 May 2020).
- Adam Thierer, A.C. Economic Perspectives Projecting the Growth and Economic Impact of the Internet of Things; George Mason University, Mercatus Center: Arlington, VA, USA, 2015. [Google Scholar]
- Irfan, M.; Ahmad, N. Internet of medical things: Architectural model, motivational factors and impediments. In Proceedings of the 2018 15th Learning and Technology Conference (L&T), Jeddah, Saudi Arabia, 25–26 February 2018; pp. 6–13. [Google Scholar]
- Gatouillat, A.; Badr, Y.; Massot, B.; Sejdić, E. Internet of Medical Things: A Review of Recent Contributions Dealing With Cyber-Physical Systems in Medicine. IEEE Internet Things J. 2018, 5, 3810–3822. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Higgins, H.; Braem, B.; Latre, B.; Blondia, C.; Moerman, I.; Saleem, S.; Rahman, Z.; Kwak, K.-S. A Comprehensive Survey of Wireless Body Area Networks. J. Med Syst. 2012, 36, 1065–1094. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Gentile, M.; Shen, C.-F.; Cheng, C.-M. Combining Point-of-Care Diagnostics and Internet of Medical Things (IoMT) to Combat the COVID-19 Pandemic. Diagnostics 2020, 10, 224. [Google Scholar] [CrossRef]
- World Health Organization. Home Care for Patients with Suspected or Confirmed COVID-19 and Management of Their Contacts; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Wilson, D.; Halperin, D.T. “Know your epidemic, know your response”: A useful approach, if we get it right. Lancet 2008, 372, 423–426. [Google Scholar] [CrossRef]
- Tizzoni, M.; Bajardi, P.; Poletto, C.; Ramasco, J.J.; Balcan, D.; Gonçalves, B.; Perra, N.; Colizza, V.; Vespignani, A. Real-time numerical forecast of global epidemic spreading: Case study of 2009 A/H1N1pdm. BMC Med. 2012, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jothi, N.; Rashid, N.A.; Husain, W. Data Mining in Healthcare—A Review. Procedia Comput. Sci. 2015, 72, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Al-Turjman, F.; Nawaz, M.H.; Ulusar, U.D. Intelligence in the Internet of Medical Things era: A systematic review of current and future trends. Comput. Commun. 2020, 150, 644–660. [Google Scholar] [CrossRef]
- El Majid, B.; Motahhir, S.; El Hammoumi, A.; Lebbadi, A.; El Ghzizal, A. Preliminary Design of a Smart Wristband Disinfectant to Help in Covid-19 Fight. Inventions 2020, 5, 32. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.-C.; Shen, C.-F.; Cheng, C.-M. Point-of-Care RNA-Based Diagnostic Device for COVID-19. Diagnotics 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Petrovan, V.; Vrajmasu, V.; Bucur, A.C.; Soare, D.S.; Radu, E.; Dimon, P.; Zaulet, M. Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples. Diagnostics 2020, 10, 472. [Google Scholar] [CrossRef]
- Beetz, C.; Skrahina, V.; Förster, T.M.; Gaber, H.; Paul, J.J.; Curado, F.; Rolfs, A.; Bauer, P.; Schäfer, S.; Weckesser, V.; et al. Rapid Large-Scale COVID-19 Testing During Shortages. Diagnostics 2020, 10, 464. [Google Scholar] [CrossRef]
- Allam, M.; Cai, S.; Ganesh, S.; Venkatesan, M.; Doodhwala, S.; Song, Z.; Hu, T.; Kumar, A.; Heit, J.; COVID-nineteen Study Group; et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics 2020, 10, 409. [Google Scholar] [CrossRef]
- Cho, H.; Ippolito, D.; Yu, Y.W. Contact Tracing Mobile Apps for COVID-19: Privacy Considerations and Related Trade-Offs. arXiv 2020, arXiv:2003.11511. [Google Scholar]
- Kim, J.; Campbell, A.S.; De Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.S.; Qazi, R.; Kwon, Y.; Jeong, J.; Yeo, W.-H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, e1901924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Lopes, P.A.; Gomes, D.V.; Marques, D.G.; Faia, P.; Góis, J.; Patrício, T.F.; Coelho, J.F.J.; Serra, A.C.; De Almeida, A.T.; Majidi, C.; et al. Soft Bioelectronic Stickers: Selection and Evaluation of Skin-Interfacing Electrodes. Adv. Healthc. Mater. 2019, 8, e1900234. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med Eng. 2014, 2014, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Havlík, J.; Fousek, O.; Ložek, M. Patient Monitoring Using Bioimpedance Signal. Comput. Vis. 2012, 7451, 171–172. [Google Scholar] [CrossRef]
- Villarejo, M.V.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. A Stress Sensor Based on Galvanic Skin Response (GSR) Controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [Green Version]
- Hadjileontiadis, L.J.; Rekanos, I.T.; Panas, S.M. Bioacoustic Signals. In Wiley Encyclopedia of Biomedical Engineering; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Cotur, Y.; Kasimatis, M.; Kaisti, M.; Olenik, S.; Georgiou, C.; Güder, F. Stretchable Composite Acoustic Transducer for Wearable Monitoring of Vital Signs. Adv. Funct. Mater. 2020, 30, 1910288. [Google Scholar] [CrossRef]
- Shahina, A.; Yegnanarayana, B. Mapping Speech Spectra from Throat Microphone to Close-Speaking Microphone: A Neural Network Approach. EURASIP J. Adv. Signal Process. 2007, 2007, 087219. [Google Scholar] [CrossRef] [Green Version]
- Makeyev, O.; Lopez-Meyer, P.; Schuckers, S.; Besio, W.; Sazonov, E. Automatic food intake detection based on swallowing sounds. Biomed. Signal Process. Control. 2012, 7, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Carson, D.; Boris, L.; Mabary, J.; Lin, Z.; Nicodème, F.; Cuttica, M.; Kahrilas, P.J.; Pandolfino, J.E.; Carlson, D. The acoustic cough monitoring and manometric profile of cough and throat clearing. Dis. Esophagus 2013, 27, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.; Rogers, J.; Xu, S. Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities. Sci. Adv. 2020, 6, eabd4794. [Google Scholar] [CrossRef]
- Laput, G.; Xiao, R.; Harrison, C. ViBand. In Proceedings of the 29th Annual Symposium on User Interface Software and Technology; Association for Computing Machinery (ACM): New York, NY, USA, 2016; pp. 321–333. [Google Scholar]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Kim, J.; Gutruf, P.; Chiarelli, A.M.; Heo, S.Y.; Cho, K.; Xie, Z.; Banks, A.; Han, S.; Jang, K.-I.; Lee, J.W.; et al. Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef] [Green Version]
- Nayak, G.; Puttamadappa, C.; Davide, O. Classification of Bio Optical signals using K- Means Clustering for Detection of Skin Pathology. Int. J. Comput. Appl. 2010, 1, 112–116. [Google Scholar] [CrossRef]
- O’hara, G.J.; Phillips, D.B.; Diego, S. Method and Apparatus for Measuring Internal Body Temperature Utilizing Infrared Emissions. U.S. Patent 4,602,642, 23 October 1984. [Google Scholar]
- Togawa, T. Body temperature measurement. Clin. Phys. Physiol. Meas. 1985, 6, 83–108. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Bao, S.-D. Fall detection by built-in tri-accelerometer of smartphone. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 184–187. [Google Scholar]
- Xiao, Z.G.; Menon, C. A Review of Force Myography Research and Development. Sensors 2019, 19, 4557. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2020, 172, 112750. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Biosignals in human-computer interaction. Interactions 2015, 23, 76–79. [Google Scholar] [CrossRef]
- Jakobsson, M. The Big Picture: Bioengineering Signals and Systems; Academic Press: Cambridge, MA, USA, 2012; pp. 3–33. ISBN 9780123849823. [Google Scholar]
- Moosavi, S.R.; Gia, T.N.; Nigussie, E.; Rahmani, A.M.; Virtanen, S.; Tenhunen, H.; Isoaho, J. End-to-end security scheme for mobility enabled healthcare Internet of Things. Future Gener. Comput. Syst. 2016, 64, 108–124. [Google Scholar] [CrossRef]
- Rahmani, A.; Gia, T.N.; Negash, B.; Anzanpour, A.; Azimi, I.; Jiang, M.; Liljeberg, P. Exploiting smart e-Health gateways at the edge of healthcare Internet-of-Things: A fog computing approach. Future Gener. Comput. Syst. 2018, 78, 641–658. [Google Scholar] [CrossRef]
- Khan, R.A.; Pathan, A.-S.K. The state-of-the-art wireless body area sensor networks: A survey. Int. J. Distrib. Sens. Netw. 2018, 14. [Google Scholar] [CrossRef] [Green Version]
- Hyun, W.; You, I.; Jang, J.; Leu, F.-Y. A Wireless Body Sensor Network and Its Applications: Rehearsal with a Smartphone. In Proceedings of the 2016 10th International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing (IMIS), Fukuoka, Japan, 6–8 July 2016; pp. 415–418. [Google Scholar]
- Ellouze, F.; Fersi, G.; Jmaiel, M. Blockchain for Internet of Medical Things: A Technical Review. In International Conference on Smart Homes and Health Telematics; Springer: Cham, Switzerland, 2020; pp. 259–267. [Google Scholar]
- Lee, J.-S.; Su, Y.-W.; Shen, C.-C. A Comparative Study of Wireless Protocols: Bluetooth, UWB, ZigBee, and Wi-Fi. In Proceedings of the IECON 2007—33rd Annual Conference of the IEEE Industrial Electronics Society, Taipei, Taiwan, 5–8 November 2007; pp. 46–51. [Google Scholar]
- Rutronik. Determination of Practical Extremes of Bluetooth Low Energy: Throughput, Energy Consumption and Maximum Range. Available online: https://www.rutronik.com/fileadmin/Rutronik/Downloads/printmedia/products/06_wireless/bluetooth5.pdf (accessed on 24 November 2020).
- Woolley, M.; Schmidt, S. Bluetooth 5 Go Faster. Go Further; Bluetooth SIG: Kirkland, WA, USA, 2016; p. 25. [Google Scholar]
- Dementyev, A.; Hodges, S.; Taylor, S.; Smith, J. Power consumption analysis of Bluetooth Low Energy, ZigBee and ANT sensor nodes in a cyclic sleep scenario. In Proceedings of the 2013 IEEE International Wireless Symposium (IWS), Beijing, China, 14–18 April 2013; pp. 1–4. [Google Scholar]
- Kelsch, N. Green Power. Water Wastes Dig. 2013, 53, 1–7. [Google Scholar]
- Asan, N.B.; Velander, J.; Redzwan, S.; Perez, M.; Hassan, E.; Blokhuis, T.J.; Voigt, T.; Augustine, R. Effect of Thickness Inhomogeneity in Fat Tissue on In-Body Microwave Propagation. In Proceedings of the 2018 IEEE International Microwave Biomedical Conference (IMBioC), Philadelphia, PA, USA, 14–15 June 2018; pp. 136–138. [Google Scholar]
- Asan, N.B.; Hassan, E.; Perez, M.D.; Shah, S.R.M.; Velander, J.; Blokhuis, T.J.; Voigt, T.; Augustine, R. Assessment of Blood Vessel Effect on Fat-Intrabody Communication Using Numerical and Ex-Vivo Models at 2.45 GHz. IEEE Access 2019, 7, 89886–89900. [Google Scholar] [CrossRef]
- Ma, C.; Huang, Z.; Wang, Z.; Zhou, L.; Li, Y. An Energy Efficient Technique Using Electric Active Shielding for Capacitive Coupling Intra-Body Communication. Sensors 2017, 17, 2056. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Oh, K.-I.; Park, H.; Kang, S. Review of capacitive coupling human body communications based on digital transmission. ICT Express 2016, 2, 180–187. [Google Scholar] [CrossRef]
- Thielens, A.; Baumbauer, C.; Anderson, M.G.; Ting, J.; Arias, A.C.; Rabaey, J.M. Feasability of On-Body Backscattering in the UHF-RFID Band using Screen-Printed Dipole Antennas. In Proceedings of the 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), Oslo, Norway, 8–10 May 2019; pp. 1–5. [Google Scholar]
- Magsi, H.; Sodhro, A.H.; Chachar, F.A.; Abro, S.A.K.; Sodhro, G.H.; Pirbhulal, S. Evolution of 5G in Internet of medical things. In Proceedings of the 2018 International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 3–4 March 2018; pp. 1–7. [Google Scholar]
- Nasri, F.; Mtibaa, A. Smart Mobile Healthcare System based on WBSN and 5G. Int. J. Adv. Comput. Sci. Appl. 2017, 8. [Google Scholar] [CrossRef]
- Alliance, Z. Zigbee and Wireless Radio Frequency Coexistence. Available online: https://www.trane.com/content/dam/Trane/Commercial/global/controls/building-mgmt/Air-Fi/ZigBee Wireless Whitepaper.pdf (accessed on 24 November 2020).
- Hernandez, M.; Kohno, R. UWB systems for body area networks in IEEE 802.15.6. In Proceedings of the 2011 IEEE International Conference on Ultra-Wideband (ICUWB), Bologna, Italy, 14–16 September 2011; pp. 235–239. [Google Scholar]
- Dynastream Innovations Inc. ANT Message Protocol and Usage. Available online: https://www.thisisant.com/developer/resources/downloads/#documents_tab (accessed on 24 November 2020).
- Yu, X.; Xia, X.; Chen, X. Design and Application of RuBee-Based Telemedicine Data Acquisition System. In Proceedings of the 2011 10th IEEE/ACIS International Conference on Computer and Information Science, Washington, DC, USA, 16–18 May 2011; pp. 365–370. [Google Scholar]
- Wong, A.C.W.; McDonagh, D.; Omeni, O.; Nunn, C.; Hernandez-Silveira, M.; Burdett, A.J. Sensium: An ultra-low-power wireless body sensor network platform: Design & application challenges. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; Volume 2009, pp. 6576–6579. [Google Scholar]
- Sagan, D. RF Integrated Circuits for Medical Applications: Meeting the Challenge of Ultra Low Power Communication, San Diego, CA, USA. 2007, p. 16. Available online: http://stf.ucsd.edu/presentations/2007/2007-08%20STF%20-%20abstract%20RF%20IC%20for%20Medical%20Apps%20-%20Meeting%20the%20Challenge%20of%20ULP%20Communications.pdf (accessed on 23 November 2020).
- Johansen, N. Software Design Specification. Z-Wave Transport-Encapsulation Command Class Specification. 6 July 2020. Available online: https://www.silabs.com/documents/login/miscellaneous/SDS13783-Z-Wave-Transport-Encapsulation-Command-Class-Specification.pdf (accessed on 23 November 2020).
- Grgurić, A.; Mošmondor, M.; Huljenić, D. The SmartHabits: An Intelligent Privacy-Aware Home Care Assistance System. Sensors 2019, 19, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Journal of Science Technology and Engineering. Near Field Communication (NFC) Technology in Smart E-Transactions. Available online: https://www.academia.edu/14208144/Near_Field_Communication_NFC_Technology_in_Smart_E_Transactions (accessed on 28 September 2020).
- Hosaka, R. An Analysis for Specifications of Medical Use RFID System as a Wireless Communication. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 2795–2798. [Google Scholar]
- Mitra, R.N.; Agrawal, D.P. 5G mobile technology: A survey. ICT Express 2015, 1, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Malakooti, M.H.; Paisana, H.; Ohm, Y.; Marques, D.G.; Lopes, P.; Piedade, A.P.; De Almeida, A.T.; Majidi, C. EGaIn-Assisted Room-Temperature Sintering of Silver Nanoparticles for Stretchable, Inkjet-Printed, Thin-Film Electronics. Adv. Mater. 2018, 30, e1801852. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.F.A.; Paisana, H.; De Almeida, A.T.; Majidi, C.; Tavakoli, M. Hydroprinted Electronics: Ultrathin Stretchable Ag–In–Ga E-Skin for Bioelectronics and Human–Machine Interaction. ACS Appl. Mater. Interfaces 2018, 10, 38760–38768. [Google Scholar] [CrossRef]
- Saada, G.; Layani, M.; Chernevousky, A.; Magdassi, S. Hydroprinting Conductive Patterns onto 3D Structures. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Alberto, J.M.M.A.V.; Leal, C.; Fernandes, C.; Lopes, P.A.; Paisana, H.; De Almeida, A.T.; Tavakoli, M. Fully Untethered Battery-free Biomonitoring Electronic Tattoo with Wireless Energy Harvesting. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Leal, C.; Lopes, P.A.; Serra, A.; Coelho, J.F.J.; De Almeida, A.T.; Tavakoli, M. Untethered Disposable Health Monitoring Electronic Patches with an Integrated Ag2O–Zn Battery, a AgInGa Current Collector, and Hydrogel Electrodes. ACS Appl. Mater. Interfaces 2019, 12, 3407–3414. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Ismailov, U.; Badier, J.-M.; Greco, F.; Ismailova, E. Conducting polymer tattoo electrodes in clinical electro- and magneto-encephalography. npj Flex. Electron. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. npj Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolò, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, L.; Tao, X.; Ding, X. Review of Flexible Temperature Sensing Networks for Wearable Physiological Monitoring. Adv. Healthc. Mater. 2017, 6, 1601371. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, X.; Wang, W.; Guo, X.; Hao, C.; Pan, W.; Liu, P.; Liu, C.; Ma, Y.; Zhang, Y.; et al. High-resolution flexible temperature sensor based graphite-filled polyethylene oxide and polyvinylidene fluoride composites for body temperature monitoring. Sens. Actuators A Phys. 2018, 278, 1–10. [Google Scholar] [CrossRef]
- Abu-Khalaf, J.; Saraireh, R.; Eisa, S.; Al-Halhouli, A. Experimental Characterization of Inkjet-Printed Stretchable Circuits for Wearable Sensor Applications. Sensors 2018, 18, 3476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef] [Green Version]
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Norton, J.J.S.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.-I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.Y. Quantum Tunneling Cantilever Accelerometer. May 1987. Available online: https://patents.google.com/patent/US4638669 (accessed on 26 May 2020).
- Huh, M.; Won, D.-J.; Gil Kim, J.; Kim, J. Simple and robust resistive dual-axis accelerometer using a liquid metal droplet. Micro Nano Syst. Lett. 2017, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Markvicka, E.J.; Jin, Y.; Majidi, C. Soft-Matter Printed Circuit Board with UV Laser Micropatterning. ACS Appl. Mater. Interfaces 2017, 9, 22055–22062. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Ayers, H.R.; Majidi, C.; Dickey, M.D. Methods to pattern liquid metals. J. Mater. Chem. C 2015, 3, 3834–3841. [Google Scholar] [CrossRef]
- Boley, J.W.; White, E.L.; Chiu, G.T.-C.; Kramer, R. Direct Writing of Gallium-Indium Alloy for Stretchable Electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Tavakoli, M.; Rocha, R.; Osorio, L.; Almeida, M.; De Almeida, A.; Ramachandran, V.; Tabatabai, A.; Lu, T.; Majidi, C. Carbon doped PDMS: Conductance stability over time and implications for additive manufacturing of stretchable electronics. J. Micromech. Microeng. 2017, 27, 035010. [Google Scholar] [CrossRef]
- Silva, A.F.; Paisana, H.; Fernandes, T.; Góis, J.; Serra, A.C.; Coelho, J.F.J.; De Almeida, A.T.; Majidi, C.; Tavakoli, M. High Resolution Soft and Stretchable Circuits with PVA/Liquid-Metal Mediated Printing. Adv. Mater. Technol. 2020, 2000343. [Google Scholar] [CrossRef]
- Fernandes, D.F.; Majidi, C.; Tavakoli, M. Digitally printed stretchable electronics: A review. J. Mater. Chem. C 2019, 7, 14035–14068. [Google Scholar] [CrossRef]
- Huang, T.-T.; Wu, W. Scalable nanomanufacturing of inkjet-printed wearable energy storage devices. J. Mater. Chem. A 2019, 7, 23280–23300. [Google Scholar] [CrossRef]
- Marques, D.G.; Lopes, P.A.; De Almeida, A.T.; Majidi, C.; Tavakoli, M. Reliable interfaces for EGaIn multi-layer stretchable circuits and microelectronics. Lab. Chip 2019, 19, 897–906. [Google Scholar] [CrossRef]
- Guo, R.; Yao, S.; Sun, X.; Liu, J. Semi-liquid metal and adhesion-selection enabled rolling and transfer (SMART) printing: A general method towards fast fabrication of flexible electronics. Sci. China Mater. 2019, 62, 982–994. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Tang, J.; Dong, S.; Lin, J.; Wang, H.; Liu, J.; Rao, W. One-Step Liquid Metal Transfer Printing: Toward Fabrication of Flexible Electronics on Wide Range of Substrates. Adv. Mater. Technol. 2018, 3. [Google Scholar] [CrossRef]
- Fallegger, F.; Schiavone, G.; Lacour, S. Conformable Hybrid Systems for Implantable Bioelectronic Interfaces. Adv. Mater. 2020, 32, e1903904. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, M.R.; De Almeida, A.T.; Tavakoli, M. Wearable and Comfortable e-Textile Headband for Long-Term Acquisition of Forehead EEG Signals. IEEE Sensors J. 2020, 20, 15107–15116. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wu, K.; Suo, Z. Photodetachable Adhesion. Adv. Mater. 2018, 31, e1806948. [Google Scholar] [CrossRef] [PubMed]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, B.; Majidi, C. EGaIn-Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Vogt, D.M.; Park, Y.-L.; Wood, R.J. Design and Characterization of a Soft Multi-Axis Force Sensor Using Embedded Microfluidic Channels. IEEE Sensors J. 2013, 13, 4056–4064. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhao, Y.; Ding, Y.; Dong, X.; Chen, L.; Cao, J.; Wang, C.; Xia, Y.; Peng, H.; Wang, Y. Multi-functional Flexible Aqueous Sodium-Ion Batteries with High Safety. Chem 2017, 3, 348–362. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Guo, Z.; Ren, J.; Wang, Y.; Peng, H. Flexible, Stretchable, and Rechargeable Fiber-Shaped Zinc-Air Battery Based on Cross-Stacked Carbon Nanotube Sheets. Angew. Chem. Int. Ed. 2015, 54, 15390–15394. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-Stretchable Zinc–Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Pan, J.; Peng, H. Stretchable lithium-air batteries for wearable electronics. J. Mater. Chem. A 2016, 4, 13419–13424. [Google Scholar] [CrossRef]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Chen, X.; Zhou, L. A metamaterial electromagnetic energy rectifying surface with high harvesting efficiency. AIP Adv. 2016, 6, 125020. [Google Scholar] [CrossRef]
- Glaser, P.E. Power from the Sun: Its Future. Science 1968, 162, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Xu, R.; Lee, S.; Jang, K.-I.; Yang, Y.; Banks, A.; Yu, K.J.; Kim, J.; Xu, S.; Ma, S.; et al. Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. USA 2016, 113, 6131–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Cong, S.; Tian, Z.; Song, Y.; Yu, L.; Lu, C.; Shao, Y.; Li, J.; Zou, G.; Rümmeli, M.H.; et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy 2019, 60, 247–256. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Zhang, H.; Shen, Y.; Zakeeruddin, S.M.; Graetzel, M.; Cheng, Y.-B.; Wang, M. A Power Pack Based on Organometallic Perovskite Solar Cell and Supercapacitor. ACS Nano 2015, 9, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Zadan, M.; Malakooti, M.H.; Majidi, C. Soft and Stretchable Thermoelectric Generators Enabled by Liquid Metal Elastomer Composites. ACS Appl. Mater. Interfaces 2020, 12, 17921–17928. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.; Parekh, D.P.; Ladd, C.; Vashaee, D.; Dickey, M.D.; Öztürk, M.C. Flexible thermoelectric generator using bulk legs and liquid metal interconnects for wearable electronics. Appl. Energy 2017, 202, 736–745. [Google Scholar] [CrossRef]
- Kim, C.S.; Lee, G.S.; Choi, H.; Kim, Y.J.; Yang, H.M.; Lim, S.H.; Lee, S.-G.; Cho, B.J. Structural design of a flexible thermoelectric power generator for wearable applications. Appl. Energy 2018, 214, 131–138. [Google Scholar] [CrossRef]
- Siddiqui, S.; Lee, H.B.; Kim, D.-I.; Duy, L.T.; Hanif, A.; Lee, N. An Omnidirectionally Stretchable Piezoelectric Nanogenerator Based on Hybrid Nanofibers and Carbon Electrodes for Multimodal Straining and Human Kinematics Energy Harvesting. Adv. Energy Mater. 2018, 8, 1701520. [Google Scholar] [CrossRef]
- Chun, J.; Kang, N.-R.; Kim, J.-Y.; Noh, M.-S.; Kang, C.-Y.; Choi, D.; Kim, S.-W.; Wang, Z.L.; Baik, J.M. Highly anisotropic power generation in piezoelectric hemispheres composed stretchable composite film for self-powered motion sensor. Nano Energy 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Joe, P.; Tuzman, O.L.; Park, K.-I.; Lee, K.J.; Shi, Y.; Huang, Y.Y.; Rogers, J.A. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extreme Mech. Lett. 2016, 9, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchet, R.; Yoon, H.-J.; Ryu, H.; Kim, M.-K.; Choi, E.-K.; Kim, D.-S.; Kim, S. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 2019, 365, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, X.; Imani, S.; Bandodkar, A.J.; Ramírez, J.; Mercier, P.P.; Wang, J. Wearable textile biofuel cells for powering electronics. J. Mater. Chem. A 2014, 2, 18184–18189. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Mohan, A.M.V.; Kurniawan, J.F.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, J.; Yin, L.; You, J.-M.; Meng, Y.S.; Wang, J. All-Printed, Stretchable Zn-Ag2 O Rechargeable Battery via Hyperelastic Binder for Self-Powering Wearable Electronics. Adv. Energy Mater. 2017, 7, 1602096. [Google Scholar] [CrossRef] [Green Version]
- Zamarayeva, A.M.; Ostfeld, A.E.; Wang, M.; Duey, J.K.; Deckman, I.; Lechêne, B.P.; Davies, G.; Steingart, D.A.; Arias, A.C. Flexible and stretchable power sources for wearable electronics. Sci. Adv. 2017, 3, e1602051. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [Green Version]
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Liu, K.; et al. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Song, M.-S.; Bao, Z.; Cui, Y. Stretchable Lithium-Ion Batteries Enabled by Device-Scaled Wavy Structure and Elastic-Sticky Separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sekhar, S.C.; Bharat, L.K.; Yu, J.S. Wearable Fabrics with Self-Branched Bimetallic Layered Double Hydroxide Coaxial Nanostructures for Hybrid Supercapacitors. ACS Nano 2017, 11, 10860–10874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, J.; Pan, Z.; Zhang, J.; Zhao, J.; Wang, X.; Zhang, C.; Yao, Y.; Lu, W.; Li, Q.; et al. Stretchable fiber-shaped asymmetric supercapacitors with ultrahigh energy density. Nano Energy 2017, 39, 219–228. [Google Scholar] [CrossRef]
- Liu, L.; Tian, Q.; Yao, W.; Li, M.; Li, Y.; Wu, W. All-printed ultraflexible and stretchable asymmetric in-plane solid-state supercapacitors (ASCs) for wearable electronics. J. Power Sour. 2018, 397, 59–67. [Google Scholar] [CrossRef]
- Ogawa, Y.; Takai, Y.; Kato, Y.; Kai, H.; Miyake, T.; Nishizawa, M. Stretchable biofuel cell with enzyme-modified conductive textiles. Biosens. Bioelectron. 2015, 74, 947–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movesense Sensor Technical Brief Dimensions. Available online: www.movesense.com (accessed on 23 September 2020).
- Park, J.; Bhat, G.; Krishnakumar, A.; Geyik, C.S.; Ogras, U.Y.; Lee, H.G. Energy per Operation Optimization for Energy-Harvesting Wearable IoT Devices. Sensors 2020, 20, 764. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It; World Health Organization, 2020; p. 1. Available online: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 14 May 2020).
- Alexander, L.K.; Keene, B.W.; Yount, B.L.; Geratz, J.D.; Small, J.; Baric, R.S. ECG changes after rabbit coronavirus infection. J. Electrocardiol. 1999, 32, 21–32. [Google Scholar] [CrossRef]
- Pew Research Center. Americans Divided on whether it’s Acceptable for the Government to Track People Who Have Tested Positive for COVID-19 through Their Cellphone | Pew Research Center. Available online: https://www.pewresearch.org/fact-tank/2020/05/04/how-americans-see-digital-privacy-issues-amid-the-covid-19-outbreak/ft-2020-05-04_privacy_02/ (accessed on 23 September 2020).
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Harris, P.R.; Stein, P.K.; Fung, G.L.; Drew, B.J. Heart rate variability measured early in patients with evolving acute coronary syndrome and 1-year outcomes of rehospitalization and mortality. Vasc. Heal. Risk Manag. 2014, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.K.; To, K.-F.; Wu, A.; Tse, G.M.; Chan, K.-F.; Lui, S.-F.; Sung, J.J.; Tam, J.S.; Tomlinson, B. Human Metapneumovirus-associated Atypical Pneumonia and SARS. Emerg. Infect. Dis. 2004, 10, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.-T.; Yu, W.-C.; Mok, N.-S.; Tsui, P.-T.; Tong, W.-L.; Stella, W.C.; Cheng, S.W.C. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int. J. Cardiol. 2005, 100, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakor, N.V.; Webster, J.G. Ground-Free ECG Recording with Two Electrodes. IEEE Trans. Biomed. Eng. 1980, 27, 699–704. [Google Scholar] [CrossRef]
- Iskandar, A.A.; Kolla, R.; Schilling, K.; Voelker, W. A wearable 1-lead necklace ECG for continuous heart rate monitoring. In Proceedings of the 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom), Munich, Germany, 14–17 September 2016; pp. 1–4. [Google Scholar]
- Posada-Quintero, H.F.; Reyes, B.A.; Burnham, K.; Pennace, J.; Chon, K.H. Low Impedance Carbon Adhesive Electrodes with Long Shelf Life. Ann. Biomed. Eng. 2015, 43, 2374–2382. [Google Scholar] [CrossRef]
- Chlaihawi, A.A.; Narakathu, B.B.; Emamian, S.; Bazuin, B.J.; Atashbar, M.Z. Development of printed and flexible dry ECG electrodes. Sens. Bio Sens. Res. 2018, 20, 9–15. [Google Scholar] [CrossRef]
- Li, M.; Xiong, W.; Li, Y. Wearable Measurement of ECG Signals Based on Smart Clothing. Int. J. Telemed. Appl. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ankhili, A.; Zaman, S.U.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D. How to Connect Conductive Flexible Textile Tracks to Skin Electrocardiography Electrodes and Protect Them Against Washing. IEEE Sensors J. 2019, 19, 11995–12002. [Google Scholar] [CrossRef]
- Lee, E.; Kim, I.; Liu, H.; Cho, G. Exploration of AgNW/PU nanoweb as ECG textile electrodes and comparison with Ag/AgCl electrodes. Fibers Polym. 2017, 18, 1749–1753. [Google Scholar] [CrossRef]
- Yoo, J.; Yan, L.; Lee, S.; Kim, H.; Yoo, H.-J. A Wearable ECG Acquisition System with Compact Planar-Fashionable Circuit Board-Based Shirt. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 897–902. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, B.; Yoo, H.-J. A Wearable Fabric Computer by Planar-Fashionable Circuit Board Technique. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 282–285. [Google Scholar]
- Jin, H.; Matsuhisa, N.; Lee, S.; Abbas, M.; Yokota, T.; Someya, T. Enhancing the Performance of Stretchable Conductors for E-Textiles by Controlled Ink Permeation. Adv. Mater. 2017, 29, 1605848. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.L.; Saleh, S.M.; Yudin, M.B.M.; Harun, F.K.C.; Sriprachuabwong, C.; Tuantranont, A.; Wicaksono, D.H. Graphene Ink-Coated Cotton Fabric-Based Flexible Electrode for Electrocardiography, Communications, Information Technology, and Biomedical Engineering (ICICI-BME). In Proceedings of the 2017 5th International Conference on Instrumentation, Bandung, Indonesia, 6–7 November 2017; pp. 73–75. [Google Scholar]
- Arquilla, K.; Webb, A.K.; Anderson, A.P. Textile Electrocardiogram (ECG) Electrodes for Wearable Health Monitoring. Sensors 2020, 20, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, S.; Balan, A. Wearable sensors for ECG measurement: A review. Sens. Rev. 2018, 38, 412–419. [Google Scholar] [CrossRef]
- Gao, Y.; Soman, V.V.; Lombardi, J.P.; Rajbhandari, P.P.; Dhakal, T.P.; Wilson, D.G.; Poliks, M.D.; Ghose, K.; Turner, J.N.; Jin, Z. Heart Monitor Using Flexible Capacitive ECG Electrodes. IEEE Trans. Instrum. Meas. 2019, 69, 4314–4323. [Google Scholar] [CrossRef]
- Zulqarnain, M.; Stanzione, S.; Van Der Steen, J.-L.P.J.; Gelinck, G.H.; Myny, K.; Cantatore, E. A Low Power Time Domain ECG Interface Based on Flexible a-IGZO TFTs. In Proceedings of the 2019 IEEE 8th International Workshop on Advances in Sensors and Interfaces (IWASI), Otranto, Italy, 13–14 June 2019; pp. 205–209. [Google Scholar]
- Yamamoto, Y.; Yamamoto, D.; Takada, M.; Naito, H.; Arie, T.; Akita, S.; Takei, K. Efficient Skin Temperature Sensor and Stable Gel-Less Sticky ECG Sensor for a Wearable Flexible Healthcare Patch. Adv. Health Mater. 2017, 6, 1700495. [Google Scholar] [CrossRef]
- Poliks, M.; Turner, J.; Ghose, K.; Jin, Z.; Garg, M.; Gui, Q.; Arias, A.; Kahn, Y.; Schadt, M.; Egitto, F. A Wearable Flexible Hybrid Electronics ECG Monitor. In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 July 2016; pp. 1623–1631. [Google Scholar]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft Microfluidic Assemblies of Sensors, Circuits, and Radios for the Skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef]
- Hafen, B.B.; Sharma, S. Oxygen Saturation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Tavakoli, M.; Turicchia, L.; Sarpeshkar, R. An Ultra-Low-Power Pulse Oximeter Implemented With an Energy-Efficient Transimpedance Amplifier. IEEE Trans. Biomed. Circuits Syst. 2009, 4, 27–38. [Google Scholar] [CrossRef]
- Kramer, M.; Lobbestael, A.; Barten, E.; Eian, J.; Rausch, G. Wearable Pulse Oximetry Measurements on the Torso, Arms, and Legs: A Proof of Concept. Mil. Med. 2017, 182, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Claverie, T. A Reflectance Sensor Holder for PPG Measurements from the Chest; Worcester Polytechnic Institute: Worcester, MA, USA, 2017. [Google Scholar]
- Schreiner, C.; Catherwood, P.; Anderson, J.; Mclaughlin, J. Blood Oxygen Level Measurement with a Chest-Based Pulse Oximetry Prototype System. In Proceedings of the IEEE Computing in Cardiology 2010, Belfast, UK, 26–29 September 2012; Volume 37, pp. 537–540. [Google Scholar]
- Shikida, M.; Yokota, T.; Naito, J.; Sato, K. Fabrication of a stent-type thermal flow sensor for measuring nasal respiration. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Wei, C.-L.; Lin, C.-F.; Tseng, I.-T. A Novel MEMS Respiratory Flow Sensor. IEEE Sensors J. 2009, 10, 16–18. [Google Scholar] [CrossRef]
- Zhou, S.Z. 2013 (54) Device for Determining Respiratory Rate and other Vital Signs. U.S. Patent 8,506,480 B2, 22 October 2019. [Google Scholar]
- Yabuki, S.; Toyama, H.; Takei, Y.; Wagatsuma, T.; Yabuki, H.; Yamauchi, M. Influences of environmental noise level and respiration rate on the accuracy of acoustic respiration rate monitoring. J. Clin. Monit. 2017, 32, 127–132. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, D. Electroactive fabrics and wearable biomonitoring devices. Autex Res. J. 2003, 3, 180–185. [Google Scholar]
- Krehel, M.; Schmid, M.; Rossi, R.M.; Boesel, L.F.; Bona, G.-L.; Scherer, L.J. An Optical Fibre-Based Sensor for Respiratory Monitoring. Sensors 2014, 14, 13088–13101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.W.; Jang, Y.W.; Lee, I.; Shin, S.; Kim, S. Wearable Respiratory Rate Monitoring using Piezo-Resistive Fabric Sensor; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 282–284. [Google Scholar]
- Zhang, H.; Zhang, J.; Hu, Z.; Quan, L.; Shi, L.; Chen, J.; Xuan, W.; Zhang, Z.; Dong, S.; Luo, J. Waist-wearable wireless respiration sensor based on triboelectric effect. Nano Energy 2019, 59, 75–83. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Pegan, J.D.; Zhang, J.; Chu, M.; Nguyen, T.; Park, S.-J.; Paul, A.; Kim, J.; Bachman, M.; Khine, M. Skin-mountable stretch sensor for wearable health monitoring. Nanoscale 2016, 8, 17295–17303. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Malyarchuk, V.; Jia, L.; Jang, K.-I.; Webb, R.C.; Fu, H.; Shi, Y.; Zhou, G.; Shi, L.; et al. Epidermal photonic devices for quantitative imaging of temperature and thermal transport characteristics of the skin. Nat. Commun. 2014, 5, 4938. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Jeong, B.; Kim, J.; Nam, V.B.; Yoon, Y.; Jung, J.; Hong, S.; Lee, H.; Eom, H.; Yeo, J.; et al. Sensitive Wearable Temperature Sensor with Seamless Monolithic Integration. Adv. Mater. 2019, 32, e1905527. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Lee, P.S. Stretchable Graphene Thermistor with Tunable Thermal Index. ACS Nano 2015, 9, 2130–2137. [Google Scholar] [CrossRef]
- Lebedev, V.; Laukhina, E.; Laukhin, V.; Somov, A.; Baranov, A.; Rovira, C.; Veciana, J. Investigation of sensing capabilities of organic bi-layer thermistor in wearable e-textile and wireless sensing devices. Org. Electron. 2017, 42, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Loh, K.J. Wearable carbon nanotube-based fabric sensors for monitoring human physiological performance. Smart Mater. Struct. 2017. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.; Phan, H.-P.; Nguyen, T.-K.; Qamar, A.; Foisal, A.R.M.; Viet, T.N.; Tran, C.-D.; Zhu, Y.; Nguyen, N.-T.; Dao, D.V. Environment-friendly carbon nanotube based flexible electronics for noninvasive and wearable healthcare. J. Mater. Chem. C 2016, 4, 10061–10068. [Google Scholar] [CrossRef]
- Koehler, D.R. Double resonator cantilever accelerometer. J. Acoust. Soc. Am. 1985, 78, 1453. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Davies, E.V.; Harlow, E.R.; Hsu, J.J.; Knighton, S.C.; Walker, T.A.; Voos, J.E.; Drummond, C.K. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front. Digit. Health 2020, 2, 8. [Google Scholar] [CrossRef]
- Using WHOOP Wearable Technology to Predict COVID-19 Risk | WHOOP. Available online: https://www.whoop.com/thelocker/predict-covid-19-risk/ (accessed on 23 September 2020).
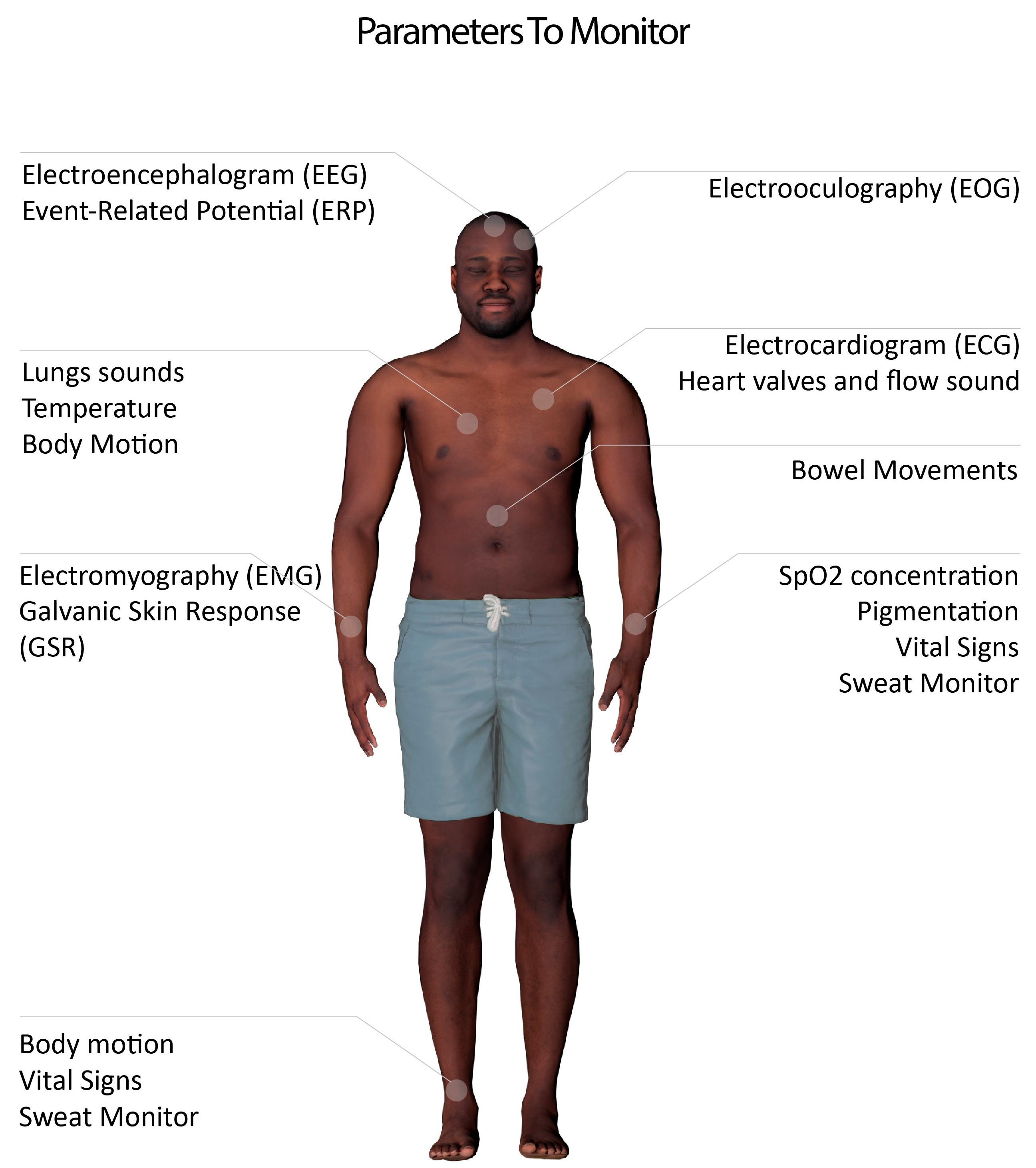






| Biopotential | Bioimpedance | Conductance | Acoustics | Optical | Others |
|---|---|---|---|---|---|
| Muscles (EMG) | Emotions | GSR | Voice | SpO2 Concentration | Temperature |
| Heart (ECG) | Body fat | Food intake | Pigmentation changes | Mechanical (myography) | |
| Brain (EEG) | Digestive System | Chemical (e.g., sweat) | |||
| Eyes (EOG) | Coughs | ||||
| Heart murmurs |
| E-Skin Patches, Current Challenges and Research Focus | ||||
|---|---|---|---|---|
| Fabrication | Long Term Use/Reliability | Interfacing | Energy Autonomy | User Data Security |
| Scalability | Biocompatibility | Soft/rigid interface | Energy harvest | Protocol security |
| Cost | Durability of materials | Microchip integration | Energy storage | Data privacy |
| Production time | Adhesion (removable/semipermanent) | Skin-interfacing | Power consumption | |
| Skin breathing | ||||
| Water-resistance/ re-application | ||||
| Solid State Parts | Voltage (V) | Typical Current (µA) | Standby/Sleep Current (µA) | Example |
|---|---|---|---|---|
| Oximeter | 1.8/3.3 | 600 | 0.7 | MAX30102 |
| Temperature Sensor | 1.9–3.6 | 90 | 0.06 | Si7050 |
| Accelerometer | 1.71–3.6 | 2 | 0.5 | LIS2DH |
| ECG Board | 1.1–2 | 100 | 0.73 | MAX30003 |
| Communication | 1.71–5.5 | 15,600 Tx, 16,400 Rx | 1.3 Deep Sleep 0.150 Hibernate 0.06 Stop | CYBLE-014008-00: EZ-BLE |
| µC | 1.71–5.5 | 2500 at 6 MHz | 1.3 Deep Sleep 0.150 Hibernate 0.06 Stop | CYBLE-014008-00: EZ-BLE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.F.; Tavakoli, M. Domiciliary Hospitalization through Wearable Biomonitoring Patches: Recent Advances, Technical Challenges, and the Relation to Covid-19. Sensors 2020, 20, 6835. https://doi.org/10.3390/s20236835
Silva AF, Tavakoli M. Domiciliary Hospitalization through Wearable Biomonitoring Patches: Recent Advances, Technical Challenges, and the Relation to Covid-19. Sensors. 2020; 20(23):6835. https://doi.org/10.3390/s20236835
Chicago/Turabian StyleSilva, André F., and Mahmoud Tavakoli. 2020. "Domiciliary Hospitalization through Wearable Biomonitoring Patches: Recent Advances, Technical Challenges, and the Relation to Covid-19" Sensors 20, no. 23: 6835. https://doi.org/10.3390/s20236835





