Integrated EC-SERS Chip with Uniform Nanostructured EC-SERS Active Working Electrode for Rapid Detection of Uric Acid
Abstract
:1. Introduction
2. Materials and Methods
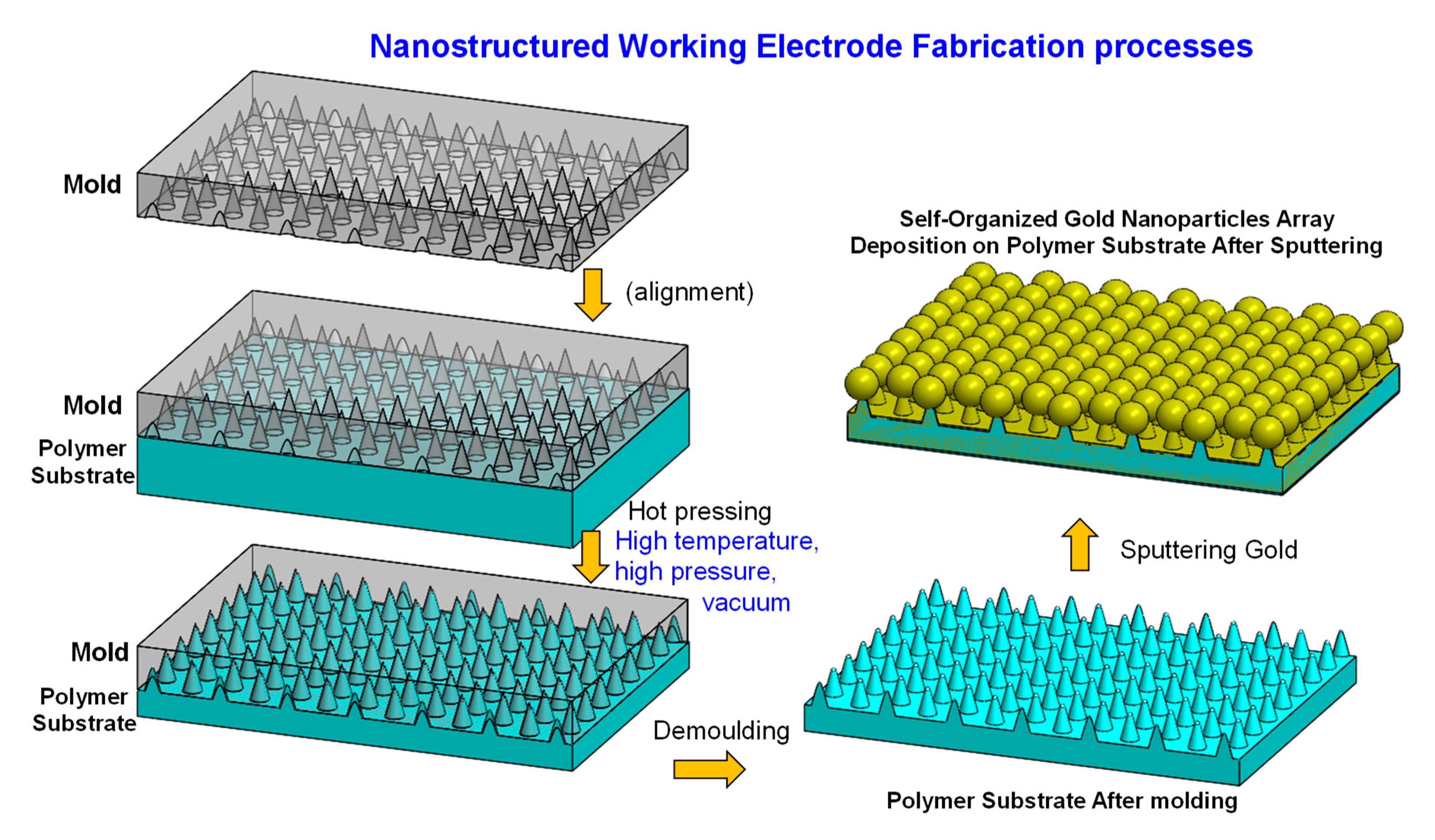
2.1. Uniform Nanostructured Working Electrode Fabrication
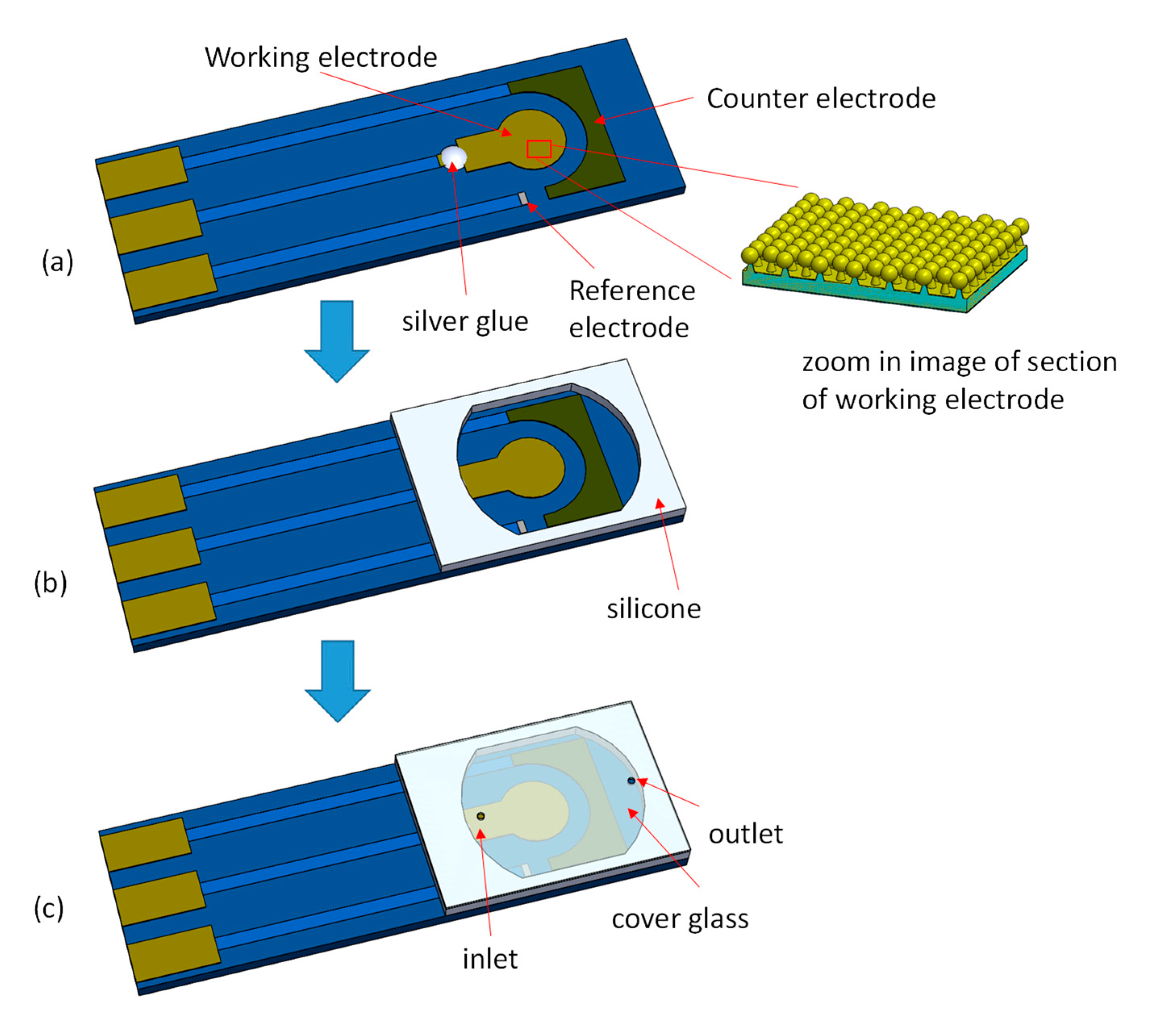
2.2. Integrated EC-SERS Chip Fabrication
2.3. Experiments
3. Results and Discussion
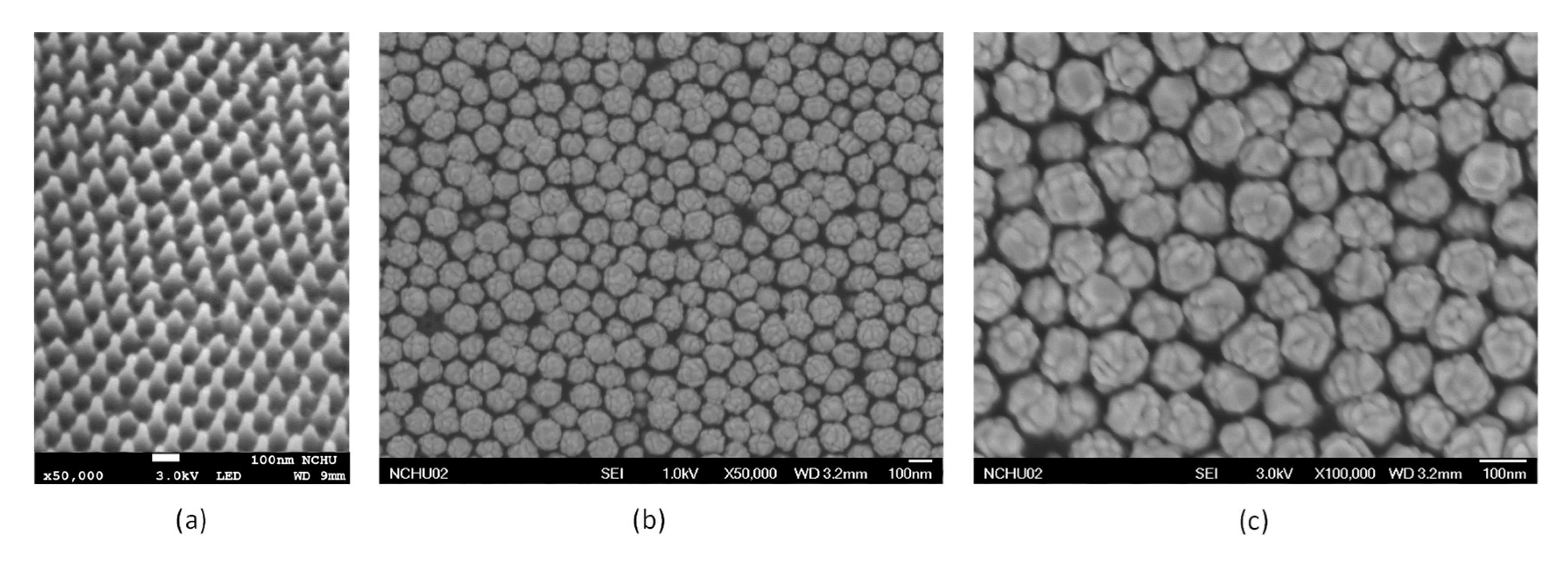
3.1. Characterization of the Developed Working Electrode Substrate
3.2. Simulations
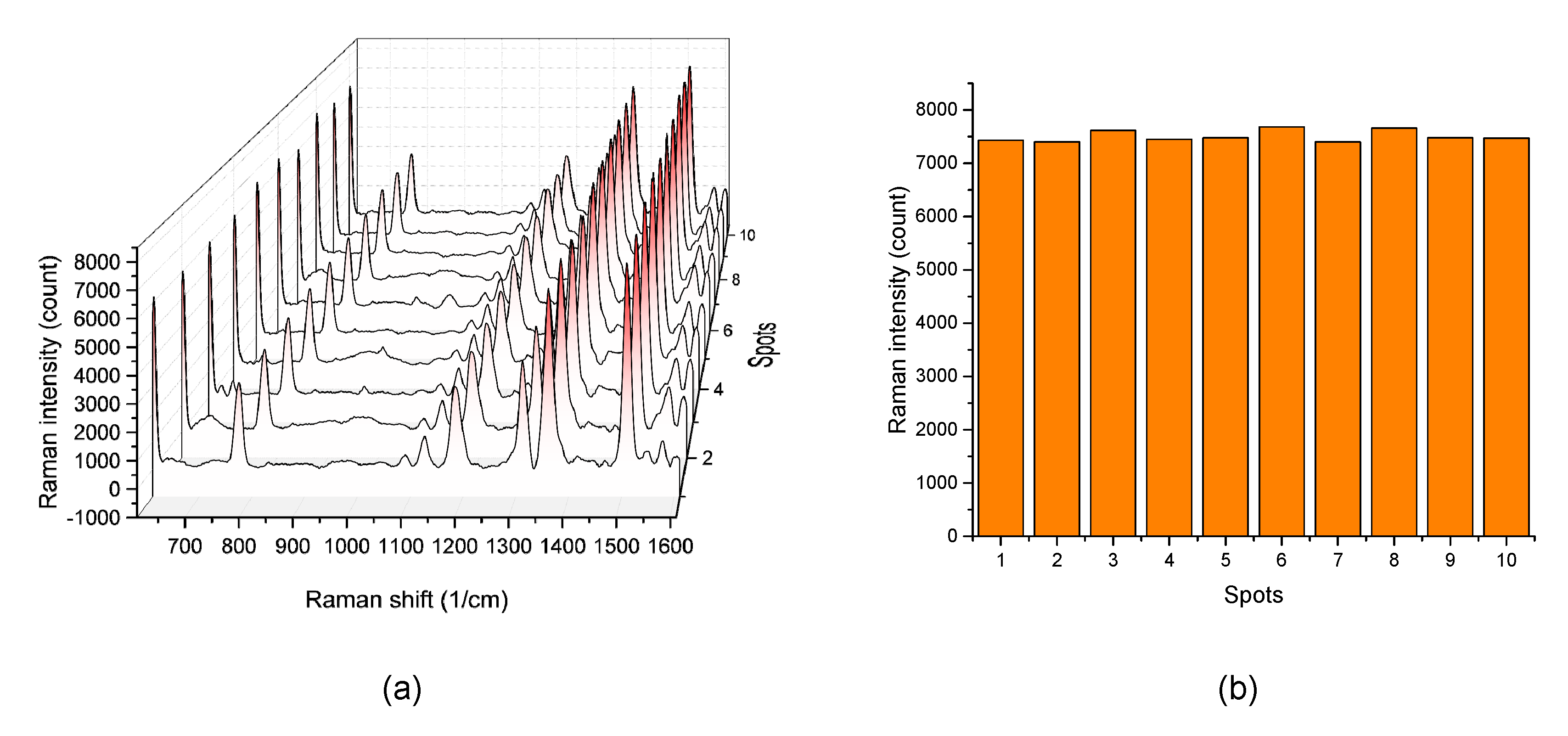
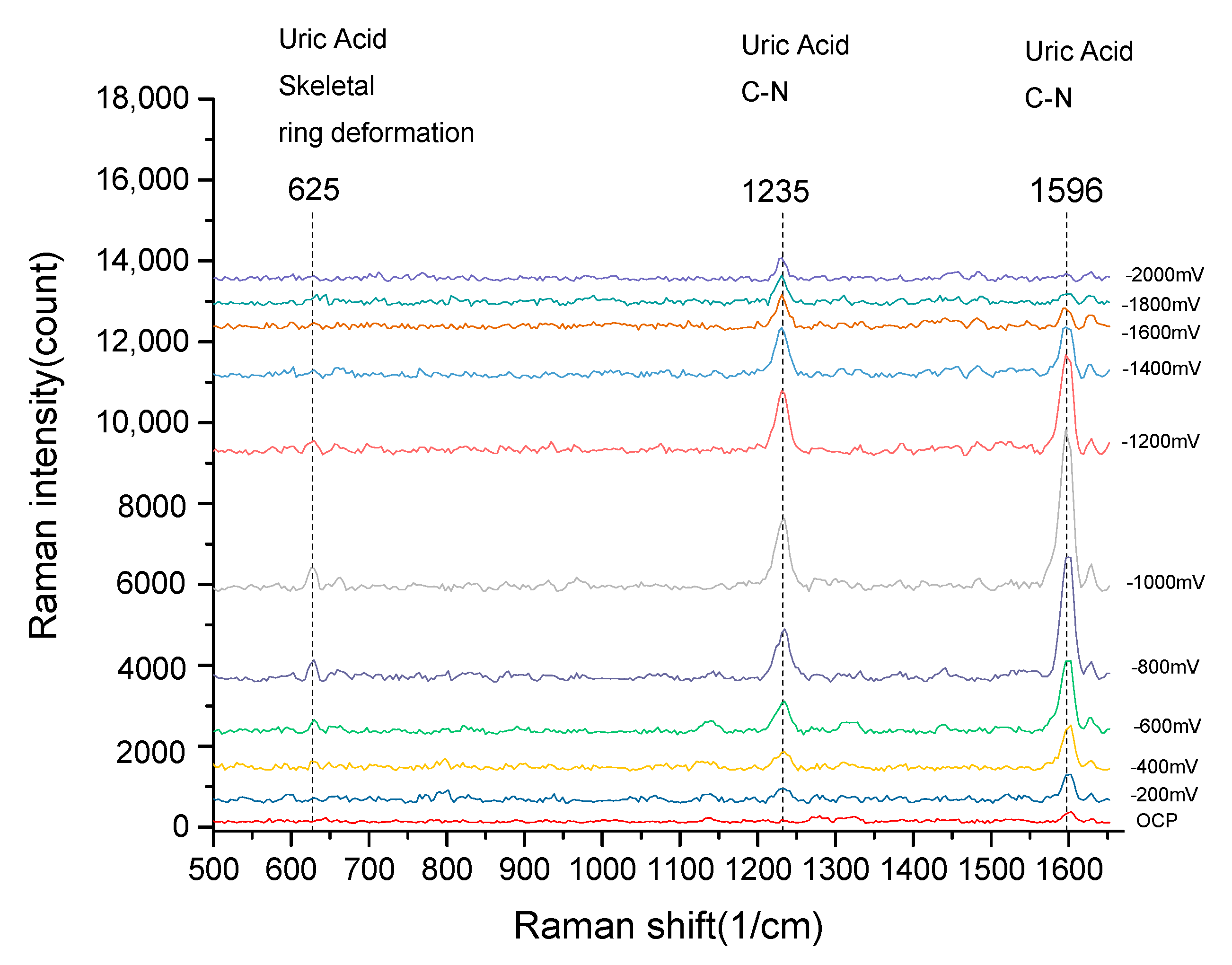
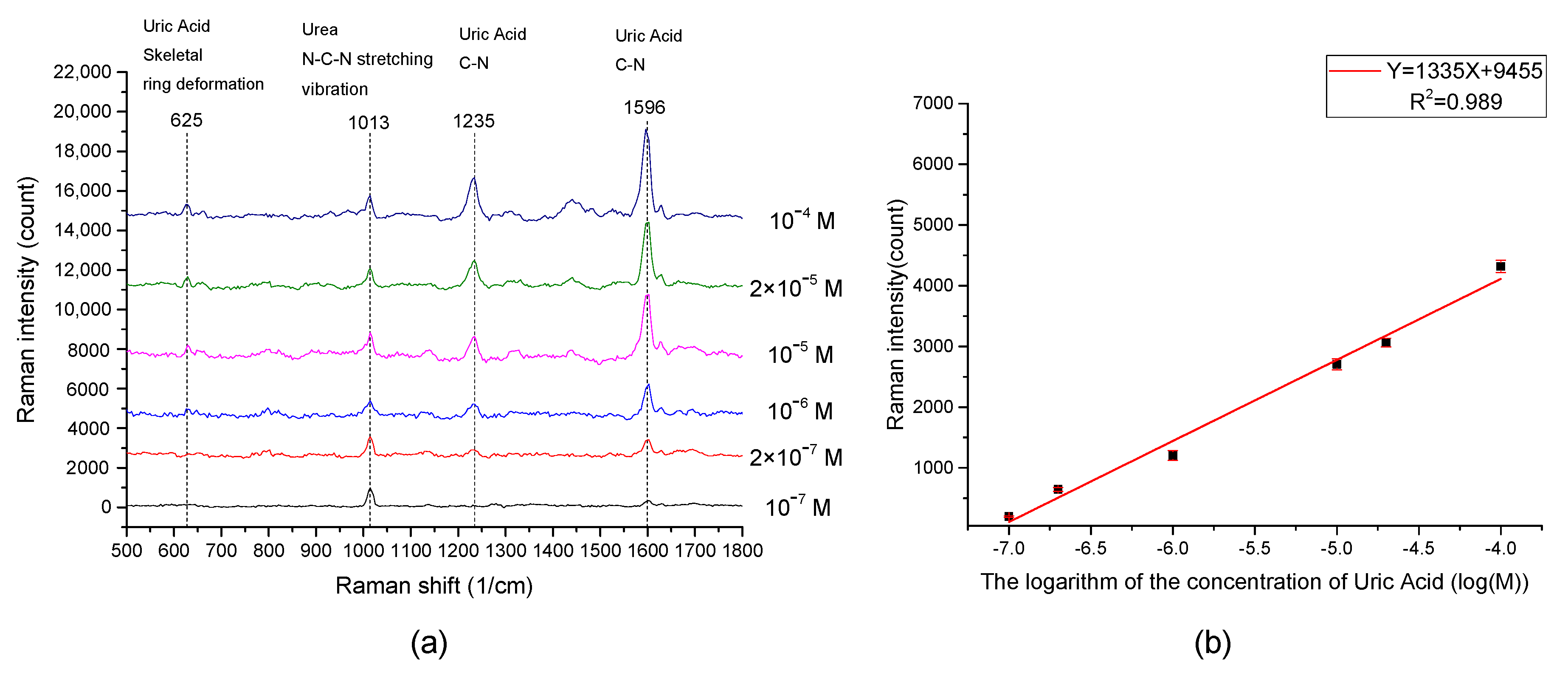
3.3. EC-SERS Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Savaj, S.; Vaziri, N.D. An overview of recent advances in pathogenesis and diagnosis of preeclampsia. Iran. J. Kidney Dis. 2012, 6, 334–338. [Google Scholar] [PubMed]
- Bainbridge, S.A.; Roberts, J.M. Uric Acid as a Pathogenic Factor in Preeclampsia. Placenta 2008, 29, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmella, M.J.; Clifton, R.G.; Althouse, A.D.; Roberts, J.M. Uric acid determination in gestational hypertension. Reprod. Sci. 2015, 22, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Norwitz, E.R.; Funai, E.; Richman, S.; Guller, S.; Lockwood, C.J.; Buhimschi, I.A. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. In Proceedings of the American Journal of Obstetrics and Gynecology; Mosby Inc.: St. Louis, MO, USA, 2005; Volume 192, pp. 734–741. [Google Scholar]
- Chen, Z.; Fang, C.; Qiu, G.; He, J.; Deng, Z. Non-enzymatic disposable test strip for detecting uric acid in whole blood. J. Electroanal. Chem. 2009, 633, 314–318. [Google Scholar] [CrossRef]
- Erden, P.E.; Kiliç, E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 2013, 107, 312–323. [Google Scholar] [CrossRef]
- Villa, J.E.L.; Poppi, R.J. A portable SERS method for the determination of uric acid using a paper-based substrate and multivariate curve resolution. Analyst 2016, 141, 1966–1972. [Google Scholar] [CrossRef] [Green Version]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute Quantification of Uric Acid in Human Urine Using Surface Enhanced Raman Scattering with the Standard Addition Method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [Green Version]
- Pucetaite, M.; Velicka, M.; Pilipavicius, J.; Beganskiene, A.; Ceponkus, J.; Sablinskas, V. Uric acid detection by means of SERS spectroscopy on dried Ag colloidal drops. J. Raman Spectrosc. 2016, 47, 681–686. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; Su, W.-N.; Chen, K.-J.; Pan, C.-J.; Rick, J.; Chang, C.-C.; Hwang, B.-J. Au@SiO2 core/shell nanoparticle assemblage used for highly sensitive SERS-based determination of glucose and uric acid. J. Raman Spectrosc. 2013, 44, 1671–1677. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; You, R.; Wu, Y.; Shen, H.; Zhu, L.; Feng, S. Superhydrophobic silver film as a SERS substrate for the detection of uric acid and creatinine. Biomed. Opt. Express 2018, 9, 4988. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Guan, C.; Shan, G.; Chen, Y.; Wang, C.; Liu, Y. A nanocomposite prepared from silver nanoparticles and carbon dots with peroxidase mimicking activity for colorimetric and SERS-based determination of uric acid. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alula, M.T.; Lemmens, P.; Bo, L.; Wulferding, D.; Yang, J.; Spende, H. Preparation of silver nanoparticles coated ZnO/Fe3O4 composites using chemical reduction method for sensitive detection of uric acid via surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2019, 1073, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, G.; Majumder, S.; Senapati, D.; Banerjee, S.; Satpati, B. Core-shell gold @silver hollow nanocubes for higher SERS enhancement and non-enzymatic biosensor. Mater. Chem. Phys. 2020, 239, 122113. [Google Scholar] [CrossRef]
- Lynk, T.P.; Sit, C.S.; Brosseau, C.L. Electrochemical Surface-Enhanced Raman Spectroscopy as a Platform for Bacterial Detection and Identification. Anal. Chem. 2018, 90, 12639–12646. [Google Scholar] [CrossRef]
- McLeod, K.E.R.; Lynk, T.P.; Sit, C.S.; Brosseau, C.L. On the origin of electrochemical surface-enhanced Raman spectroscopy (EC-SERS) signals for bacterial samples: The importance of filtered control studies in the development of new bacterial screening platforms. Anal. Methods 2019, 11, 924–929. [Google Scholar] [CrossRef]
- Bindesri, S.D.; Alhatab, D.S.; Brosseau, C.L. Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) fabric-based plasmonic sensor for point-of-care diagnostics. Analyst 2018, 143, 4128–4135. [Google Scholar] [CrossRef]
- Velička, M.; Adomavičiūtė, S.; Zacharovas, E.; Šablinskas, V. Application of label-free SERS and EC-SERS for detection of traces of drugs in biological fluids. In Proceedings of the Plasmonics in Biology and Medicine XVII; Vo-Dinh, T., Ho, H.-P.A., Ray, K., Eds.; SPIE: Bellingham, WA, USA, 2020; Volume 11257, p. 28. [Google Scholar]
- Willets, K.A. Probing nanoscale interfaces with electrochemical surface-enhanced Raman scattering. Curr. Opin. Electrochem. 2019, 13, 18–24. [Google Scholar] [CrossRef]
- Greene, B.H.C.; Alhatab, D.S.; Pye, C.C.; Brosseau, C.L. Electrochemical-Surface Enhanced Raman Spectroscopic (EC-SERS) Study of 6-Thiouric Acid: A Metabolite of the Chemotherapy Drug Azathioprine. J. Phys. Chem. C 2017, 121, 8084–8090. [Google Scholar] [CrossRef]
- Zaleski, S.; Clark, K.A.; Smith, M.M.; Eilert, J.Y.; Doty, M.; VanDuyne, R.P. Identification and Quantification of Intravenous Therapy Drugs Using Normal Raman Spectroscopy and Electrochemical Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2017, 89, 2497–2504. [Google Scholar] [CrossRef]
- Zhao, L.; Blackburn, J.; Brosseau, C.L. Quantitative detection of uric acid by electrochemical-surface enhanced raman spectroscopy using a multilayered Au/Ag substrate. Anal. Chem. 2015, 87, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Tsai, M.-S. Tunable Silver Nanoparticle Arrays by Hot Embossing and Sputter Deposition for Surface-Enhanced Raman Scattering. Appl. Sci. 2019, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Runge, J.M. The Metallurgy of Anodizing Aluminum; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Brzózka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G.D. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Tsai, M.-S. Fabrication of 3D nano-hemispherical cavity array plasmonic substrate for SERS applications. Int. J. Optomechatronics 2018, 12, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, S.; Perales-Rondon, J.V.; Heras, A.; Colina, A. Determination of uric acid in synthetic urine by using electrochemical surface oxidation enhanced Raman scattering. Anal. Chim. Acta 2019, 1085, 61–67. [Google Scholar] [CrossRef]
- Wu, S.; Shen, Y.; Jin, C. Surface-enhanced Raman scattering induced by the coupling of the guided mode with localized surface plasmon resonances. Nanoscale 2019, 11, 14164–14173. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Lu, J.; Song, Y.; Lei, F.; Du, X.; Huo, Y.; Xu, S.; Li, C.; Ning, T.; Yu, J.; Zhang, C. Electric Field-Modulated Surface Enhanced Raman Spectroscopy by PVDF/Ag Hybrid. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Litti, L.; Meneghetti, M. Predictions on the SERS enhancement factor of gold nanosphere aggregate samples. Phys. Chem. Chem. Phys 2019, 21, 15515. [Google Scholar] [CrossRef]
- LeRu, E.C.; Etchegoin, P.G. Quantifying SERS enhancements. MRS Bull. 2013, 38, 631–640. [Google Scholar] [CrossRef]
- LeRu, E.C.; Blackie, E.; Meyer, M.; Etchegoint, P.G. Surface enhanced raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- He, Y.; Song, C.; Que, L. Nanoforest-based SERS sensor fabricated using a maskless process for detecting chemical and pathogen. Microsyst. Technol. 2019. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals. Nat. Publ. Gr. 2015. [Google Scholar] [CrossRef]
- Caro, C.; Gámez, F.; Zaderenko, A.P. Preparation of surface-enhanced raman scattering substrates based on immobilized silver-capped nanoparticles. J. Spectrosc. 2018, 2018. [Google Scholar] [CrossRef]
- Sree Satya Bharati, M.; Byram, C.; Soma, V.R. Femtosecond Laser Fabricated Ag@Au and Cu@Au Alloy Nanoparticles for Surface Enhanced Raman Spectroscopy Based Trace Explosives Detection. Front. Phys. 2018, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Goodall, B.L.; Robinson, A.M.; Brosseau, C.L. Electrochemical-surface enhanced Raman spectroscopy (E-SERS) of uric acid: A potential rapid diagnostic method for early preeclampsia detection. Phys. Chem. Chem. Phys. 2013, 15, 1382–1388. [Google Scholar] [CrossRef]




| Reference Value | Found ± s (μM) | Accuracy | Precision | |
|---|---|---|---|---|
| Recovery (%) | Er (%) | |||
| Level 1 (5 μM) | 4.15 ± 0.42 | 83 | −7 | Level 1 (5 μM) |
| Level 2 (50 μM) | 48.52 ± 4.71 | 97 | −3 | Level 2 (50 μM) |
| Level 3 (95 μM) | 99.75 ± 8.35 | 105 | +5 | Level 3 (95 μM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Hsiao, H.-C. Integrated EC-SERS Chip with Uniform Nanostructured EC-SERS Active Working Electrode for Rapid Detection of Uric Acid. Sensors 2020, 20, 7066. https://doi.org/10.3390/s20247066
Huang C-Y, Hsiao H-C. Integrated EC-SERS Chip with Uniform Nanostructured EC-SERS Active Working Electrode for Rapid Detection of Uric Acid. Sensors. 2020; 20(24):7066. https://doi.org/10.3390/s20247066
Chicago/Turabian StyleHuang, Chu-Yu, and Hung-Che Hsiao. 2020. "Integrated EC-SERS Chip with Uniform Nanostructured EC-SERS Active Working Electrode for Rapid Detection of Uric Acid" Sensors 20, no. 24: 7066. https://doi.org/10.3390/s20247066






