Effect of Thiol Molecular Structure on the Sensitivity of Gold Nanoparticle-Based Chemiresistors toward Carbonyl Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thiol Ligand Syntheses
2.3. Thiol-Functionalized AuNP Synthesis
2.4. Fabrication of Interdigitated Electrodes and Thiol-Coated AuNP Chemiresistors
2.5. Sensor Measurements
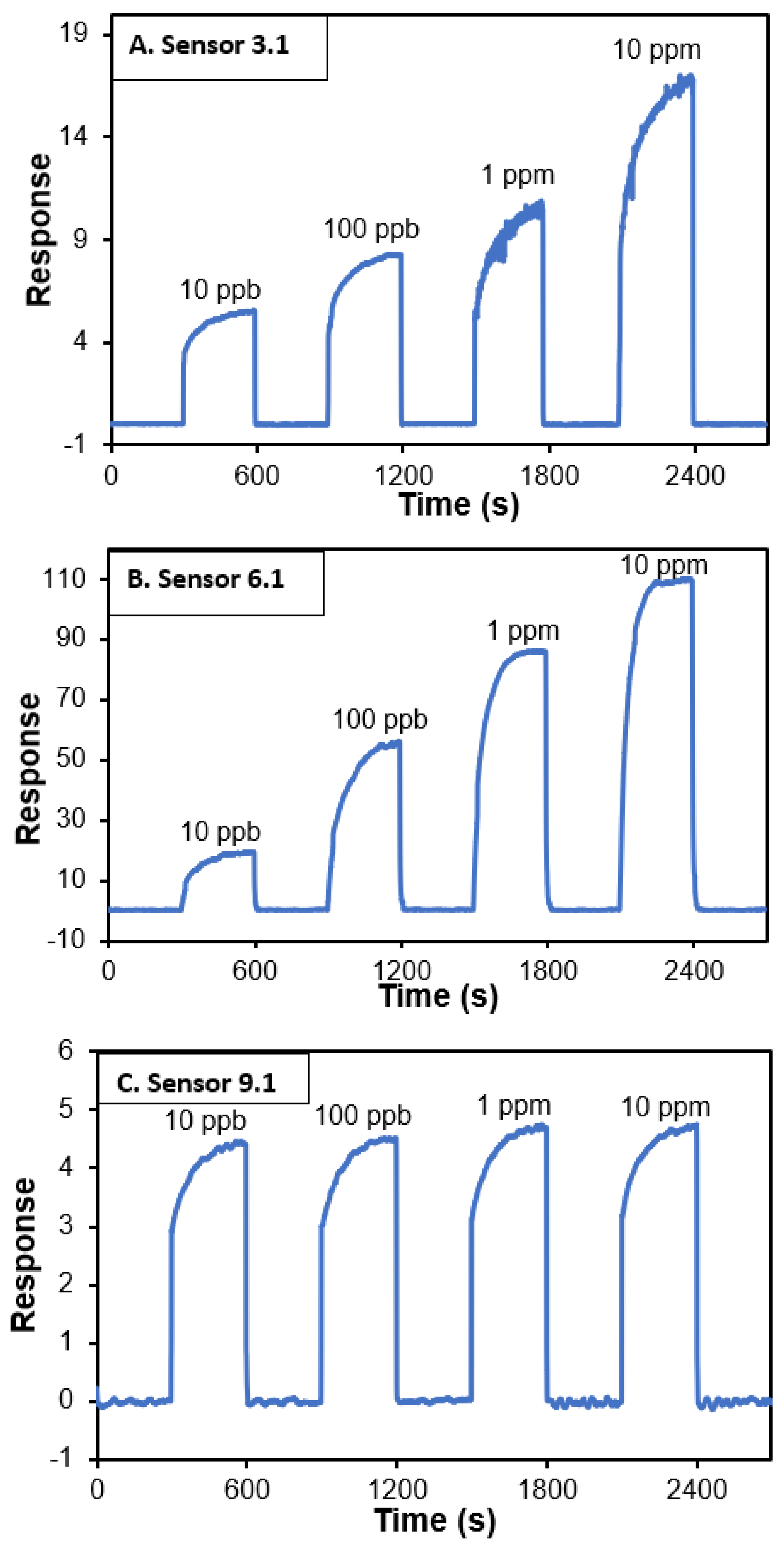
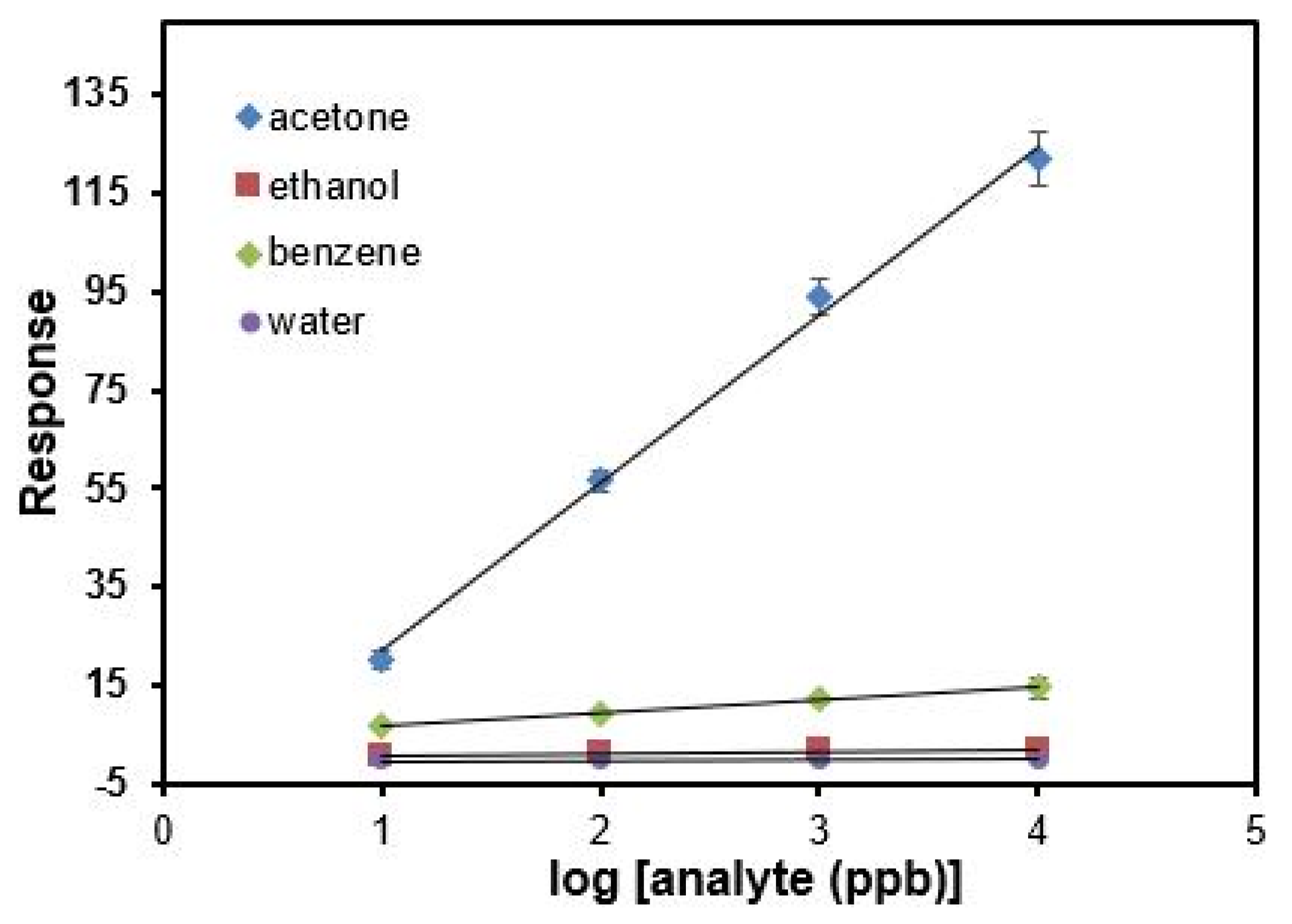
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, P.; Xia, H.; Dai, Y.Z.; Yang, H.; Liu, T. Microsensor based on gold nanoparticles for fast and sensitive ortho-xylene detection. IEEE Sens. J. 2020, 20, 12552–12557. [Google Scholar] [CrossRef]
- Tang, C.; Ku, K.H.; Luo, S.X.; Concellon, A.; Wu, Y.C.; Lu, R.Q.; Swager, T.M. Chelating phosphine ligand stabilized AuNPs in methane detection. ACS Nano 2020, 14, 11605–11612. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, F.J.; Zamborini, F.P. Chemiresistive sensing with chemically modified metal and alloy nanoparticles. Small 2012, 8, 174–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.; Agasti, S.; Kim, C.; Li, X.; Rotello, V. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Wohltjen, H.; Snow, A.W. Colloidal metal-insulator-metal ensemble chemiresistor sensor. Anal. Chem. 1998, 70, 2856–2859. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Peled, N.; Haick, H. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Grzelczak, M.; Liz-Marzan, L.; Klajn, R. Stimuli-response self-assembly of nanoparticles. Chem. Soc. Rev. 2019, 48, 1342–1361. [Google Scholar] [CrossRef] [Green Version]
- Kareem, H.; Shan, S.; Wu, Z.P.; Velasco, L.; Moseman, K.; O’Brien, C.; Tran, D.T.; Lee, I.C.; Maswadeh, Y.; Yang, L.; et al. Catalytic oxidation of propane over palladium alloyed with gold: An assessment of the chemical and intermediate species. Catal. Sci. Technol. 2018, 8, 6228–6240. [Google Scholar]
- Sibakoti, T.R.; Jasinski, J.B.; Nantz, M.H.; Zamborini, F.P. Iodine activation: A general method for catalytic enhancement of thiolate monolayer-protected metal clusters. Nanoscale 2020, 12, 12027–12037. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.P. A novel approach to a fully inkjet printed SnO2-based gas sensor on a flexible foil. J. Mater. Chem. C 2019, 7, 12343–12353. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Li, P.; Li, G.; Lian, K.; Zhang, W.; Zhuiykov, S.; Hu, J. Pr6O11-functionalized SnO2 flower-like architectures for highly efficient, stable, and selective acetone detection. IEEE Sens. J. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-dimensional oxide nanostructures as gas-sensing materials: Review and issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [Green Version]
- Comini, E. Metal oxide nanowire chemical sensors: Innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Bhowmik, B.; Manjuladevi, V.; Gupta, R.K.; Bhattacharyya, P. Highly selective low-temperature acetone sensor based on hierarchical 3-D TiO2 nanoflowers. IEEE Sens. J. 2016, 16, 3488–3495. [Google Scholar] [CrossRef]
- Rella, R.; Spadavecchia, J.; Manera, M.G.; Capone, S.; Taurino, A.; Martino, M.; Caricato, A.P.; Tunno, T. Acetone and ethanol solid-state gas sensors based on TiO2 nanoparticles thin film deposited by matrix assisted pulsed laser evaporation. Sens. Actuators B 2007, 127, 426–431. [Google Scholar] [CrossRef]
- Park, J.; Shen, X.; Wang, G. Solvothermal synthesis and gas-sensing performance of Co3O4 hollow nanospheres. Sens. Actuators B 2009, 136, 494–498. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Robinson, J.A. Chemical vapor detection using single-walled carbon nanotubes. Chem. Soc. Rev. 2006, 35, 790–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Button, B.K.; Wepsiec, K.; Dmitriev, S.; Kolmakov, A. Toward the nanoscopic “Electronic nose”: Hydrogen vs carbon monoxide discrimination with an array of individual metal oxide nano- and mesowire sensors. Nano Lett. 2006, 6, 1584–1588. [Google Scholar] [CrossRef]
- Pinto, N.J.; Ramos, I.; Rojas, R.; Wang, P.C.; Johnson, A.T., Jr. Electric response of isolated electrospun polyaniline nanofibers to vapors of aliphatic alcohols. Sens. Actuators B 2008, 129, 621–627. [Google Scholar] [CrossRef]
- Li, C.; Chartuprayoon, N.; Bosze, W.; Low, K.; Lee, K.H.; Nam, J.; Myung, N.V. Electrospun polyaniline/poly(ethylene oxide) composite nanofibers based gas sensor. Electroanalysis 2014, 26, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Nakhleh, M.K.; Broza, Y.Y.; Haick, H. Monolayer-capped gold nanoparticles for disease detection from breath. Nanomedicine 2014, 9, 1991–2002. [Google Scholar] [CrossRef]
- Zhou, Y.; Khachatryan, W.; Multhoff, G.; Gao, H.L. Recent Advances in Gold Nanoformulations for Cancer Therapy. Curr. Drug Metab. 2018, 19, 768–780. [Google Scholar]
- Ibañez, F.J.; Zamborini, F.P. Chemiresistive sensing of volatile organic compounds with films of surfactant-stabilized gold and gold-silver alloy nanoparticles. ACS Nano 2008, 2, 1543–1552. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef]
- Han, L.; Shi, X.; Wu, W.; Kirk, F.L.; Luo, J.; Wang, L.; Mott, D.; Cousineau, L.; Lim, S.I.-I.; Lu, S.; et al. Nanoparticle-structured sensing array materials and pattern recognition for VOC detection. Sens. Actuators B 2005, 106, 431–441. [Google Scholar] [CrossRef]
- Krasteva, N.; Besnard, I.; Guse, B.G.; Bauer, R.E.; Müllen, K.; Yasuda, A.; Vossmeyer, T. Self-assembled gold nanoparticle/dendrimer composite films for vapor sensing applications. Nano Lett. 2002, 2, 551–555. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Zellers, E.T. Dual-chemiresistor GC detector employing monolayer-protected metal nanocluster interfaces. Anal. Chem. 2002, 74, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- IOM. Secondhand Smoke Exposure and Cardiovascular Effects; The National Academies: Washington, DC, USA, 2010; p. 228. [Google Scholar]
- U.S. EPA. National Air Toxics Trends Station Work Plan Template; U.S. Environment Protection Agency: Washington, DC, USA, 2011.
- Kaftory, M.; Kapon, M.; Botoshansky, M. Role of hydrogen bonding in determining the crystal structrues of the adducts between acetone and urea derivatives. Chem. Mater. 1994, 6, 1245–1249. [Google Scholar] [CrossRef]
- Xie, Z.; Raju, M.V.R.; Stewart, A.C.; Nantz, M.H.; Fu, X.-A. Imparting sensitivity and selectivity to a gold nanoparticle chemiresistor through thiol monolayer functionalization for sensing acetone. RSC Adv. 2018, 8, 35618–35624. [Google Scholar] [CrossRef] [Green Version]
- Paulini, R.; Frankamp, B.L.; Rotello, V.M. Effects of branched ligands on the structure and stability of monolayers on gold nanoparticles. Langmuir 2002, 18, 2368–2373. [Google Scholar] [CrossRef]
- Park, S.; Yousaf, M.N. An interfacial oxime reaction to immobilize ligands and cells in patterns and gradients to photoactive surfaces. Langmuir 2008, 24, 6201–6207. [Google Scholar] [CrossRef]
- Steemers, L.; Wanner, M.J.; Lutz, M.; Hiemstra, H.; van Maarseveen, J.H. Synthesis of spiro quasi[1]catenanes and quasi[1] rotaxanes via a templated backfolding strategy. Nat. Commun. 2017, 8, 15392. [Google Scholar] [CrossRef]
- Willwacher, J.; Rakshit, S.; Glorius, F. Investigating N-methoxy-N’-aryl ureas in oxidative C-H olefination reactions: An unexpected oxidation behaviour. Org. Biomol. Chem. 2011, 9, 4736–4740. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Li, M.; Biswas, S.; Nantz, M.H.; Higashi, R.M.; Fu, X.-A. Preconcentration and analysis of trace volatile carbonyl compounds. Anal. Chem. 2012, 84, 1288–1293. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2006; pp. 104–105. [Google Scholar]
- Smith, M.B. March’s Advanced Organic Chemistry, 7th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 182–184. [Google Scholar]
- Grekov, A.P.; Veselov, V.Y. The α-effect in the chemistry of organic compounds. Russ. Chem. Rev. 1978, 47, 631–648. [Google Scholar] [CrossRef]
- Edwards, J.O.; Pearson, R.G. The factors determining nucleophilic reactivities. J. Am. Chem. Soc. 1962, 84, 16–21. [Google Scholar] [CrossRef]
- Ruiz-Chica, A.J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Characterization by Raman spectroscopy of conformational changes on guanine-cytosine and adenine-thymine oligonucleotides induced by aminooxy analogues of spermidine. J. Raman Spectrosc. 2004, 35, 93–100. [Google Scholar] [CrossRef]
- Bordwell, F.G.; Ji, G.Z. Effects of structural changes on acidities and homolytic bond dissociation energies of the H-N bonds in amidines, carboxamides, and thiocarboxamides. J. Am. Chem. Soc. 1991, 113, 8398–8401. [Google Scholar] [CrossRef]

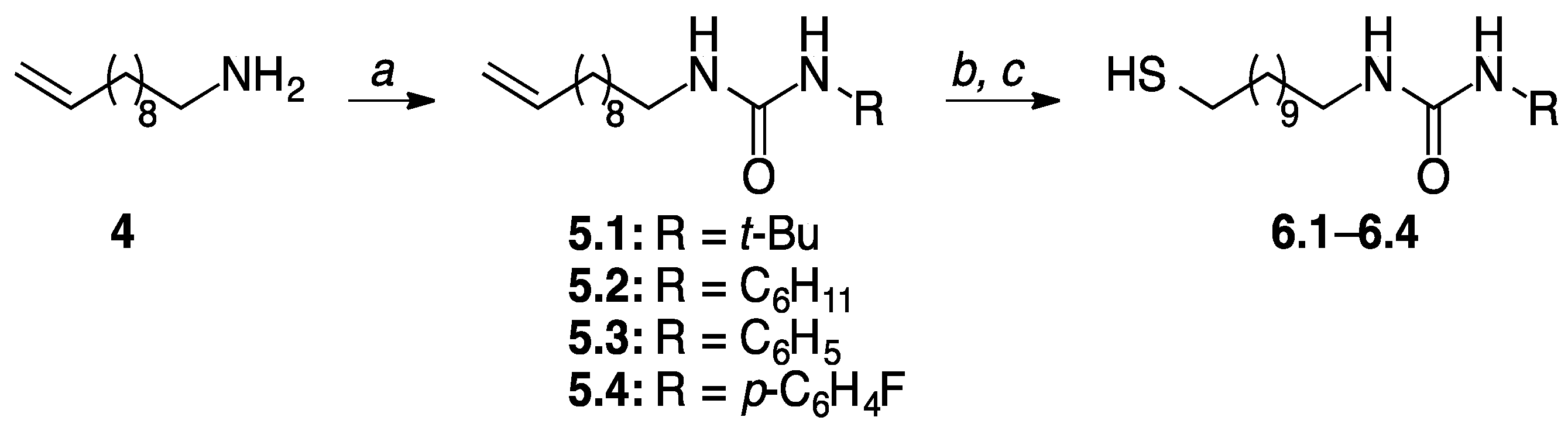
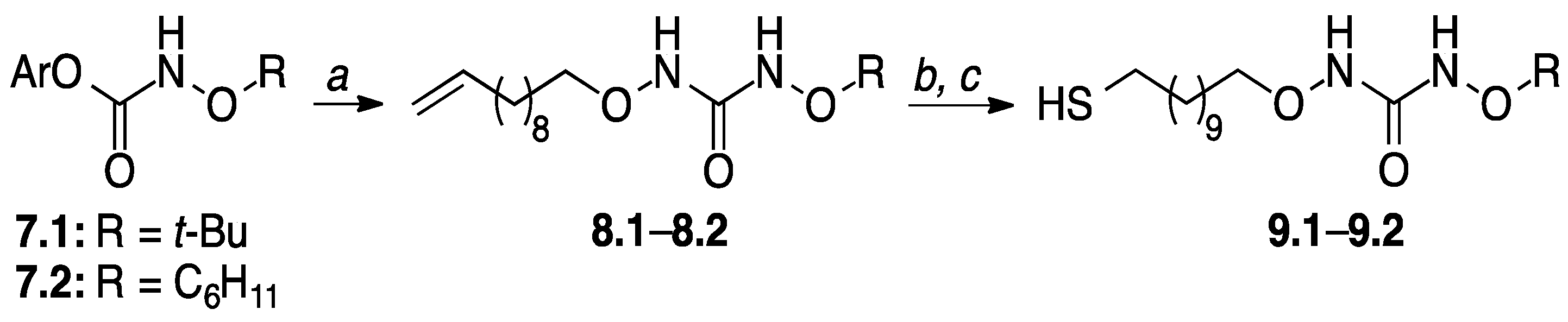
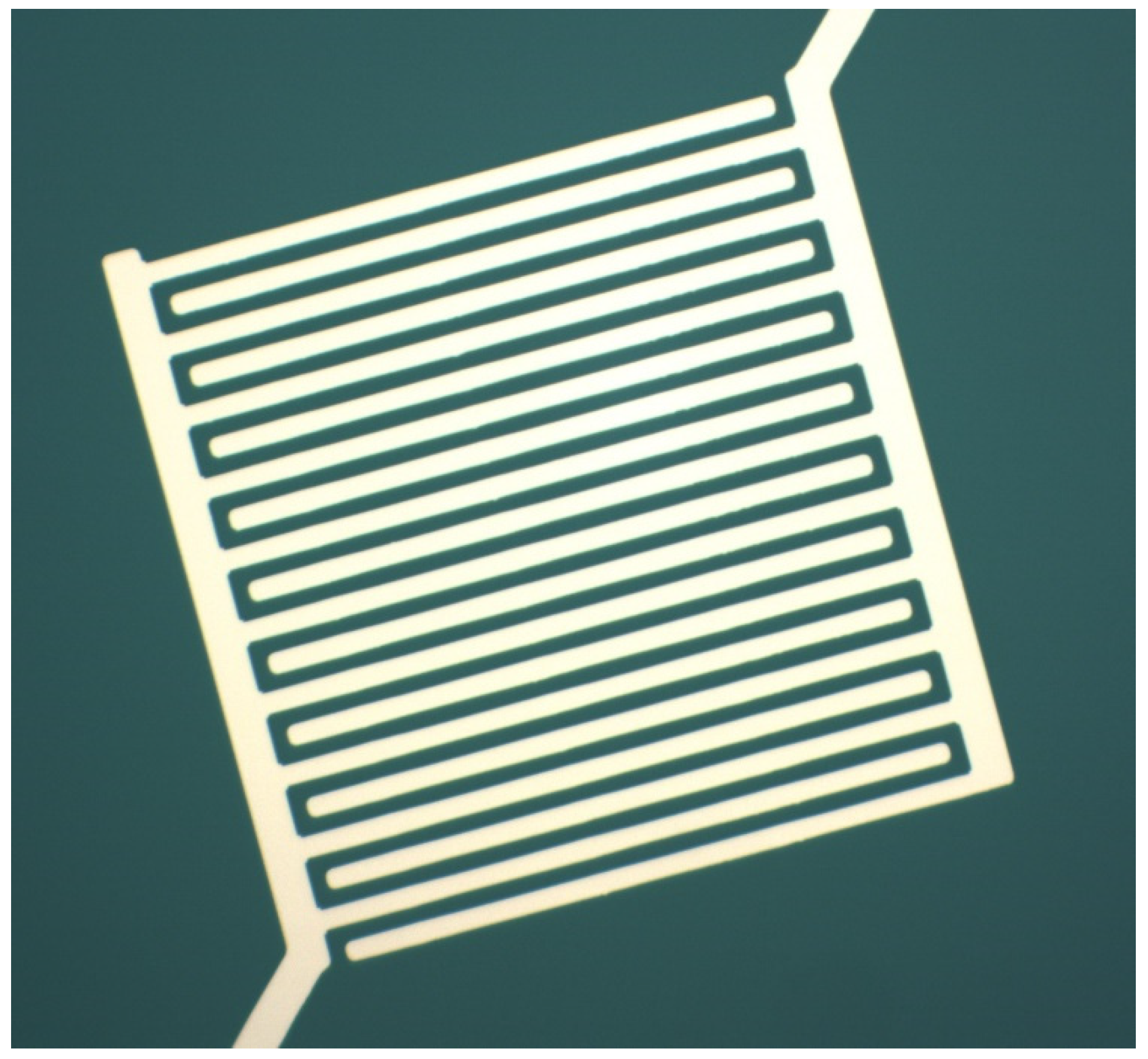
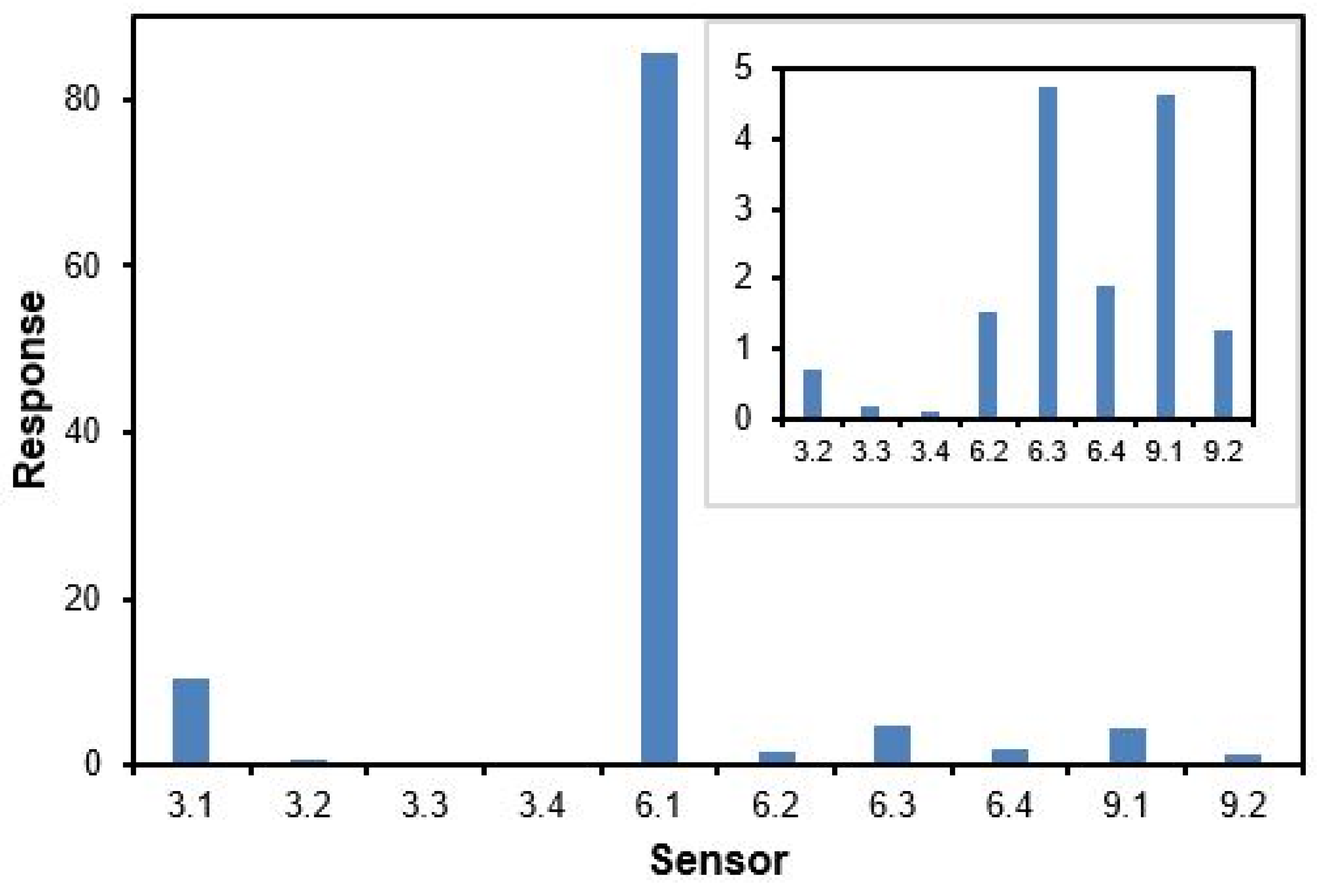
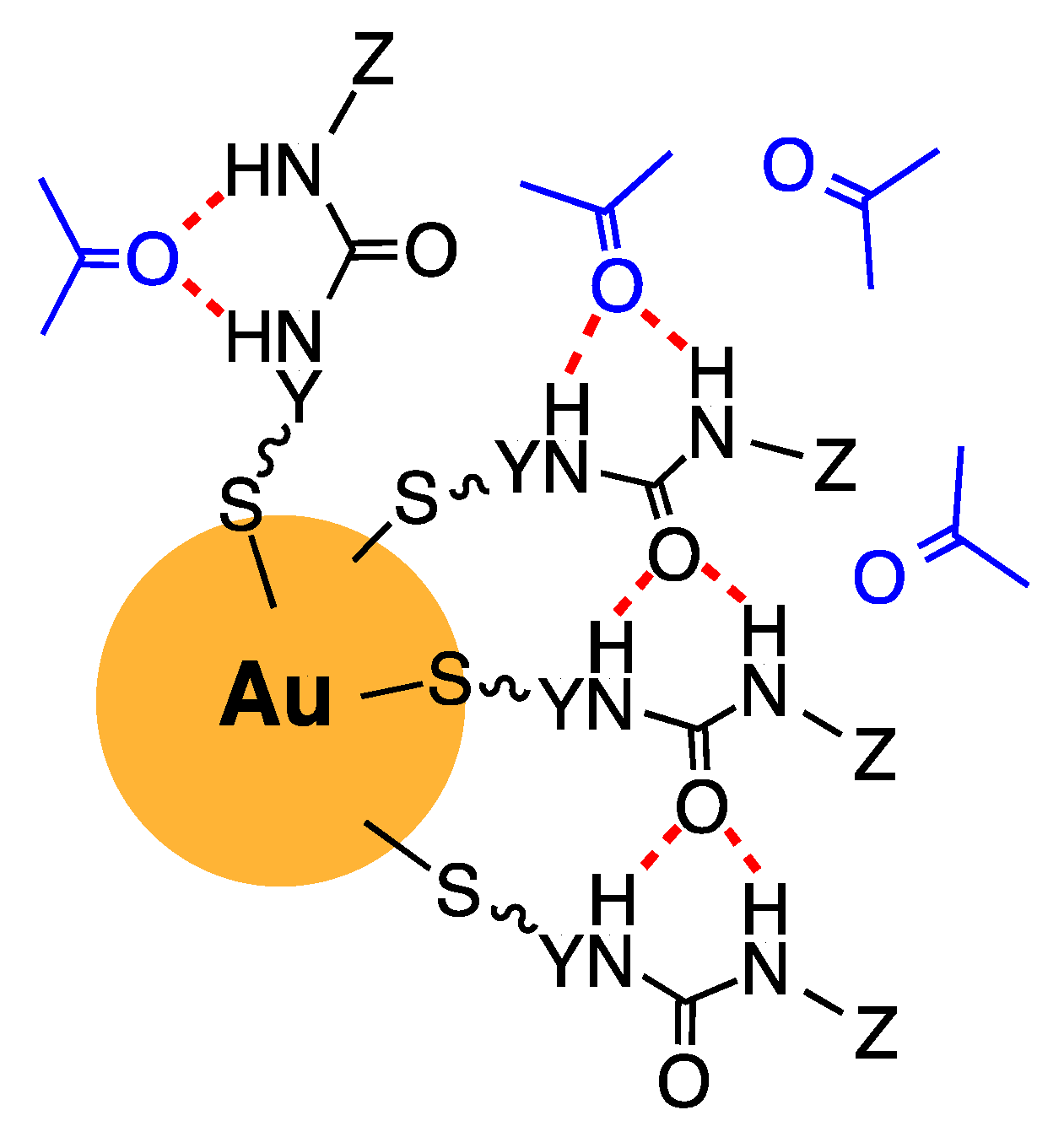
| Series I Alkoxy Alkyl (3.1–3.4) | Series II Dialkyl (6.1–6.4) | Series III Dialkoxy (9.1–9.2) |
|---|---|---|
 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Ramakrishnam Raju, M.V.; Adhihetty, P.K.; Fu, X.-A.; Nantz, M.H. Effect of Thiol Molecular Structure on the Sensitivity of Gold Nanoparticle-Based Chemiresistors toward Carbonyl Compounds. Sensors 2020, 20, 7024. https://doi.org/10.3390/s20247024
Xie Z, Ramakrishnam Raju MV, Adhihetty PK, Fu X-A, Nantz MH. Effect of Thiol Molecular Structure on the Sensitivity of Gold Nanoparticle-Based Chemiresistors toward Carbonyl Compounds. Sensors. 2020; 20(24):7024. https://doi.org/10.3390/s20247024
Chicago/Turabian StyleXie, Zhenzhen, Mandapati V. Ramakrishnam Raju, Prasadanie K. Adhihetty, Xiao-An Fu, and Michael H. Nantz. 2020. "Effect of Thiol Molecular Structure on the Sensitivity of Gold Nanoparticle-Based Chemiresistors toward Carbonyl Compounds" Sensors 20, no. 24: 7024. https://doi.org/10.3390/s20247024
APA StyleXie, Z., Ramakrishnam Raju, M. V., Adhihetty, P. K., Fu, X.-A., & Nantz, M. H. (2020). Effect of Thiol Molecular Structure on the Sensitivity of Gold Nanoparticle-Based Chemiresistors toward Carbonyl Compounds. Sensors, 20(24), 7024. https://doi.org/10.3390/s20247024





