LAPKaans: Tool-Motion Tracking and Gripping Force-Sensing Modular Smart Laparoscopic Training System
Abstract
1. Introduction
2. Materials and Methods
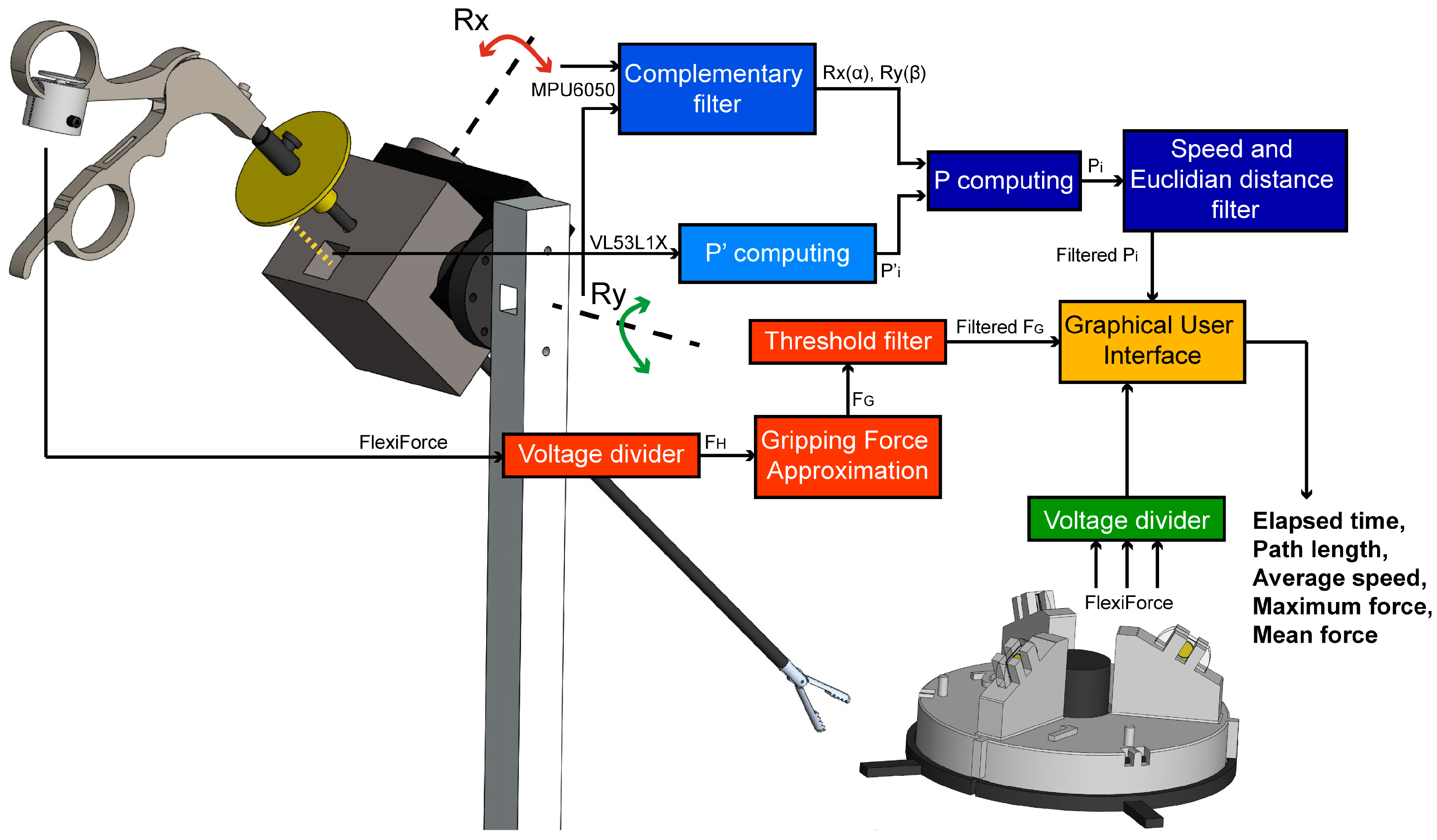
2.1. Tool-Motion Tracking Module
2.2. Gripping Force Sensing Module
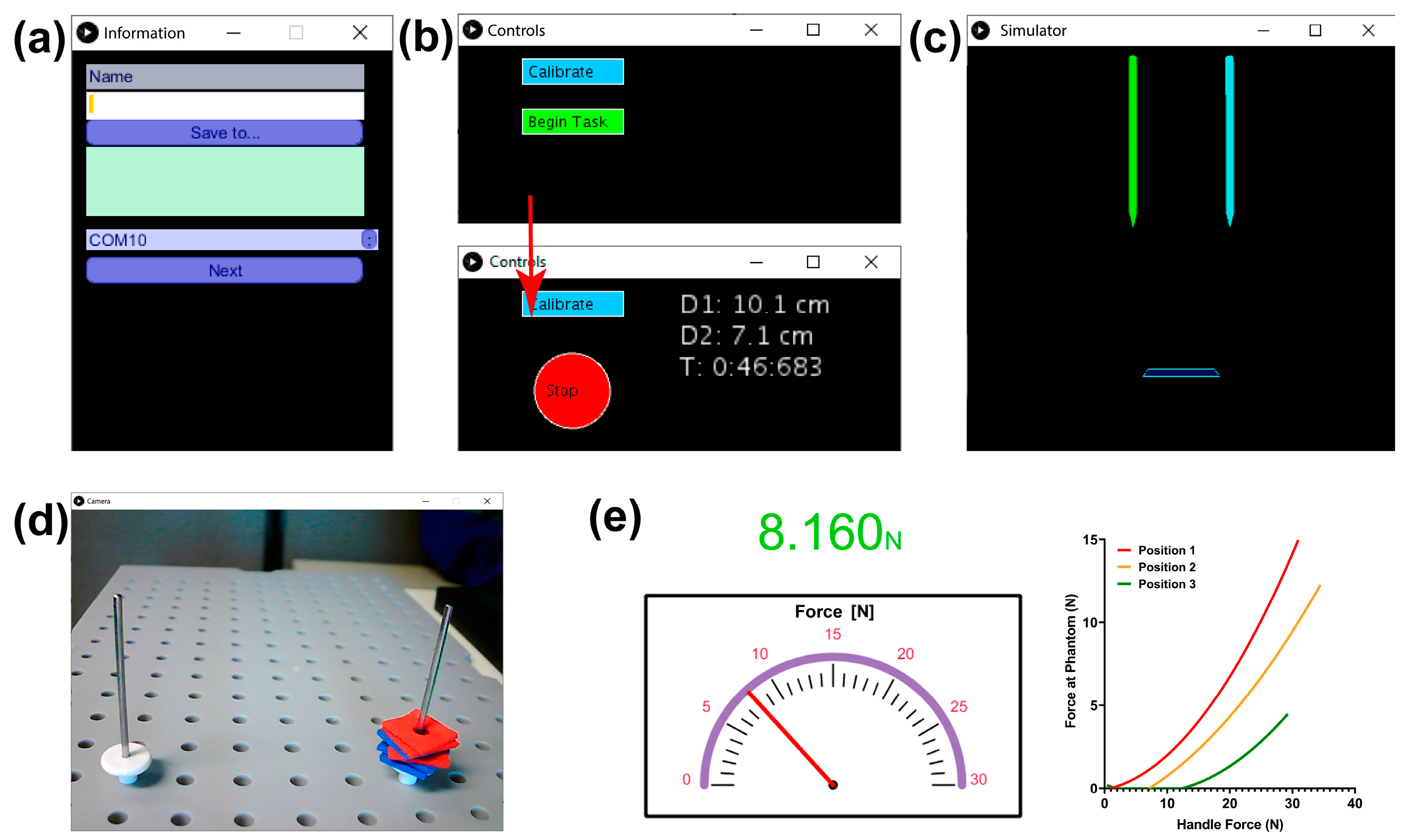
2.3. Force Training Module
2.4. Data Acquisition and Processing
2.5. Graphical User Interface Module
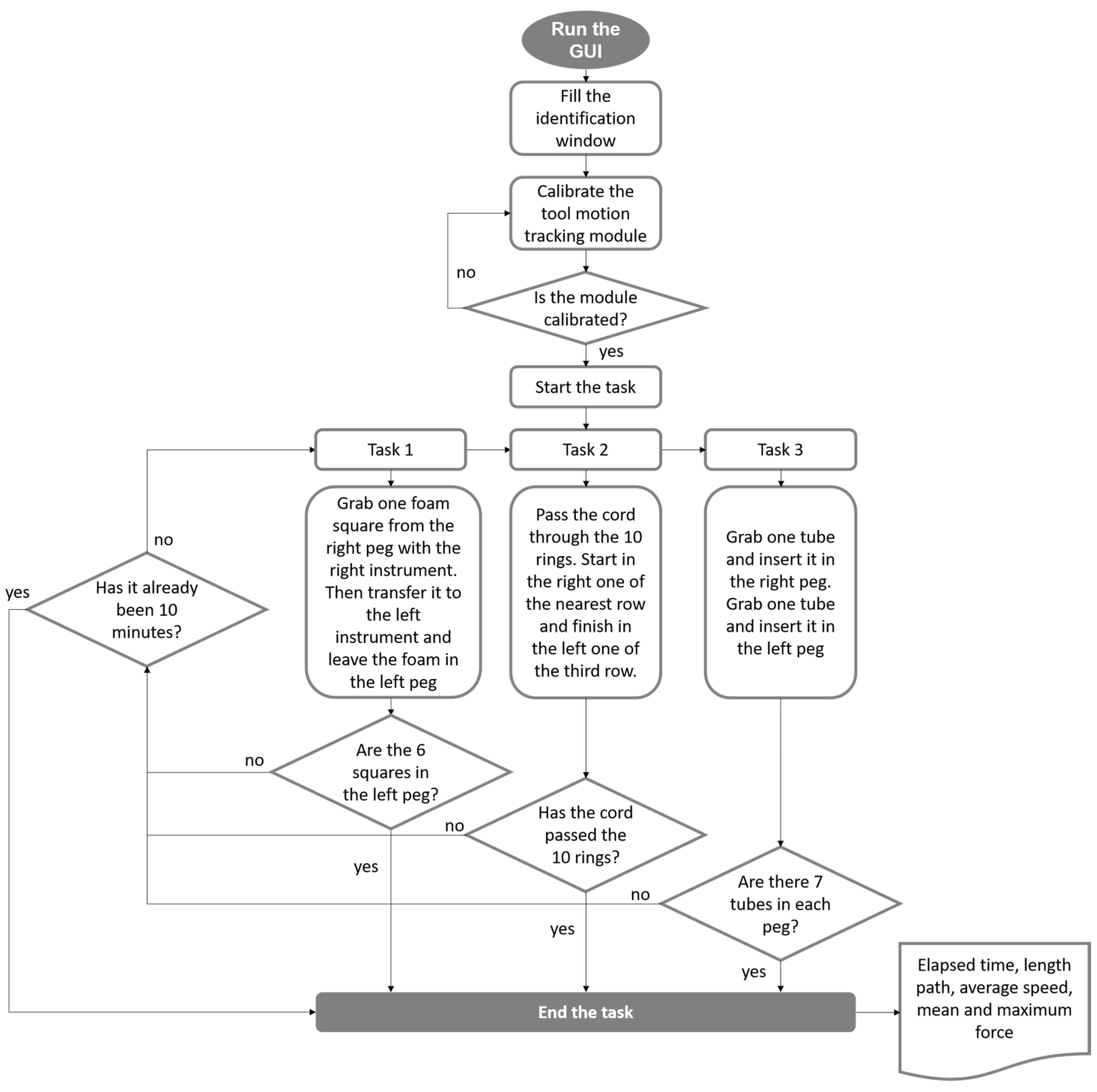
2.6. Validation in Medical Training Context
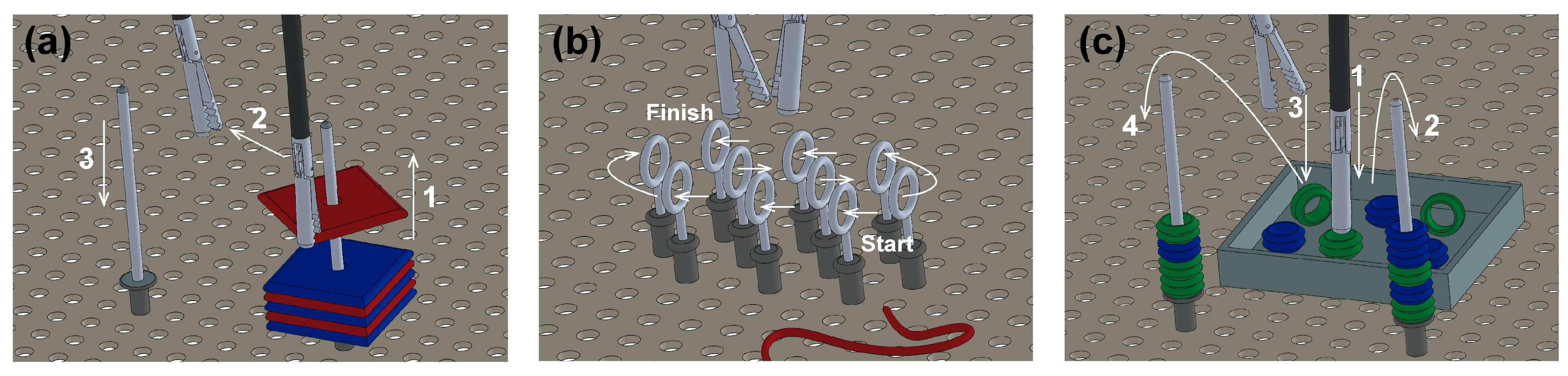
- Peg transfer: the subjects must grip one foam square from a right peg with the right instrument. Once the object is taken out from the peg, the subject must transfer it to the left instrument. Finally, the object must be delivered into the left peg. If a foam square falls, the subject must grip it with the same instrument being used. The task should be done for each of the six (Figure 5a).
- Object transfer: a thread must be inserted through ten rings in a zig-zag array, without any instrument use restriction. The task must be initiated in the right ring nearest to the user and continue horizontally to the two left consecutive rings. Next in the second line, beginning in the left ring and advancing to the right. Finally, in the farthest line, execute like in the first row (from right to left) (Figure 5b).
- Pea on a peg: small plastic tubes must be gripped from a box and be inserted in a peg, alternating between the right and the left one. For the right peg, the pipes must be taken with the right instrument. Likewise, for the left peg, the pipes must be taken with the left instrument. In the end, seven tubes must be inserted into each peg. The color of the gripped object is not important (Figure 5c).
2.7. Statistical Analysis
3. Results
3.1. Tool-Motion Tracking Module
3.2. Gripping Force Sensing Module
3.3. Force Training Module
3.4. Validation in Medical Training Context
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Protocol for Validation in Medical Training Context
References
- Markets, R. Global Laparoscopy and Endoscopy Devices Market, 2025—Focus on Surgical Procedures (Cholecystectomy and Hysterectomy) and Product Types (Arthroscopes, Neuroendoscopes, Cystoscope, and Bronchoscopes). Available online: http://www.globenewswire.com/news-release/2018/09/19/1572863/0/en/Global-Laparoscopy-and-Endoscopy-Devices-Market-2025-Focus-on-Surgical-Procedures-Cholecystectomy-and-Hysterectomy-and-Product-Types-Arthroscopes-Neuroendoscopes-Cystoscope-and-Bro.html (accessed on 7 October 2020).
- Buia, A.; Stockhausen, F.; Hanisch, E. Laparoscopic surgery: A qualified systematic review. World J. Methodol. 2015, 5, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Våpenstad, C.; Buzink, S.N. Procedural virtual reality simulation in minimally invasive surgery. Surg. Endosc. 2013, 27, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Wijn, R.P.W.F.; Persoon, M.C.; Schout, B.M.A.; Martens, E.J.; Scherpbier, A.J.J.A.; Hendrikx, A.J.M. Virtual Reality Laparoscopic Nephrectomy Simulator Is Lacking in Construct Validity. J. Endourol. 2010, 24, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Operative Laparoscopy: Overview, Periprocedural Care, Technique. Available online: https://emedicine.medscape.com/article/1848486-overview (accessed on 25 May 2020).
- Dubois, P.; Thommen, Q.; Jambon, A.C. In vivo measurement of surgical gestures. IEEE Trans. Biomed. Eng. 2002, 49, 49–54. [Google Scholar] [CrossRef]
- Yiasemidou, M.; de Siqueira, J.; Tomlinson, J.; Glassman, D.; Stock, S.; Gough, M. “Take-home” box trainers are an effective alternative to virtual reality simulators. J. Surg. Res. 2017, 213, 69–74. [Google Scholar] [CrossRef]
- Demi, B.; Ortmaier, T.; Seibold, U. The touch and feel in minimally invasive surgery. In Proceedings of the International Workshop on Haptic Audio-Visual Environments and Their Applications, Ottawa, ON, Canada, 1 October 2005; p. 6. [Google Scholar]
- Trejos, A.L.; Patel, R.V.; Naish, M.D. Force sensing and its application in minimally invasive surgery and therapy: A survey. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1435–1454. [Google Scholar] [CrossRef]
- Nisky, I.; Huang, F.; Milstein, A.; Pugh, C.M.; Mussa-ivaldi, F.A.; Karniel, A. Perception of Stiffness in Laparoscopy—The Fulcrum Effect. Stud. Health Technol. Inform. 2012, 173, 313–319. [Google Scholar]
- Gallagher; McClure; McGuigan; Crothers; Browning Virtual Reality Training in Laparoscopic Surgery: A Preliminary Assessment of Minimally Invasive Surgical Trainer Virtual Reality (MIST VR). Endoscopy 1999, 31, 310–313. [CrossRef]
- Puangmali, P.; Althoefer, K.; Seneviratne, L.D.; Murphy, D.; Dasgupta, P. State-of-the-Art in Force and Tactile Sensing for Minimally Invasive Surgery. IEEE Sens. J. 2008, 8, 371–381. [Google Scholar] [CrossRef]
- Tavakoli, M.; Patel, R.V.; Moallem, M. Robotic suturing forces in the presence of haptic feedback and sensory substitution. In Proceedings of the 2005 Conference on Control Applications, 2005 CCA, Ottawa, ON, Canada, 28–31 August 2005; pp. 1–6. [Google Scholar]
- Barrie, J.; Jayne, D.G.; Neville, A.; Hunter, L.; Hood, A.J.; Culmer, P.R. Real-Time Measurement of the Tool-Tissue Interaction in Minimally Invasive Abdominal Surgery: The First Step to Developing the Next Generation of Smart Laparoscopic Instruments. Surg. Innov. 2016, 23, 463–468. [Google Scholar] [CrossRef]
- Adamsen, S.; Hansen, O.H.; Funch-Jensen, P.; Schulze, S.; Stage, J.G.; Wara, P. Bile duct injury during laparoscopic cholecystectomy: A prospective nationwide series. J. Am. Coll. Surg. 1997, 184, 571–578. [Google Scholar]
- Deziel, D.J.; Millikan, K.W.; Economou, S.G.; Doolas, A.; Ko, S.T.; Airan, M.C. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am. J. Surg. 1993, 165, 9–14. [Google Scholar] [CrossRef]
- Hugh, T.B. New strategies to prevent laparoscopic bile duct injury—Surgeons can learn from pilots. Surgery 2002, 132, 826–835. [Google Scholar] [CrossRef] [PubMed]
- van der Voort, M.; Heijnsdijk, E.A.; Gouma, D.J. Bowel injury as a complication of laparoscopy. Br. J. Surg. 2004, 91, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ido, K.; Kumagai, M.; Isoda, N.; Hozumi, M.; Nagamine, N.; Ono, K.; Shibusawa, H.; Togashi, K.; Sugano, K. Laparoscopic adhesiolysis for recurrent small bowel obstruction: Long-term follow-up. Gastrointest. Endosc. 2001, 54, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.; Klein, J.; Teber, D.; Schulze, M.; Frede, T. Mechanical Simulators for Training for Laparoscopic Surgery in Urology. J. Endourol. 2007, 21, 252–262. [Google Scholar] [CrossRef]
- Aggarwal, R.; Moorthy, K.; Darzi, A. Laparoscopic skills training and assessment. Br. J. Surg. 2004, 91, 1549–1558. [Google Scholar] [CrossRef]
- Seymour, N.E.; Gallagher, A.G.; Roman, S.A.; O’Brien, M.K.; Bansal, V.K.; Andersen, D.K.; Satava, R.M. Virtual Reality Training Improves Operating Room Performance: Results of a Randomized, Double-Blinded Study. Ann. Surg. 2002, 236, 458–464. [Google Scholar] [CrossRef]
- Bann, S.; Darzi, A.; Munz, Y.; Kumar, B.D.; Moorthy, K. Laparoscopic virtual reality and box trainers: Is one superior to the other? Surg. Endosc. 2004, 18, 485–494. [Google Scholar] [CrossRef]
- Tholey, G.; Pillarisetti, A.; Green, W.; Desai, J.P. Design, Development, and Testing of an Automated Laparoscopic Grasper with 3-D Force Measurement Capability. In Medical Simulation; Cotin, S., Metaxas, D., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3078, pp. 38–48. ISBN 978-3-540-22186-9. [Google Scholar]
- Soriero, D.; Atzori, G.; Barra, F.; Pertile, D.; Massobrio, A.; Conti, L.; Gusmini, D.; Epis, L.; Gallo, M.; Banchini, F.; et al. Development and Validation of a Homemade, Low-Cost Laparoscopic Simulator for Resident Surgeons (LABOT). Int. J. Environ. Res. Public Health 2020, 17, 323. [Google Scholar] [CrossRef]
- Zihni, A.M.; Ohu, I.; Cavallo, J.A.; Ousley, J.; Cho, S.; Awad, M.M. FLS tasks can be used as an ergonomic discriminator between laparoscopic and robotic surgery. Surg. Endosc. 2014, 28, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Travascio, F.; Onar-Thomas, A.; Asfour, S. Effect of Visual Display Location on Human Performance in Simulated Laparoscopic Tasks. J. Ergon. 2014, 4. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Higgins, W. A Comparison of Complementary and Kalman Filtering. IEEE Trans. Aerosp. Electron. Syst. 1975, AES-11, 321–325. [Google Scholar] [CrossRef]
- FlexiForce Integration Guides. Available online: https://www.tekscan.com/flexiforce-integration-guides (accessed on 18 June 2020).
- Hardon, S.F.; Horeman, T.; Bonjer, H.J.; Meijerink, W.J.H.J. Force-based learning curve tracking in fundamental laparoscopic skills training. Surg. Endosc. 2018, 32, 3609–3621. [Google Scholar] [CrossRef] [PubMed]
- McDougall, E.M.; Corica, F.A.; Boker, J.R.; Sala, L.G.; Stoliar, G.; Borin, J.F.; Chu, F.T.; Clayman, R.V. Construct Validity Testing of a Laparoscopic Surgical Simulator. J. Am. Coll. Surg. 2006, 202, 779–787. [Google Scholar] [CrossRef]
- Gallagher, A.G.; Smith, C.D.; Bowers, S.P.; Seymour, N.E.; Pearson, A.; McNatt, S.; Hananel, D.; Satava, R.M. Psychomotor skills assessment in practicing surgeons experienced in performing advanced laparoscopic procedures. J. Am. Coll. Surg. 2003, 197, 479–488. [Google Scholar] [CrossRef]
- Baumgart, F. Stiffness—An unknown world of mechanical science? Injury 2000, 31, 14–84. [Google Scholar] [CrossRef]
- Ikuta, K.; Kato, T.; Ooe, H.; Ando, S. “Surgery recorder system” for recording position and force of forceps during laparoscopic surgery. In Proceedings of the 2007 International Conference on Advanced Intelligent Mechatronics, ETH Zürich, Switzerland, 4–7 September 2007; pp. 1–6. [Google Scholar]
- Brown, J.D.; Rosen, J.; Moreyra, M.; Sinanan, M.; Hannaford, B. Computer-controlled motorized endoscopic grasper for in vivo measurement of soft tissue biomechanical characteristics. Stud. Health Technol. Inform. 2002, 85, 71–73. [Google Scholar]
- Tatar, F.; Mollinger, J.R.; Bastemeijer, J.; Bossche, A. Time of flight technique used for measuring position and orientation of laparoscopic surgery tools. In Proceedings of the Sensors, Vienna, Austria, 24–27 October 2004; pp. 1480–1483. [Google Scholar]
- Wolpert, S.; Bosseau Murray, W.; Gorman, P.J.; Bholat, O.S. Movement trajectories in laparoscopic tools. In Proceedings of the First Joint BMES/EMBS Conference. 1999 Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society (Cat. No.99CH37015), Atlanta, GA, USA, 13–16 October 1999; Volume 2, p. 861. [Google Scholar]
- Yamaguchi, S.; Yoshida, D.; Kenmotsu, H.; Yasunaga, T.; Konishi, K.; Ieiri, S.; Nakashima, H.; Tanoue, K.; Hashizume, M. Objective assessment of laparoscopic suturing skills using a motion-tracking system. Surg. Endosc. 2011, 25, 771–775. [Google Scholar] [CrossRef]
- Berkelman, P.; Ma, J. A Compact Modular Teleoperated Robotic System for Laparoscopic Surgery. Int. J. Robot. Res. 2009, 28, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Joerger, G.; Huang, A.; Bass, B.; Dunkin, B.; Garbey, M. Global Laparoscopy Positioning System with a Smart Trocar. In Proceedings of the 2017 17th International Conference on Bioinformatics and Bioengineering (BIBE), Washington, DC, USA, 23–25 October 2017; pp. 359–366. [Google Scholar]
- Nakamoto, M.; Nakada, K.; Sato, Y.; Konishi, K.; Hashizume, M.; Tamura, S. Intraoperative Magnetic Tracker Calibration Using a Magneto-Optic Hybrid Tracker for 3-D Ultrasound-Based Navigation in Laparoscopic Surgery. IEEE Trans. Med. Imaging 2008, 27, 255–270. [Google Scholar] [CrossRef]
- Viriyasiripong, S.; Lopez, A.; Mandava, S.H.; Lai, W.R.; Mitchell, G.C.; Boonjindasup, A.; Powers, M.K.; Silberstein, J.L.; Lee, B.R. Accelerometer Measurement of Head Movement During Laparoscopic Surgery as a Tool to Evaluate Skill Development of Surgeons. J. Surg. Educ. 2016, 73, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Rodríguez, O.; Sánchez, R.; Benítez, G.; Pena, R.; Salamo, O.; Baez, V. Laparoscopic Surgery Skills Evaluation: Analysis Based on Accelerometers. JSLS 2014, 18, e2014.00234. [Google Scholar] [CrossRef]
- Thai, M.T.; Phan, P.T.; Hoang, T.T.; Wong, S.; Lovell, N.H.; Do, T.N. Advanced Intelligent Systems for Surgical Robotics. Adv. Intell. Syst. 2020, 2, 1900138. [Google Scholar] [CrossRef]
- Araki, A.; Makiyama, K.; Yamanaka, H.; Ueno, D.; Osaka, K.; Nagasaka, M.; Yamada, T.; Yao, M. Comparison of the performance of experienced and novice surgeons: Measurement of gripping force during laparoscopic surgery performed on pigs using forceps with pressure sensors. Surg. Endosc. 2017, 31, 1999–2005. [Google Scholar] [CrossRef]
- van den Dobbelsteen, J.J.; Lee, R.A.; van Noorden, M.; Dankelman, J. Indirect measurement of pinch and pull forces at the shaft of laparoscopic graspers. Med. Biol. Eng. Comput. 2012, 50, 215–221. [Google Scholar] [CrossRef][Green Version]
- Fontanelli, G.A.; Buonocore, L.R.; Ficuciello, F.; Villani, L.; Siciliano, B. A novel force sensing integrated into the trocar for minimally invasive robotic surgery. In Proceedings of the 2017 International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 131–136. [Google Scholar]
- Peirs, J.; Clijnen, J.; Reynaerts, D.; Brussel, H.V.; Herijgers, P.; Corteville, B.; Boone, S. A micro optical force sensor for force feedback during minimally invasive robotic surgery. Sens. Actuators Phys. 2004, 115, 447–455. [Google Scholar] [CrossRef]
- Trejos, A.L.; Patel, R.V.; Naish, M.D.; Lyle, A.C.; Schlachta, C.M. A Sensorized Instrument for Skills Assessment and Training in Minimally Invasive Surgery. J. Med. Devices 2009, 3, 041002. [Google Scholar] [CrossRef]
- Yamanaka, H.; Makiyama, K.; Osaka, K.; Nagasaka, M.; Ogata, M.; Yamada, T.; Kubota, Y. Measurement of the Physical Properties during Laparoscopic Surgery Performed on Pigs by Using Forceps with Pressure Sensors. Adv. Urol. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Callaghan, D.; Semenova, Y.; Rajan, G.; Farrell, G. Photonic crystal fiber strain sensors for laparoscopic surgical devices. In Proceedings of the 2012 Photonics Global Conference (PGC), Singapore, 13–16 December 2012; pp. 1–4. [Google Scholar]
- Hanna, G.B.; Drew, T.; Arnold, G.; Fakhry, M.; Cuschieri, A. Development of force measurement system for clinical use in minimal access surgery. Surg. Endosc. 2008, 22, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Barrie, J.; Russell, L.; Hood, A.J.; Jayne, D.G.; Neville, A.; Culmer, P.R. An in vivo analysis of safe laparoscopic grasping thresholds for colorectal surgery. Surg. Endosc. 2018, 32, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovic, B.; Fahy, A.S.; Carrillo, B.; Nasr, A.; Gerstle, J.T.; Azzie, G. Development of an Open-Source Laparoscopic Simulator Capable of Motion and Force Assessment: High Tech at Low Cost. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Reddy, N.P.; Batur, P. Forces in surgical tools: Comparison between laparoscopic and surgical forceps. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1997; Volume 1, pp. 223–224. [Google Scholar]
- Cavusoglu, M.C.; Tendick, F.; Cohn, M.; Sastry, S.S. A laparoscopic telesurgical workstation. IEEE Trans. Robot. Autom. 1999, 15, 728–739. [Google Scholar] [CrossRef]
- PC-ISO: A Biocompatible Polycarbonate 3D Printing Material. Available online: https://www.stratasys.com/materials/search/pc-iso (accessed on 26 May 2020).
- Tholey, G.; Desai, J.P. A Modular, Automated Laparoscopic Grasper with Three-Dimensional Force Measurement Capability. In Proceedings of the 2007 International Conference on Robotics and Automation, Roma, Italy, 10–14 April 2007; pp. 250–255. [Google Scholar]
- Flores-Villalba, E.; Díaz-Elizondo, J.A.; Leyva-Alvizo, A.; Fernandez-Rangel, E.; Villegas-Cabello, O.; del Real-Romo, Z. Evaluación de un simulador quirúrgico en función de su desempeño al ser utilizado por residentes con diferentes grados de experiencia. Cir. Cir. 2012, 80, 157–161. [Google Scholar]
- LAP-X, Laparoscopy Simulator|MEDICAL-X. Available online: https://www.medical-x.com/products/lap_x/ (accessed on 26 May 2020).
- Laplay Laparoscopic Trainer Simulator Suture Practice Pad 1080 HD Straight-Bar Camera for Doctor Medical Students Nurse Vet. Available online: https://franceconfiance.com/products/laplay-laparoscopic-trainer-simulator-suture-practice-pad-1080-hd-straight-bar-camera-for-doctor-medical-students-nurse-vet (accessed on 26 May 2020).
- Estebanez, B.; del Saz-Orozco, P.; Rivas, I.; Bauzano, E.; Munoz, V.F.; Garcia-Morales, I. Maneuvers recognition in laparoscopic surgery: Artificial Neural Network and hidden Markov model approaches. In Proceedings of the 2012 4th RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1164–1169. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivas-Alanis, L.H.; Calzada-Briseño, R.A.; Segura-Ibarra, V.; Vázquez, E.V.; Diaz-Elizondo, J.A.; Flores-Villalba, E.; Rodriguez, C.A. LAPKaans: Tool-Motion Tracking and Gripping Force-Sensing Modular Smart Laparoscopic Training System. Sensors 2020, 20, 6937. https://doi.org/10.3390/s20236937
Olivas-Alanis LH, Calzada-Briseño RA, Segura-Ibarra V, Vázquez EV, Diaz-Elizondo JA, Flores-Villalba E, Rodriguez CA. LAPKaans: Tool-Motion Tracking and Gripping Force-Sensing Modular Smart Laparoscopic Training System. Sensors. 2020; 20(23):6937. https://doi.org/10.3390/s20236937
Chicago/Turabian StyleOlivas-Alanis, Luis H., Ricardo A. Calzada-Briseño, Victor Segura-Ibarra, Elisa V. Vázquez, Jose A. Diaz-Elizondo, Eduardo Flores-Villalba, and Ciro A. Rodriguez. 2020. "LAPKaans: Tool-Motion Tracking and Gripping Force-Sensing Modular Smart Laparoscopic Training System" Sensors 20, no. 23: 6937. https://doi.org/10.3390/s20236937
APA StyleOlivas-Alanis, L. H., Calzada-Briseño, R. A., Segura-Ibarra, V., Vázquez, E. V., Diaz-Elizondo, J. A., Flores-Villalba, E., & Rodriguez, C. A. (2020). LAPKaans: Tool-Motion Tracking and Gripping Force-Sensing Modular Smart Laparoscopic Training System. Sensors, 20(23), 6937. https://doi.org/10.3390/s20236937






