Undoped and Nickel-Doped Zinc Oxide Thin Films Deposited by Dip Coating and Ultrasonic Spray Pyrolysis Methods for Propane and Carbon Monoxide Sensing Applications
Abstract
1. Introduction
2. Experimental Procedure
2.1. ZnO Preparation Process
2.1.1. Films Deposited by Sol-gel Dip Coating
2.1.2. Films Deposited by Ultrasonic Spray Pyrolysis
2.2. Films Characterization
3. Results and Discussion
3.1. Sensing Mechanism
3.2. Structural Properties
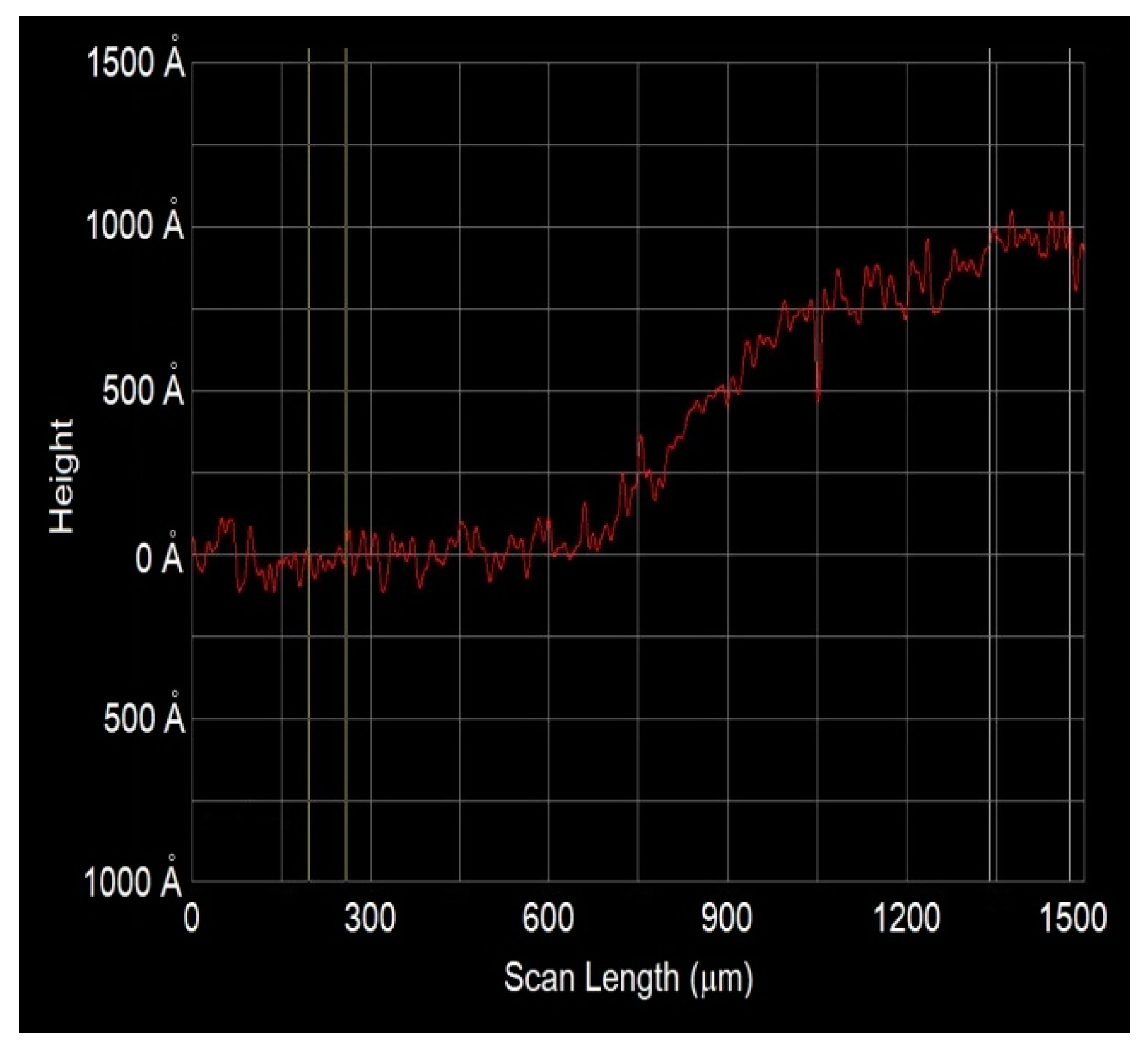
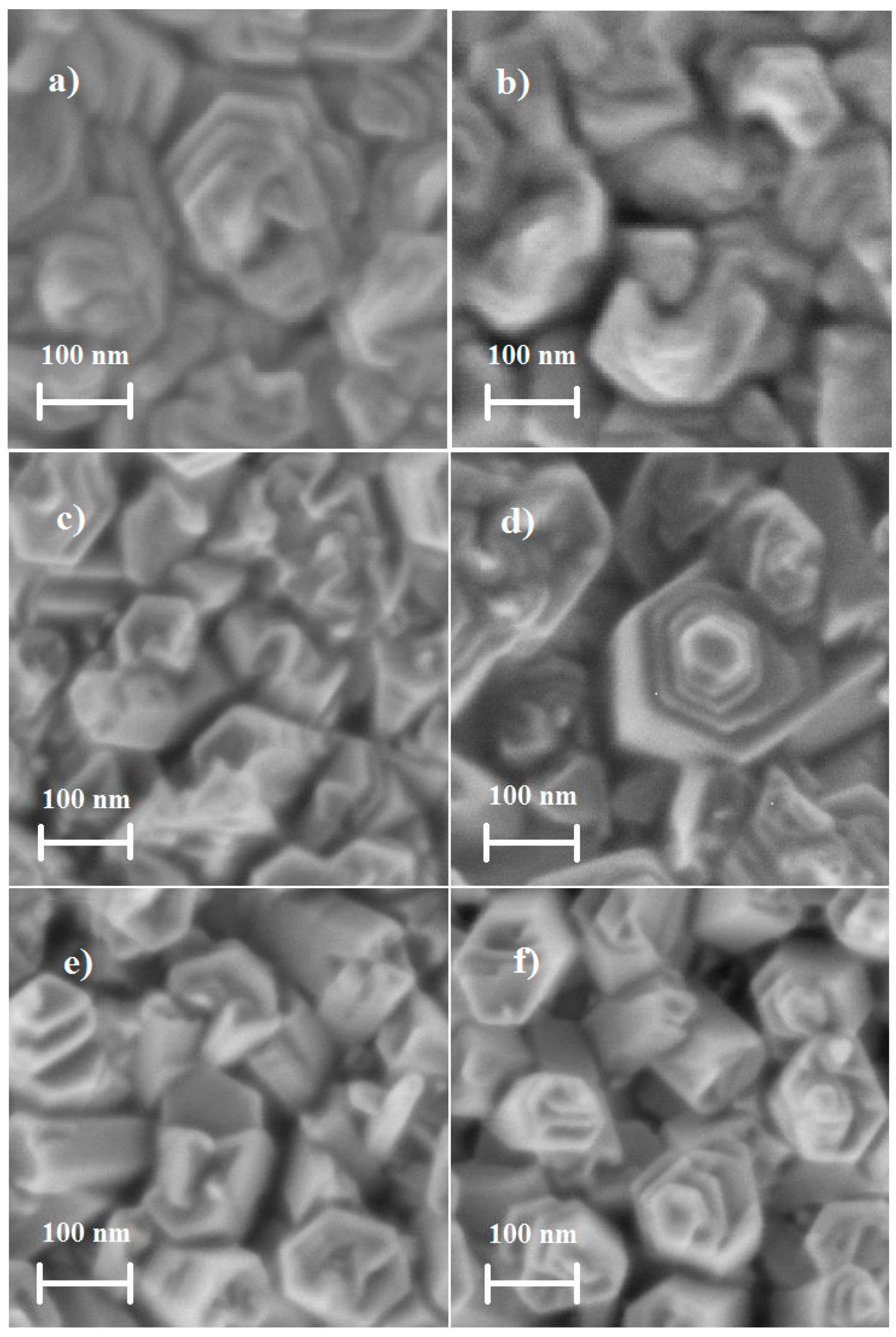
3.3. Morphological Properties for ZnO Films Deposited by Dip Coating and USP
3.4. Secondary Ion Mass Spectrometry Measurements
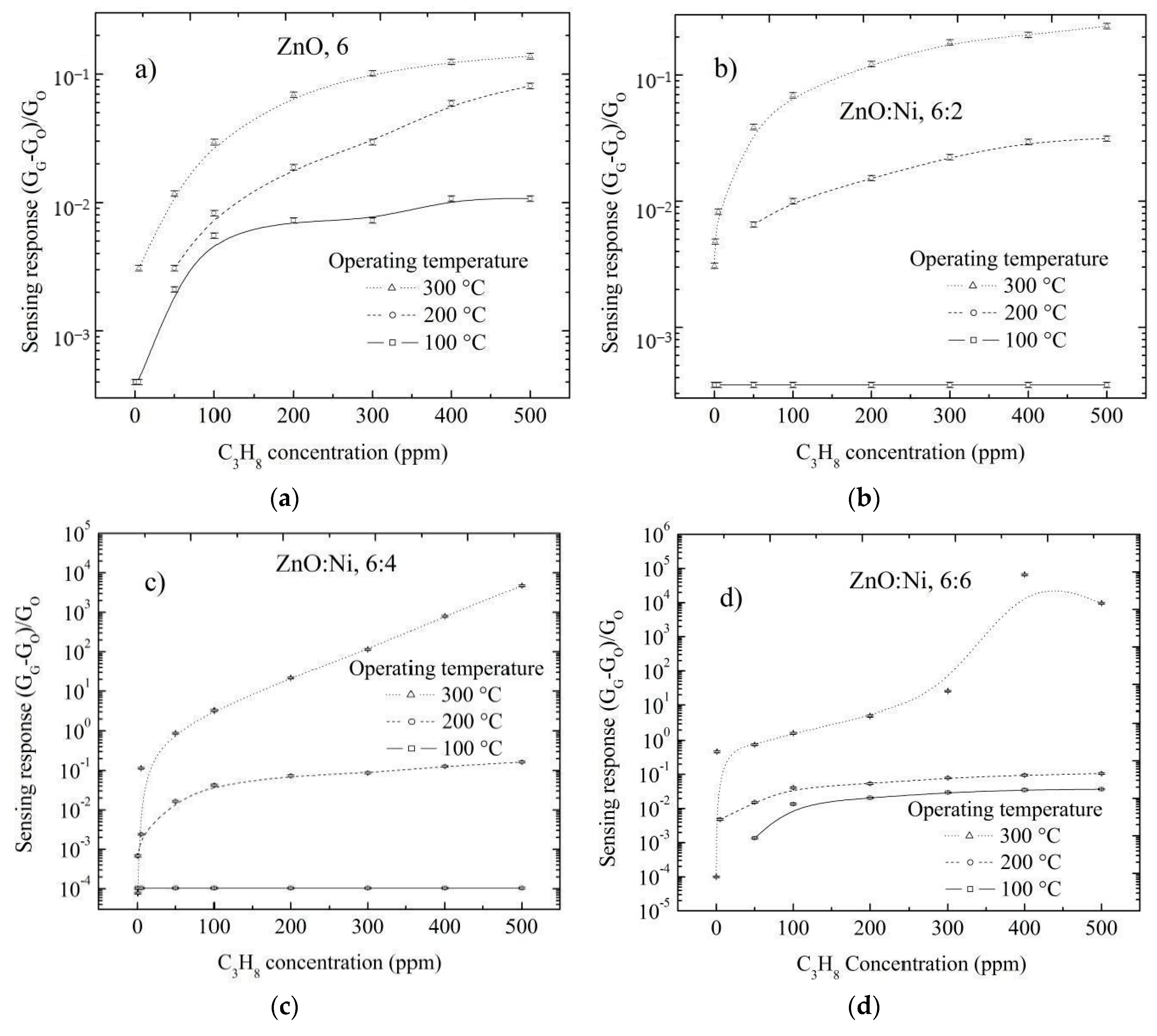
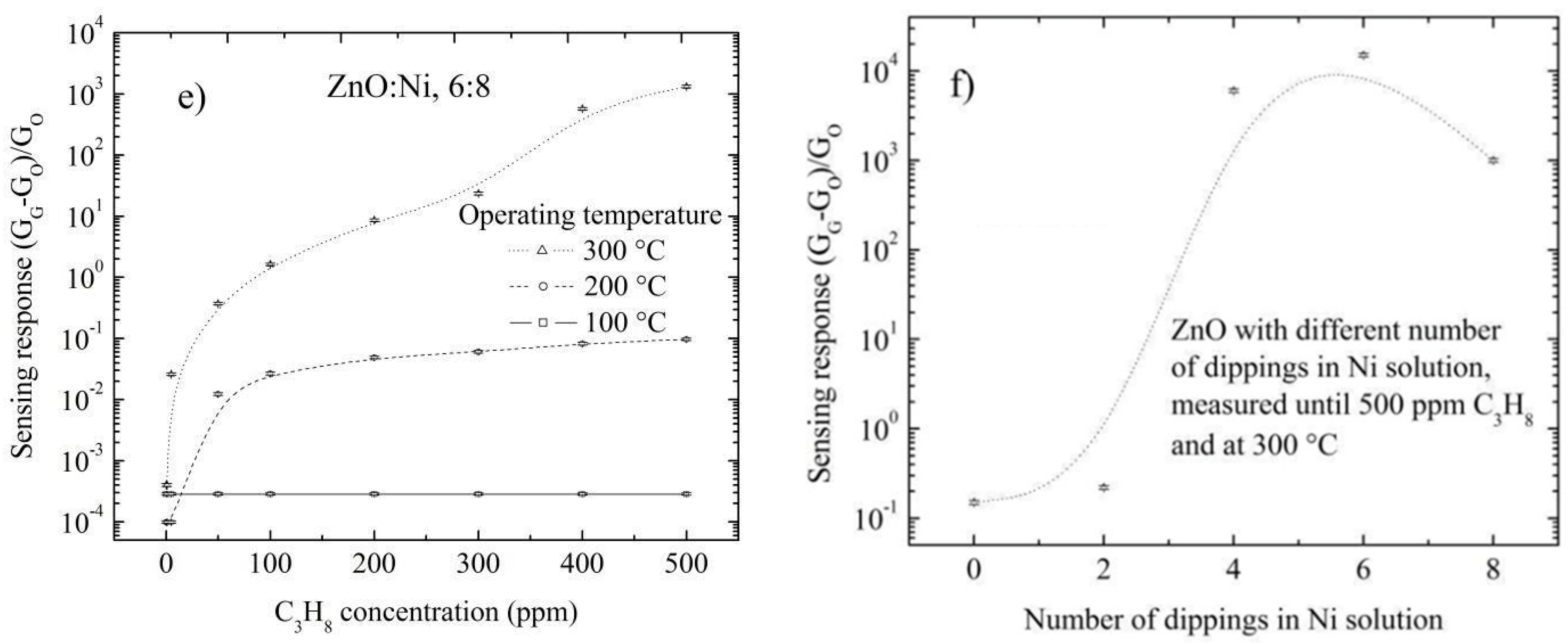
3.5. Sensing Properties
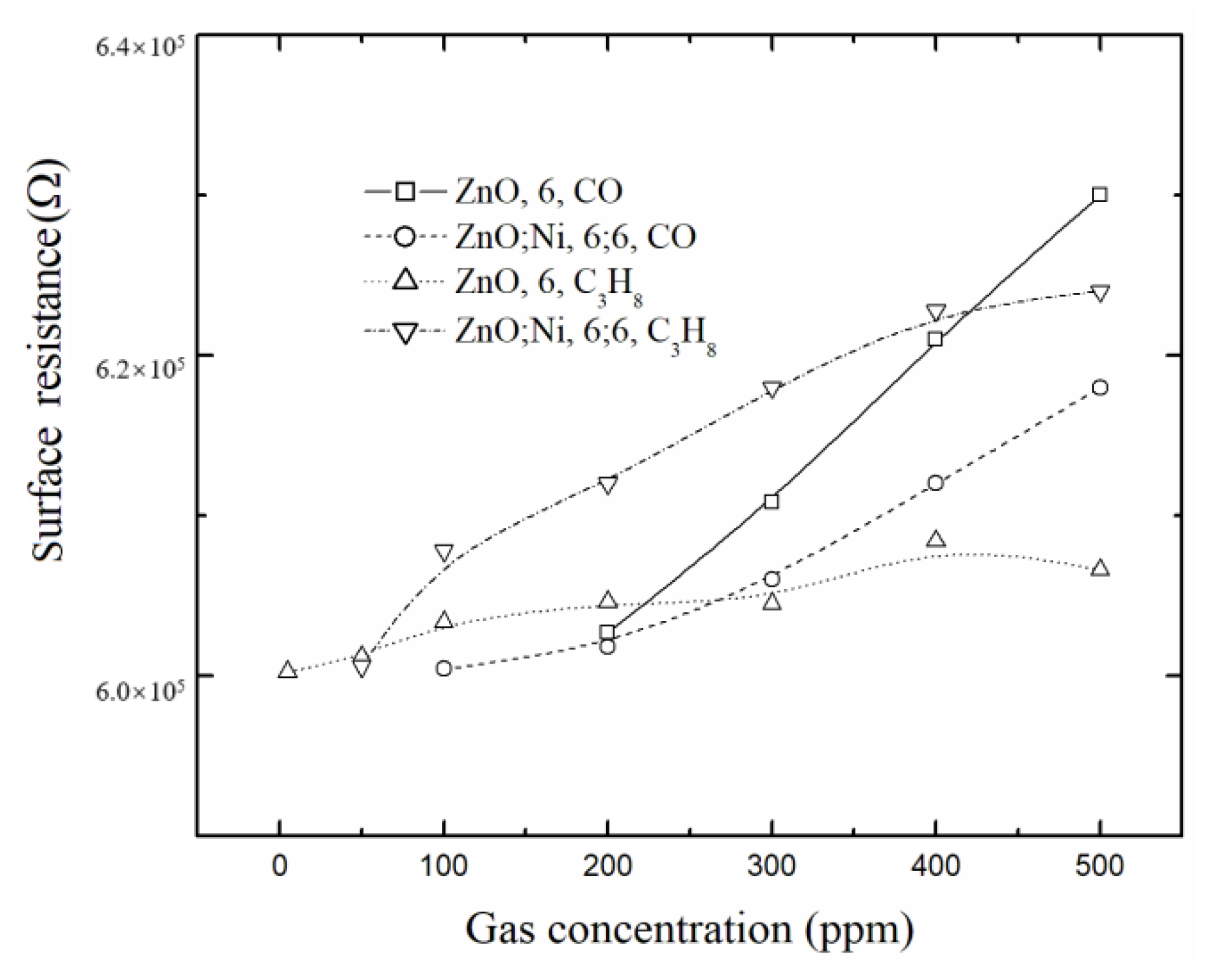
3.5.1. Selectivity
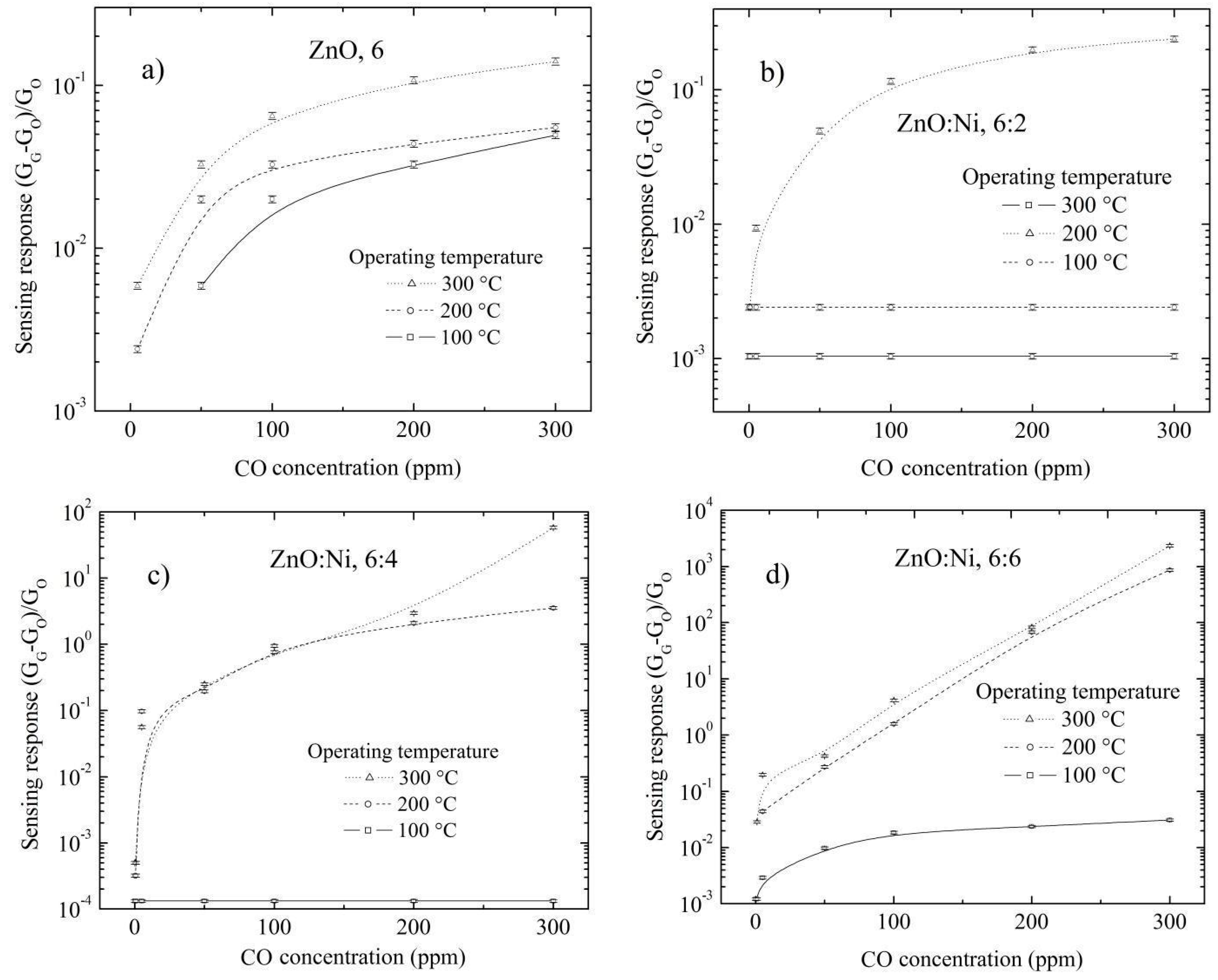
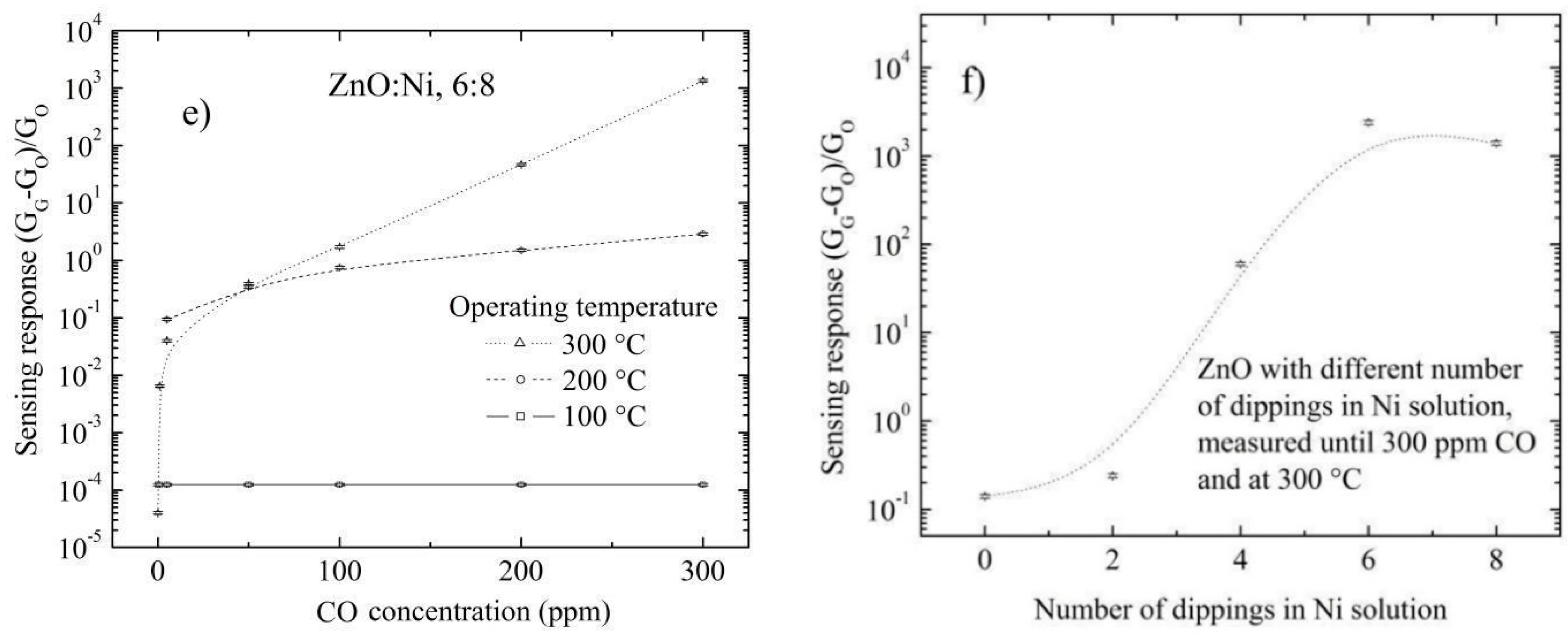
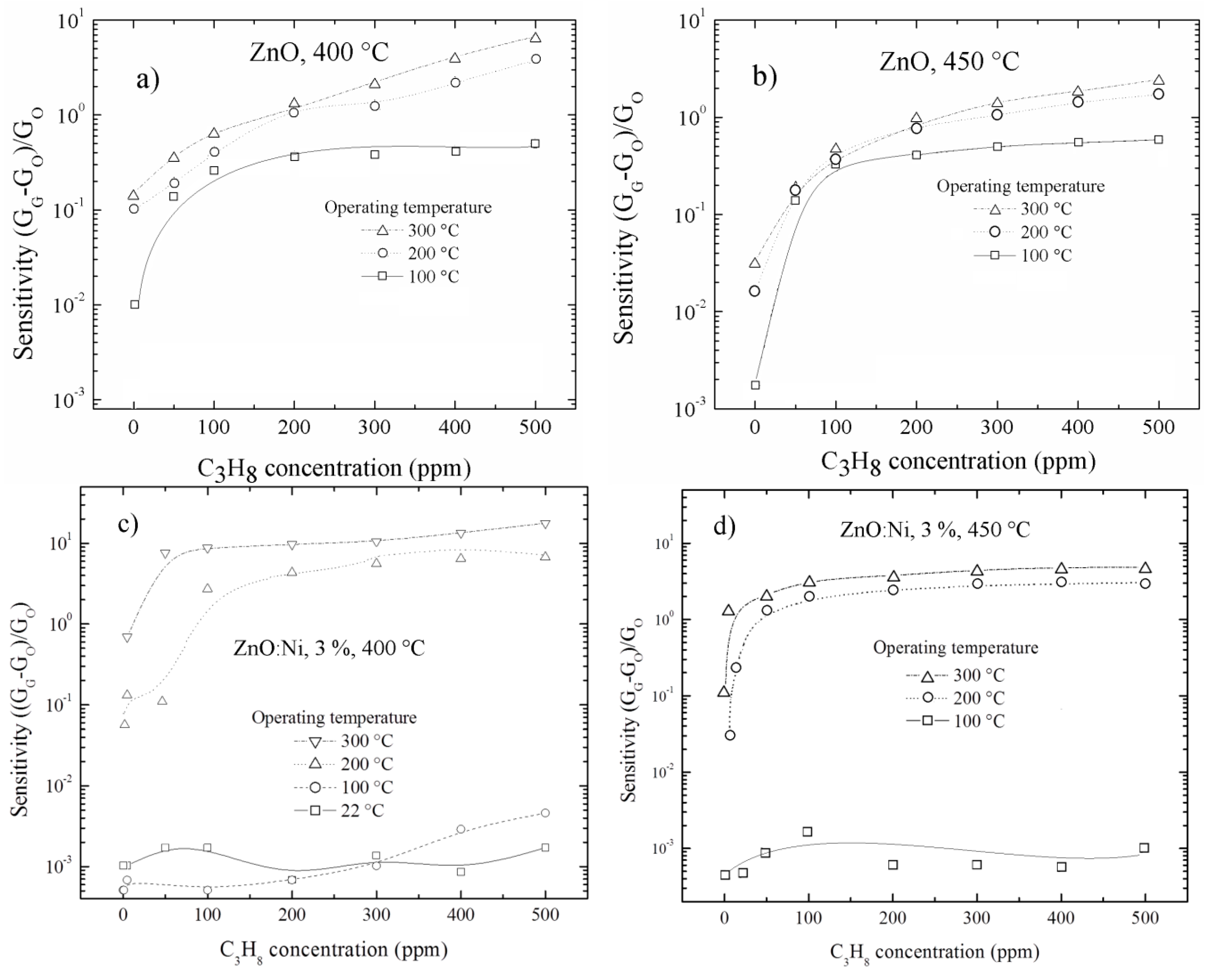
3.5.2. Doping Concentration Dependence CO and Propane Response
3.5.3. Effect of Surface Morphology on the Sensing Response
3.5.4. Temperature Dependence CO and Propane Sensing Response
3.5.5. Transient Resistance at Optimum Doping Concentration for CO and Propane Gas
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamiri, R.; Singh, B.; Belsley, M.S.; Ferreira, J.M.F. Structural and dielectric properties of Al-doped ZnOnanostructures. Ceram. Int. 2014, 40, 6031–6036. [Google Scholar] [CrossRef]
- Bdikin, K.; Silibin, M.; Ayouchi, R.; Schwarz, R.; Gavrilov, S.; Gracio, J.; Kholkin, A.L. Local electromechanical properties of ZnO thin films and microcrystals. Mater. Res. Soc. Symp. Proc. 2010, 1256, 1–6. [Google Scholar] [CrossRef]
- Mang, A.; Reimann, K.; Rübenacke, S. Band gaps, crystal-field splitting, spin-orbit coupling, and exciton binding energies in ZnO under hydrostatic pressure. Solid State Commun. 1995, 94, 251–254. [Google Scholar] [CrossRef]
- Jarzebzki, Z.M. Oxide Semiconductors; Pamplin, R.B., Ed.; Pergamon Press: Oxford, UK, 1973. [Google Scholar]
- Ho, C.C.; Lai, L.W.; Lee, C.T.; Yang, K.C.; Lai, B.T.; Liu, D.S. Transparent cosputtered ITO–ZnO electrode ohmic contact to n-type ZnO for ZnO/GaNheterojunction light-emitting diode. J. Phys. D Appl. Phys. 2013, 46, 1–7. [Google Scholar] [CrossRef]
- Velmurugan, P.; Park, J.H.; Lee, S.M.; Yi, Y.J.; Cho, M.; Jang, J.S.; Myung, H.; Bang, K.S.; Oh, B.T. Eco-friendly approach towards green synthesis of zinc oxide nanocrystals and its potential applications. Artif. Cell Nanomed. B 2016, 44, 1537–1543. [Google Scholar] [CrossRef]
- Madhusoodanan, K.N.; Vinalkkumar, T.V.; Vijayakumar, K.P. Gas sensing application of nanocrystalline zinc oxide thin films prepared by spray pyrolysis. Bull. Mater. Sci. 2015, 38, 583–591. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1439855119. [Google Scholar]
- Khantoul, A.R.; Sebais, M.; Rahal, B.; Boudine, B.; Halimi, O. Structural and Optical Properties of ZnO and Co Doped ZnO Thin Films Prepared by Sol-Gel. Acta Phys. Pol. A 2018, 133, 114–117. [Google Scholar] [CrossRef]
- Braker, W.; Mossman, A.L. Matheson Gas Data Book, 6th ed.; Matheson Gas Products: Secaucus, NJ, USA, 1980; pp. 615–623. [Google Scholar]
- ACGIH. Propane. In Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1991; pp. 1286–1287. [Google Scholar]
- Smoot, E.C.; Hickerson, W.L. Propane gas dangers and strategies for prevention of injuries. J. Burn Care Res. 1996, 17, 273–279. [Google Scholar] [CrossRef]
- Cleveland, C.J.; Morris, C. Section 45—Climate Change. In Handbook of Energy; Elsevier Science: New York, NY, USA, 2014; pp. 805–820. [Google Scholar]
- Boikess, R.S.; Edelson, E. Chemical Principles, 2nd ed.; Harper & Row Publishers: New York, NY, USA, 1981; pp. 672–673. [Google Scholar]
- Brown, T.L.; LeMay, H.E., Jr. Chemistry: The Central Science, 3rd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1985; pp. 390–392. [Google Scholar]
- Guillén-Bonilla, H.; Flores-Martínez, M.; Rodríguez-Betancourtt, V.M.; Guillen-Bonilla, A.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Olvera Amador, M.; De la, L.; Santoyo-Salazar, J. A Novel Gas Sensor Based on MgSb2O6 Nano rods to IndicateVariations in Carbon Monoxide and Propane Concentrations. Sensors 2016, 16, 177. [Google Scholar] [CrossRef]
- Florido, E.A.; Solidum, S.A. Butane/Propane Gas Sensing Using Zinc Oxide Film Grown by Successive Ionic Layer Adhesion and Reaction. Key Eng. Mater. 2016, 705, 273–277. [Google Scholar] [CrossRef]
- Ma, X.; Shen, J.; Chen, Y.; Chen, C.; Sun, L.; Zhang, X.; Ruan, S. Synthesis and enhanced gas sensing properties of Au-nanoparticle decorated CdS nanowires. RSC Adv. 2016, 6, 70907–70912. [Google Scholar] [CrossRef]
- Khadayate, R.S.; Patil, P.P. CO gas sensing properties of screen printed SnO2 thick films. IEEE Sens. J. 2016, 16, 6839–6845. [Google Scholar]
- Lim, H.J.; Lee, D.; Oh, Y.J. Gas sensing properties of ZnO thin films prepared by microcontact printing. Sens. Actuators A Phys. 2006, 125, 405–410. [Google Scholar] [CrossRef]
- Shinde, V.R.; Gujar, T.P.; Lokhande, C.D. LPG sensing properties of ZnO films prepared by spray pyrolysis method: Effect of molarity of precursor solution. Sens. Actuators B Chem. 2007, 120, 551–559. [Google Scholar] [CrossRef]
- Dhahri, R.; HjiriL, M.; MirH, L.E.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Qu, Y.; Gessert, T.A.; Coutts, T.J.; Noufi, R. Study of ion beam-sputtered, ZnO films as a function of deposition temperature. J. Vac. Sci. Technol. 1994, 12, 1507–1512. [Google Scholar] [CrossRef]
- Takai, O.; Futsuhara, M.; Shimizu, G.; Lungu, C.P.; Nozue, J. Nanostructure of ZnO thin films prepared by reactive rf magnetron sputtering. Thin Solid Films 1998, 318, 117–119. [Google Scholar] [CrossRef]
- Dikovska, A.O.; Atanasov, P.A.; Vasilev, C.; Dimitrov, I.G.; Stoyanchov, T.R. Thin ZnO films produced by pulsed laser deposition. J. Optoelectron. Adv. Mater. 2005, 7, 1329–1334. [Google Scholar] [CrossRef]
- Abdulgafour, H.I.; Hassan, Z.; Yam, F.K.; Chin, C.W. Sensing devices based on ZnO hexagonal tube-like nanostructures grown on p-GaN heterojunction by wet thermal evaporation. Thin Solid Films 2013, 540, 212–220. [Google Scholar] [CrossRef]
- Dali, Y.; Ming, H.; Shenyu, L.; Jiran, L.; Yaqiao, W.; Shangyun, M. Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 2014, 115, 297–305. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Thermally evaporated copper oxide films: A view of annealing effect on physical and gas sensing properties. Ceram. Int. 2017, 43, 7057–7064. [Google Scholar] [CrossRef]
- Hu, J.; Gordon, R.G. Textured aluminum-doped zinc oxide thin films from atmospheric pressure chemical-vapor deposition. J. Appl. Phys. 1992, 71, 880–890. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Reszka, A.; Sobczak, K.; Wojciechowski, T.; Fronc, K. The synthesis, characterization and ZnS surface passivation of polycrystalline ZnO films obtained by the spin-coating method. J. Alloys Compd. 2017, 695, 1196–1204. [Google Scholar] [CrossRef]
- Manthina, V.; Agrios, A.G. Single-pot ZnO nanostructure synthesis by chemical bath deposition and their applications. Nano-Struct. Nano-Objects 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Muthukrishnan, K.; Vanaraja, M.; Boomadevi, S.; Karn, R.K.; Pandiya, K. Studies on acetone sensing characteristics of ZnO thin film prepared by sol–gel dip coating. J. Alloys Compd. 2016, 673, 138–143. [Google Scholar] [CrossRef]
- Krishnan, V.G.; Elango, P. Influence of Ba doping concentration on the physical properties and gas sensing performance of ZnO nanocrystalline films: Automated nebulizer spray pyrolysis (ANSP) method. Optik 2017, 141, 83–89. [Google Scholar] [CrossRef]
- Kozhukharov, S.; Tchaoushev, S. Spray pyrolysis equipment for various applications. J. Univ. Chem. Technol. Metall. 2014, 48, 111–118. [Google Scholar]
- Ochoa, M.; Durães, L.; Beja, A.M.; Portugal, A.T. NanocrystallineZnO Thin Films—Influence of Sol-gel Conditions on the Underlying Chemistry and Film Microstructure and Transparency. Mater. Today Proc. 2015, 2, 49–56. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Stambolova, I.; Blaskova, V.; Dimitrov, O. Influence of different process parameters upon microstructure of ZnO films prepared by spray pyrolysis. Rev. Roumchim. 2013, 58, 843–853. [Google Scholar]
- Viguié, J.C.; Spitz, J. Chemical Vapor Deposition at Low Temperatures. Electrochem. Soc. 1975, 122, 585–588. [Google Scholar] [CrossRef]
- Ludowise, M.J. Metsdorganijcchemica; vapor deposition of HI-V semiconductors. J. Appl. Phys. 1985, 58, R31–R55. [Google Scholar] [CrossRef]
- Paraguay, F.; Estrada, W.; Acosta, D.R.; Andrade, E.; Miki-Yoshida, M. Growth, structure and optical characterization of high quality ZnO thin films obtained by spray pyrolysis. Thin Solid Films 1999, 350, 192–202. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; González-Vidal, J.L.; Torres, G.A.; Rodríguez-Baez, J.; Maldonado, A.; De la Luz Olvera, M.; Acosta, D.R.; Avendaño-Alejo, M.; Castañeda, L. Chromium and Ruthenium-Doped Zinc Oxide Thin Films for Propane Sensing Applications. Sensors 2013, 13, 3432–3444. [Google Scholar] [CrossRef]
- Morrison, S.R. The Chemical Physics of Surfaces, Chapter 1; Stanford Research Institute Menlo Park: Menlo Park, CA, USA, 1977. [Google Scholar]
- MorrIson, S.R. Semiconductor gas sensors. Sens. Actuators 1982, 2, 329–341. [Google Scholar] [CrossRef]
- Liao, L.; Lu, H.B.; Li, J.C.; He, H.; Wang, D.F.; Fu, D.J.; Liu, C. Size dependence of gas response of ZnO nanorods. J. Phys. Chem. 2007, 111, 1900–1903. [Google Scholar] [CrossRef]
- Tomas, T. Automotive suitability of air quality gas sensors. Sens. Actuators B Chem. 2012, 170, 40–44. [Google Scholar] [CrossRef]
- Long, Y.; Jian, L.; Weijun, L.; Fei, Z.; Dongcheng, H. A photo-induced ZnO coated mesh for on-demand oil/water separation based on switchable wettability. Mater. Lett. 2016, 163, 247–249. [Google Scholar] [CrossRef]
- Gonzalez-Chavarri, J.; Castro-Hurtado, L.; Castaño, E.; Mandayo, G.G. High-sensitivity Indoor-air-quality Sensor through Localized Growth of ZnO Nanostructures. Procedia Eng. 2014, 87, 983–986. [Google Scholar] [CrossRef][Green Version]
- Ryzhikov, A.; Jońca, J.; Kahn, M.; Fajerwerg, K.; Chaudret, B.; Chapelle, A.; Ménini, P.; Shim, C.H.; Gaudon, A.; Fau, P. Organometallic synthesis of ZnO nanoparticles for gas sensing: Towards selectivity through nanoparticles morphology. J. Nanopart. Res. 2015, 17, 280–290. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Jayaprakash, R.; Pinna, N.; Donato, N.; Bonavita, A.; Micali, G.; Neri, G. CO gas sensing of ZnO nanostructures synthesized by an assisted microwave wet chemical route. Sens. Actuators B Chem. 2009, 143, 198–204. [Google Scholar] [CrossRef]
- Samarasekara, P.; Yapa, N.U.S.; Kumara, N.T.R.N.; Perera, M.V.K. CO2 gas sensitivity of sputtered zinc oxide thin films. Bull. Mater. Sci. 2007, 30, 113–116. [Google Scholar] [CrossRef]
- Banerjee, A.; Haldar, A.K.; Mondal, J.; Sen, A.; Maiti, M.S. Bi-layer functionally gradient thick film semiconducting methane sensor. Bull. Mater. Sci. 2002, 25, 497–500. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Groppelli, S.; Nelli, P.; Quaranta, F.; Valentini, A.; Vasanelli, L. Oxygen gas-sensing characteristics for ZnO(Li) sputtered thin films. Sens. Actuators B Chem. 1992, 7, 747–751. [Google Scholar] [CrossRef]
- Liu, F.T.; Gao, S.F.; Pei, S.K.; Tseng, S.C.; Liu, C.H. ZnO nanorod gas sensor for NO2 detection. J. Taiwan Inst. Chem. E 2009, 40, 528–532. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, H.I.; Hsu, C.S.; Huang, C.C.; Wu, J.S.; Chou, P.C.; Liu, W.C. Characteristics of ZnO nanorods-based ammonia gas sensors with a cross-linked configuration. Sens. Actuators B Chem. 2015, 221, 491–498. [Google Scholar] [CrossRef]
- Gledhill, S.; Grimm, A.; Greiner, D.; Bohne, W.; Lux-Steiner, M.; Fischer, C.H. Doping induced structural and compositional changes in ZnO spray pyrolysed films and the effects on optical and electrical properties. Thin Solid Films 2011, 519, 4293–4298. [Google Scholar] [CrossRef]
- Hyung-Sin, M.; Sung-Eun, K.; Woo-Chang, C. Methane Gas Sensing Properties of the Zinc Oxide Nanowhisker-derived Gas Sensor. Trans. Electr. Electron. Mater. 2012, 13, 106–109. [Google Scholar] [CrossRef]
- Xu, J.; Han, J.; Zhang, Y.; Sun, Y.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B Chem. 2008, 132, 334–339. [Google Scholar] [CrossRef]
- Mazinguea, T.; Escoubas, L.; Spalluto, L.; Flory, F.; Socol, G.; Ristoscu, C.; Axente, E.; Grigorescu, S.; Mihailescu, I.N. Nanostructured ZnO coatings grown by pulsed laser deposition for optical gas sensing of butane. J. Appl. Phys. 2005, 98, 074312–1–074312–6. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Stakhanova, S.V.; Straumal, P.B.; Bulatov, M.F.; Schutz, G.; Tietze, T.; Goering, E.; Baretzky, B. Grain boundaries as a source of ferromagnetism and increased solubility of Ni in nanograined ZnO. Rev. Adv. Mater. Sci. 2015, 41, 61–71. [Google Scholar]
- Rambu, A.P.; Ursu, L.; Iftimie, N.; Nica, V.; Dobromir, M.; Iacomia, F. Study on Ni-doped ZnO films as gas sensors. Appl. Surf. Sci. 2013, 280, 598–604. [Google Scholar] [CrossRef]
- McMurdie, H.F.; Morris, M.; Evans, E.; Paretzkin, B.; Wong-Ng, W.; Ettlinger, L.; Hubbard, C. Standard X-ray diffraction powder patterns from the JCPDS research associateship. Powder Diffr. 1986, 1, 64–77. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Ahmad, A.A.; Al-Bataineh, Q.M.; Bani-Salameh, A.A.; Abdullah, H.S.; Qattan, I.A.; Albataineh, Z.M.; Telfah, A.D. Optical, Structural, and Crystal Defects Characterizations of Dip Synthesized (Fe-Ni) Co-Doped ZnO Thin Films. Materials 2020, 13, 1737. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Leitão Muniz, F.T.; Ribeiro Miranda, M.A.; dos Santos, C.M.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Cryst. A 2016, 72, 1–6. [Google Scholar] [CrossRef]
- Ilicana, S.; Caglara, Y.; Caglara, M.; Yakuphanoglu, F. Electrical Conductivity, Optical and Structural Properties of Indium-Doped ZnO Nanofiber Thin Film Deposited by Spray Pyrolysis Method. Phys. E 2006, 35, 131–138. [Google Scholar] [CrossRef]
- Khan, Z.R.; Ulfequar, M.Z.; Khan, M.S. Optical and Structural Properties of Thermally Evaporated Cadmium Sulphide Thin Films on Silicon (100) Wafers. Mater. Sci. Eng. B 2010, 174, 145–149. [Google Scholar] [CrossRef]
- Ong, H.C.; Zhu, A.X.E.; Du, G.T. Dependence of the exitonic transition energies and mosaicity on residual strain in ZnO thin films. J. Appl. Phys. Lett. 2002, 80, 941–948. [Google Scholar] [CrossRef]
- Madelung, O.; Rössler, U.; Schulz, M. (Eds.) II-VI and I-VII Compounds; Semimagnetic Compounds. Landolt-Börnstein—Group III Condensed Matter (Numerical Data and Functional Relationships in Science and Technology), Volume 41B; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Bhati, V.S.; Ranwa, S.; Rajamani, S.; Kumari, K.; Raliya, R.; Biswas, P.; Kumar, M. Improved Sensitivity with Low Limit of Detection of a Hydrogen Gas Sensor Based on rGO-Loaded Ni-Doped ZnO Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 11116–11124. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Ma, Y.; Fan, H. Ni-doped ZnO nanorods gas sensor: Enhanced gas-sensing properties, AC and DC electrical behaviors. Sens. Actuators B Chem. 2014, 199, 403–409. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Zhang, L. Crystalline Size Effects on Texture Coefficient, Electrical and Optical Properties of Sputter-deposited Ga-doped ZnO Thin Films. J. Mater. Sci. Technol. 2015, 31, 175–181. [Google Scholar] [CrossRef]
- Smith, A.; Rodriguez-Clemente, R. Morphological differences in ZnO films deposited by the pyrosol technique: Effect of HCl. Thin Solid Films 1999, 345, 192–196. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, J.; Xie, Z.; Wang, J.; Su, X. Synthesis of monodisperse WO3•2H2O nanospheres by microwave hydrothermal process with l (+) tartaric acid as a protective agent. Mater. Lett. 2008, 62, 2992–2994. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H. Morphologies of zinc oxide particles and their effects on photocatalysis. Chemosphere 2003, 51, 129–137. [Google Scholar] [CrossRef]
- Sinkó, K.; Szabó, G.; Zrínyi, M. Liquid-phase synthesis of cobalt oxide nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 4127–4135. [Google Scholar] [CrossRef]
- Schaepe, K.; Jungnickel, H.; Heinrich, T.; Tentschert, J.; Luch, A.; Wolfgang, E.S. Book Chapter 4.7—Secondary ion mass spectrometry. In Characterization of Nanoparticles, Measurement Processes for Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 481–509. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; Karthik, T.V.K.; Olvera, M.d.l.L.; García-Barrientos, A.; Pérez-Cortez, O.; Vega-Pérez, J.; Maldonado, A.; Pérez-Hernández, R.; Rodríguez-Lugo, V. ZnO thin films as propane sensors: Band structure models to explicate the dependence between the structural and morphological properties on gas sensitivity. J. Phys. Chem. Solids 2017, 106, 16–28. [Google Scholar] [CrossRef]







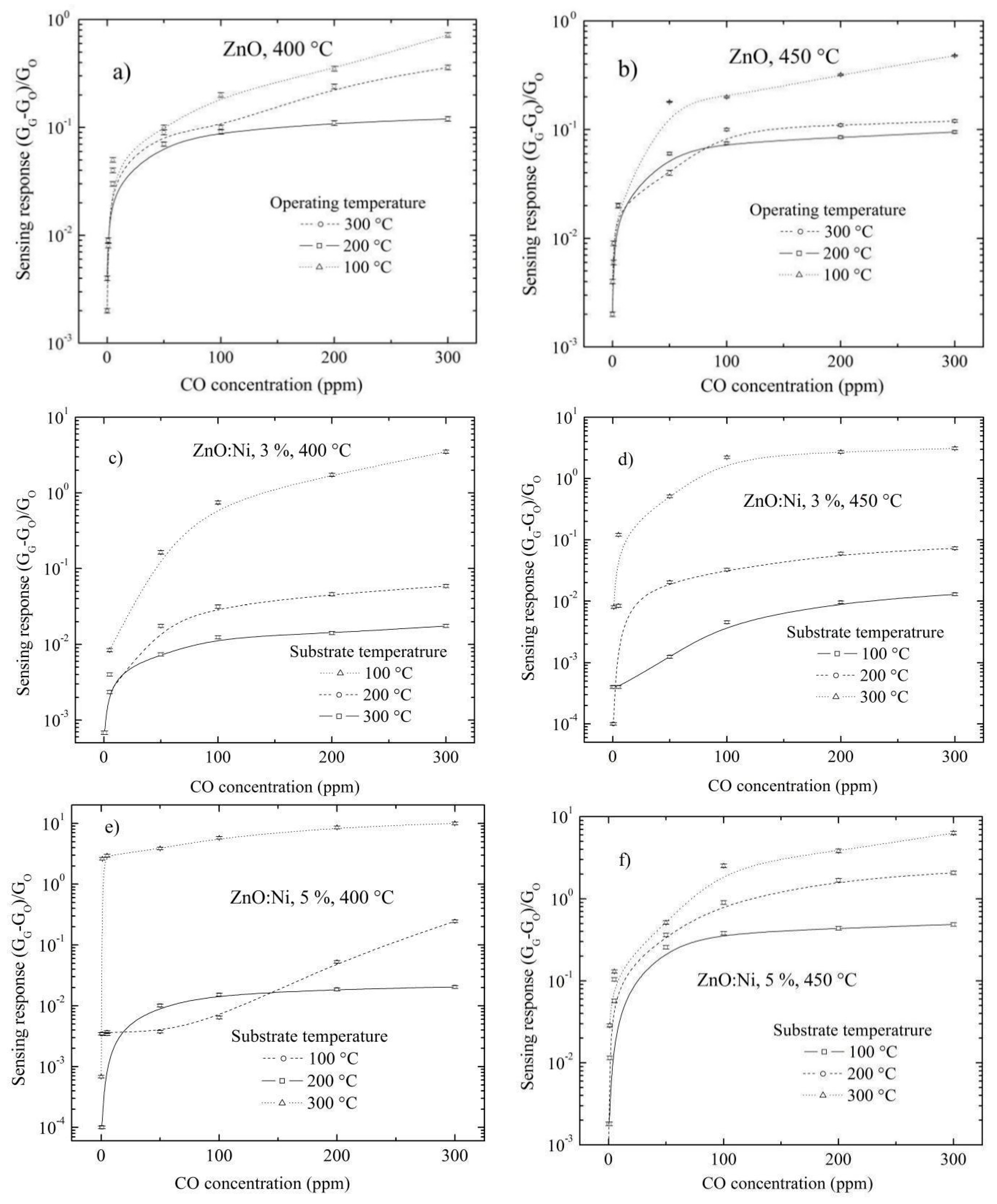
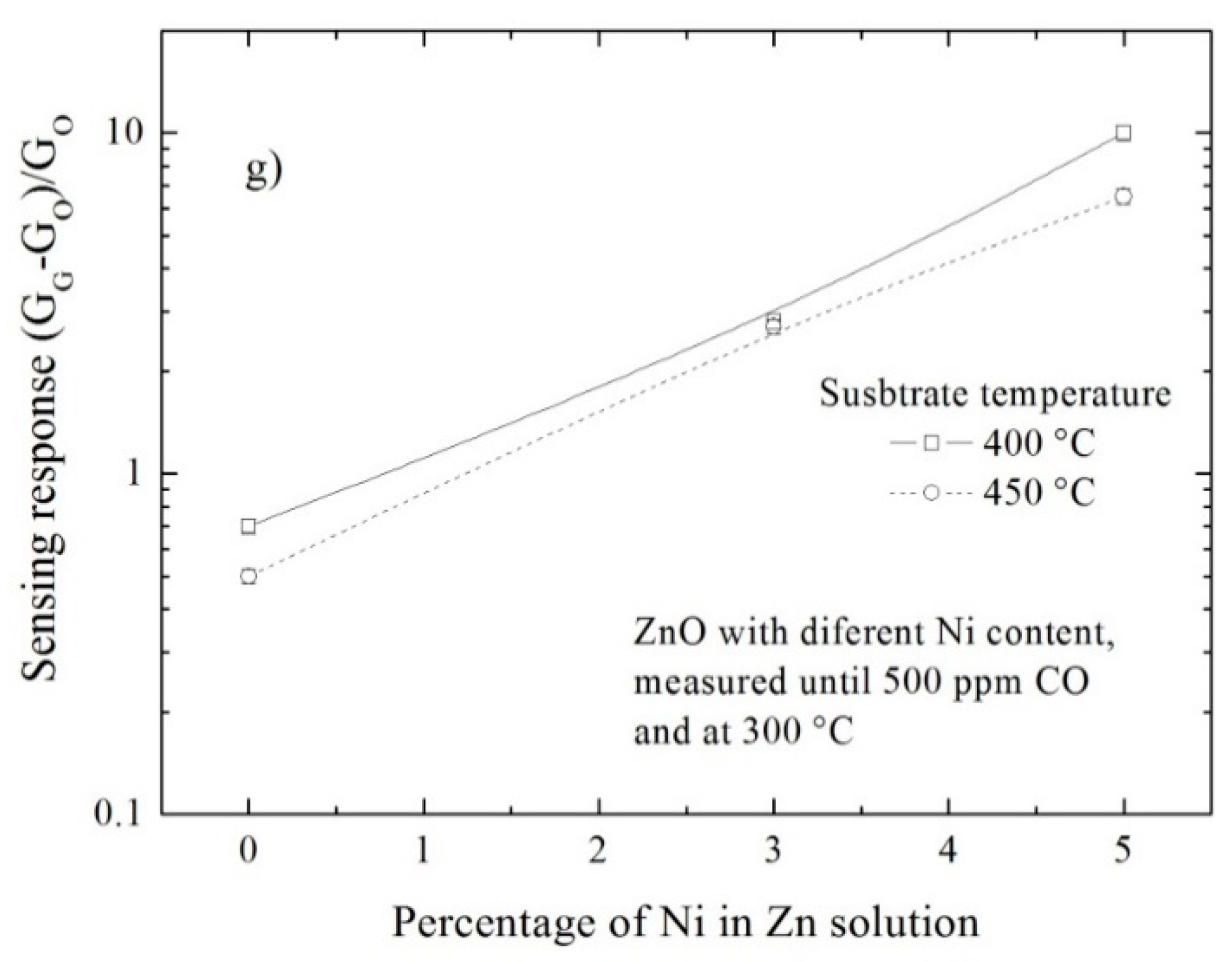

| Sensor Material | Synthesis Process | Doping Solution (at. %) | Type of Gas | Operating Temperature (°C) | Gas Concentration | Sensing Response |
|---|---|---|---|---|---|---|
| ZnO:Ni [21] | Sol-gel | 5 | Carbon oxide | 350 | 80 (ppm) | 5.5 |
| ZnO [22] | Spray pyrolysis | --- | liquid petroleum gas | 377 | 0.4 (vol %) | 32.5 |
| ZnO:Ni | Sol-gel | --- | Carbon oxide | 300 | 300 | ≈3200 |
| ZnO:Ni | Spray pyrolysis ultrasonic | 5 | Propane | 400 | 500 | ≈25 |
| Gas Concentration (ppm) | Carbon MonoxidePressure (mBar) | Propanepressure (mBar) |
|---|---|---|
| 0 | 0.7 | 0.53 |
| 1 | 8.6 | 2.7 |
| 50 | 43 | 27 |
| 100 | 86 | 55 |
| 200 | 170 | 110 |
| 300 | 260 | 160 |
| 400 | 350 | 220 |
| 500 | 430 | 270 |
| Method | Film | D (nm) | c (Å) | a (Å) | TC (002) | Thickness Film (nm) | ||
|---|---|---|---|---|---|---|---|---|
| ZnO, 6 | ≈ 35 | 5.21 | --- | 1.42 | 99 | |||
| ZnO:Ni, 6:2 | ≈ 34 | 5.22 | --- | 1.43 | 119 | |||
| Dip Coating | ZnO:Ni, 6:4 | ≈ 36 | 5.21 | --- | 1.48 | 137 | ||
| ZnO:Ni, 6:6 | ≈ 34 | 5.22 | --- | 1.45 | 156 | |||
| ZnO:Ni, 6:8 | ≈ 36 | 5.21 | --- | 1.45 | 169 | |||
| ZnO, 400 °C | ≈ 36 | 5.23 | 3.2 | 1.97 | 160 | |||
| ZnO, 450 °C | ≈ 52 | 5.23 | 3.2 | 2.39 | 164 | |||
| USP | ZnO:Ni, 3%, 400 °C | ≈ 24 | 5.27 | 3.14 | 3.18 | 150 | ||
| ZnO:Ni, 3%, 450 °C | ≈ 49 | 5.20 | 3.26 | 2.09 | 156 | |||
| ZnO:Ni, 5%, 400 °C | ≈ 27 | 5.28 | 3.14 | 3.41 | 155 | |||
| ZnO:Ni, 5%, 450 °C | ≈ 50 | 5.19 | --- | 2.53 | 160 | |||
| Method | Film | δ 10−4 (nm)−2 | εzz (%) | |||||
| ZnO, 6 | 8.1 | 0.19 | ||||||
| ZnO:Ni, 6:2 | 8.6 | 0.32 | ||||||
| Dip Coating | ZnO:Ni, 6:4 | 8.7 | 0.32 | |||||
| ZnO:Ni, 6:6 | 8.7 | 0.31 | ||||||
| ZnO:Ni, 6:8 | 8.7 | 0.32 | ||||||
| ZnO, 400 °C | 7.7 | 0.57 | ||||||
| ZnO, 450 °C | 3.7 | 0.57 | ||||||
| USP | ZnO:Ni, 3%, 400 °C | 17.3 | 1.34 | |||||
| ZnO:Ni, 3%, 450 °C | 4.1 | 0 | ||||||
| ZnO:Ni, 5%, 400 °C | 13.7 | 1.53 | ||||||
| ZnO:Ni, 5%, 450 °C | 4 | 0 | ||||||
| Method | Film | Bulk Concentration (Ni atoms/cm3) |
|---|---|---|
| ZnO:Ni 6:8 | 7.97 × 1020 | |
| Dip coating | ZnO:Ni 6:4 | 4.61 × 1020 |
| ZnO:Ni 6:2 | 3.03 × 1020 | |
| ZnO:Ni, 5%, 450 °C | 6.14 × 1019 | |
| USP | ZnO:Ni, 5%, 400 °C | 5.23 × 1019 |
| ZnO:Ni, 3%, 450 °C | 2.84 × 1019 | |
| ZnO:Ni, 3%, 400 °C | 8.57 × 1018 |
| Method | Depositions Conditions | Grain Shape | Average Grain Size (nm) | Volume Occupied by the Grains | Effective Surface Area (μm2) |
|---|---|---|---|---|---|
| Undoped ZnO | Round | 40–80 | 45.6 | 0.53 | |
| Dip Coating | ZnO:Ni 6:2 | Round | 60–100 | 48.5 | 0.53 |
| ZnO:Ni 6:4 | Round | 60–100 | 50.5 | 0.53 | |
| ZnO:Ni 6:8 | Round | 100–150 | 56.9 | 0.54 | |
| ZnO, 400 °C | Irregular | 30–150 | 46.7 | 0.58 | |
| ZnO, 450 °C | Irregular | 40–200 | 49.1 | 0.61 | |
| USP | ZnO:Ni, 3%, 400 °C | Irregular | 10–150 | 43.2 | 0.56 |
| ZnO:Ni, 3%, 450 °C | Irregular | 10–250 | 44.9 | 0.58 | |
| ZnO:Ni, 5%, 400 °C | Irregular | 10–150 | 51.8 | 0.55 | |
| ZnO:Ni, 5%, 450 °C | Irregular | 10–150 | 52.1 | 0.56 |
| Gas Concentration (ppm) | Surface Resistance ZnO:Ni 6:6, Dip Coating C3H8, 300 °C (MΩ) | Surface resistance ZnO:Ni 6:6, Dip coating CO, 300 °C (MΩ) | Surface Resistance ZnO:Ni, 5%, 400 °C, USP C3H8, 300 °C (MΩ) | Surface Resistance ZnO:Ni, 5%, 400 °C, USP CO, 300 °C (MΩ) |
|---|---|---|---|---|
| 0 | 330 | 334 | 293 | 290 |
| 5 | 225 | 300 | 291 | 288 |
| 10 | 212 | 295 | 255 | 62 |
| 50 | 189 | 210 | 133 | 80 |
| 100 | 126 | 60 | 73 | 59 |
| 200 | 54.12 | 3 | 58 | 35 |
| 300 | 12.36 | 0.12 | 26 | 32 |
| 400 | 0.005 | 22 | ||
| 500 | 0.034 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthik, T.V.K.; Olvera, M.d.l.L.; Maldonado, A.; Biswal, R.R.; Gómez-Pozos, H. Undoped and Nickel-Doped Zinc Oxide Thin Films Deposited by Dip Coating and Ultrasonic Spray Pyrolysis Methods for Propane and Carbon Monoxide Sensing Applications. Sensors 2020, 20, 6879. https://doi.org/10.3390/s20236879
Karthik TVK, Olvera MdlL, Maldonado A, Biswal RR, Gómez-Pozos H. Undoped and Nickel-Doped Zinc Oxide Thin Films Deposited by Dip Coating and Ultrasonic Spray Pyrolysis Methods for Propane and Carbon Monoxide Sensing Applications. Sensors. 2020; 20(23):6879. https://doi.org/10.3390/s20236879
Chicago/Turabian StyleKarthik, Tangirala Venkata Krishna, María de la Luz Olvera, Arturo Maldonado, Rajesh Roshan Biswal, and Heberto Gómez-Pozos. 2020. "Undoped and Nickel-Doped Zinc Oxide Thin Films Deposited by Dip Coating and Ultrasonic Spray Pyrolysis Methods for Propane and Carbon Monoxide Sensing Applications" Sensors 20, no. 23: 6879. https://doi.org/10.3390/s20236879
APA StyleKarthik, T. V. K., Olvera, M. d. l. L., Maldonado, A., Biswal, R. R., & Gómez-Pozos, H. (2020). Undoped and Nickel-Doped Zinc Oxide Thin Films Deposited by Dip Coating and Ultrasonic Spray Pyrolysis Methods for Propane and Carbon Monoxide Sensing Applications. Sensors, 20(23), 6879. https://doi.org/10.3390/s20236879







