Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples
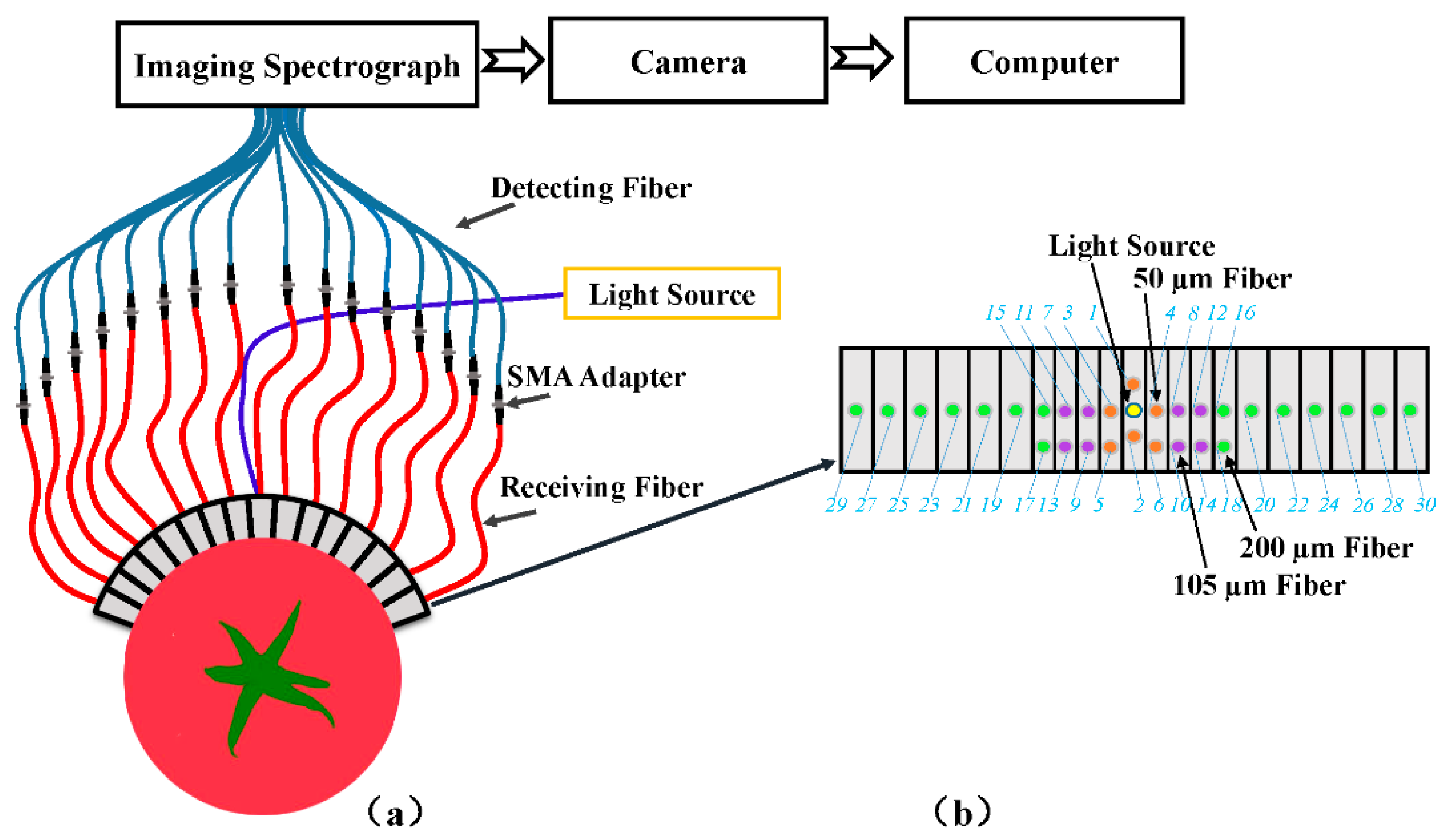
2.2. Spatially Resolved (SR) Spectra Measurement
2.3. Quality Analysis of Tomato Fruit
2.4. Tomato Maturity Classification Models
3. Results and Discussion
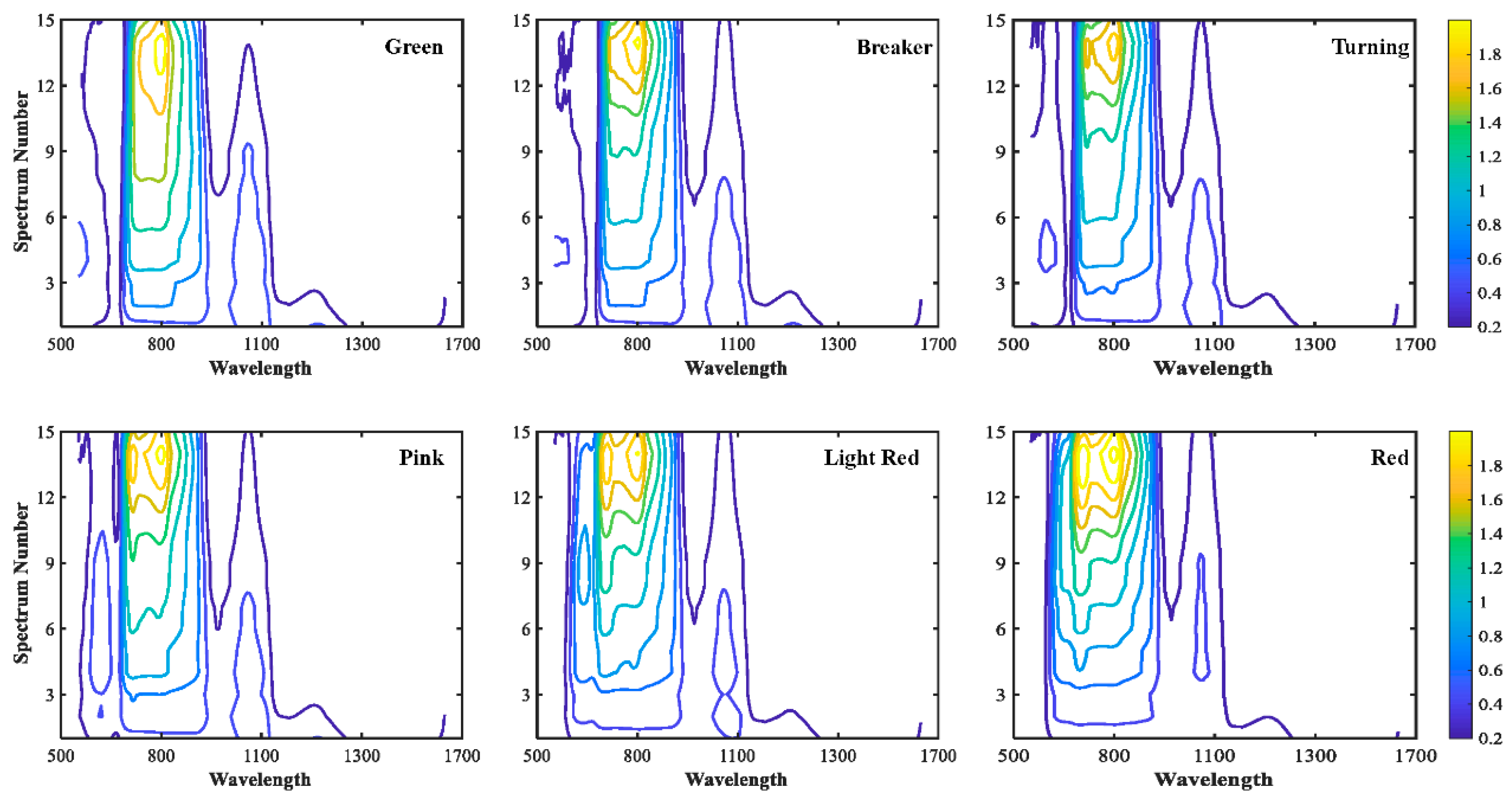
3.1. Differences of Quality Attributes for Tomatoes at Six Maturity Stages
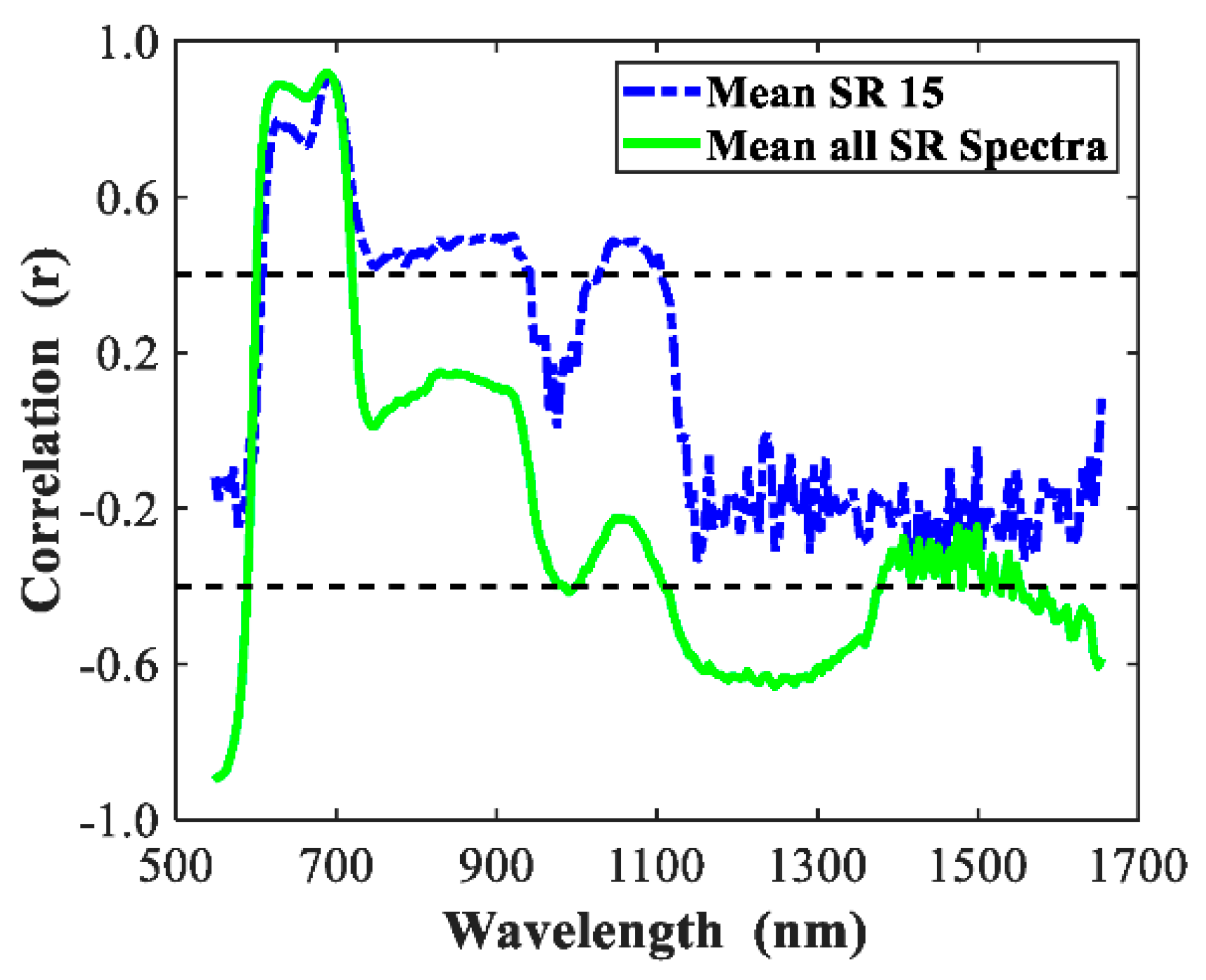
3.2. Spectral Features
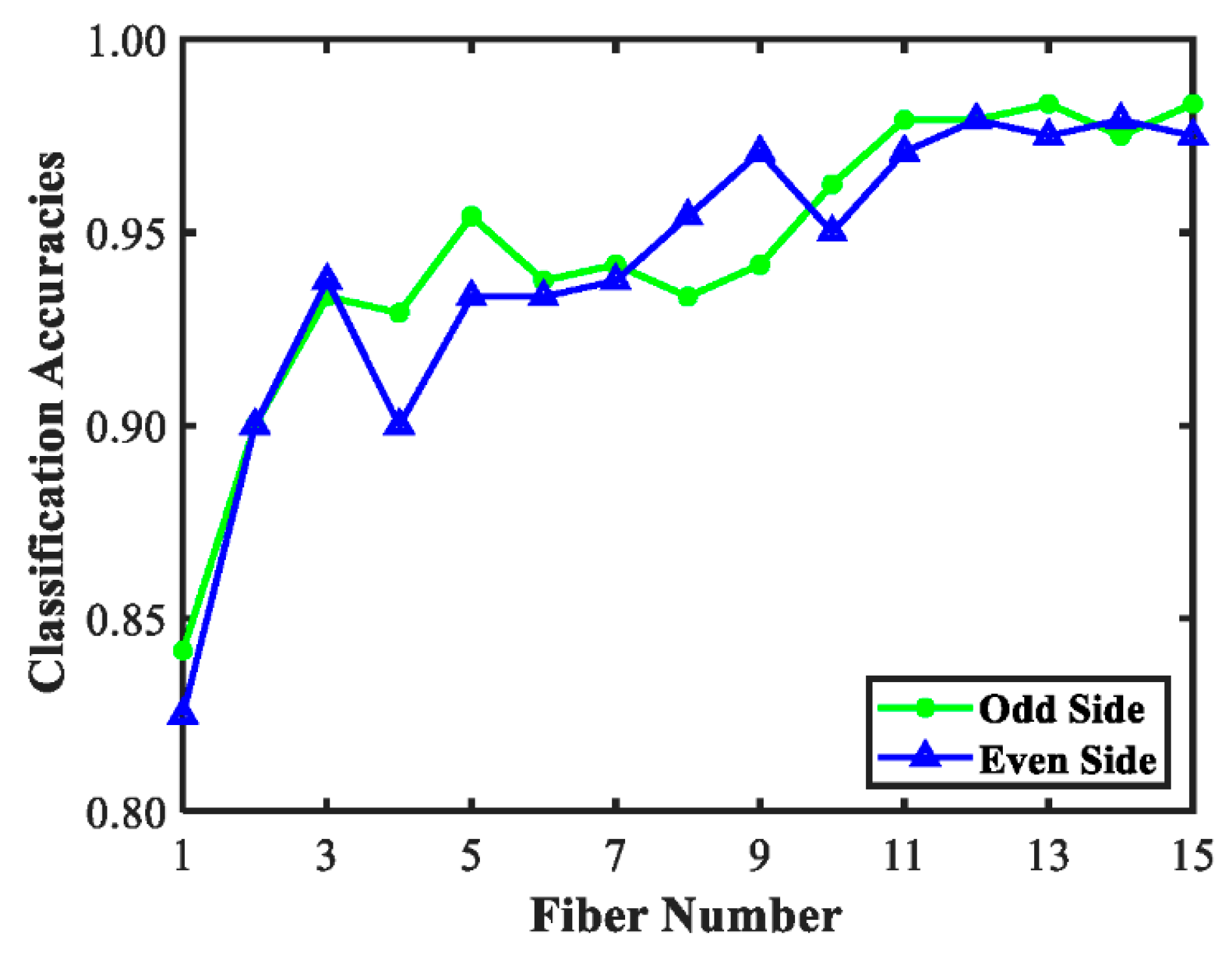
3.3. Discrimination Models for Tomato Maturity
3.4. Feature Extraction
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.; He, C.; Lu, R.; Mendoza, F.; Cen, H. Ripeness evaluation of ‘Sun Bright’ tomato using optical absorption and scattering properties. Postharvest Biol. Technol. 2015, 103, 27–34. [Google Scholar] [CrossRef]
- Xie, L.; Ying, Y.; Ying, T.; Yu, H.; Fu, X. Discrimination of transgenic tomatoes based on visible/near-infrared spectra. Anal. Chim. Acta 2007, 584, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Measurement and evaluation of tomato maturity using magnetic resonance imaging. Postharvest Biol. Technol. 2012, 67, 37–43. [Google Scholar] [CrossRef]
- Takahashi, N.; Maki, H.; Nishina, H.; Takayama, K. Evaluation of Tomato Fruit Color Change with Different Maturity Stages and Storage Temperatures Using Image Analysis. IFAC Proc. Vol. 2013, 46, 147–149. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Liu, Y.; Yang, Y.-T.; Zhang, Z.-W.; Chen, K.-J. Assessment of Tomato Color by Spatially Resolved and Conventional Vis/NIR Spectroscopies. Spectrosc. Spectr. Anal. 2019, 39, 3585–3591. [Google Scholar]
- Arakeri, M.P. Computer Vision Based Fruit Grading System for Quality Evaluation of Tomato in Agriculture industry. Procedia Comput. Sci. 2016, 79, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Arefi, A.; Motlagh, A.M.; Mollazade, K.; Teimourlou, R.F. Recognition and localization of ripen tomato based on machine vision. Aust. J. Crop Sci. 2011, 5, 1144. [Google Scholar]
- Wan, P.; Toudeshki, A.; Tan, H.; Ehsani, R. A methodology for fresh tomato maturity detection using computer vision. Comput. Electron. Agric. 2018, 146, 43–50. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Zeng, W.; Lu, X. An improved method for single image super-resolution based on deep learning. Signal Image Video Process. 2018, 13, 557–565. [Google Scholar] [CrossRef]
- Shen, L.; Wang, H.; Liu, Y.; Liu, Y.; Zhang, X.; Fei, Y. Prediction of Soluble Solids Content in Green Plum by Using a Sparse Autoencoder. Appl. Sci. 2020, 10, 3769. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, X.; Ru, Y.; Chen, Q.; Xu, L.; Zhou, H. Sweetness detection and grading of peaches and nectarines by combining short- and long-wave fourier-transform near-infrared spectroscopy. Anal. Lett. 2020, 1–20. [Google Scholar] [CrossRef]
- Ecarnot, M.; Tessarotto, L.; Chervin, C. Rapid phenotyping of the tomato fruit model, Micro-Tom, with a portable VIS-NIR spectrometer. Plant Physiol. Biochem. 2013, 70, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilahun, S.; Park, D.S.; Mu, H.S.; Hwang, I.G.; Kim, S.H.; Han, R.C.; Jeong, C.S. Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biol. Technol. 2018, 136, 50–56. [Google Scholar] [CrossRef]
- Alenazi, M.M.; Shafiq, M.; Alsadon, A.A.; Alhelal, I.M.; Saad, M.A.O. Non-destructive Assessment of Flesh Firmness and Dietary Antioxidants of Greenhouse-grown Tomato (Solanum lycopersicum L.) at Different Fruit Maturity Stages. Saudi J. Biol. Sci. 2020, 27, 2839–2846. [Google Scholar] [CrossRef]
- Sirisomboon, P.; Tanaka, M.; Kojima, T.; Williams, P. Nondestructive estimation of maturity and textural properties on tomato ‘Momotaro’ by near infrared spectroscopy. J. Food Eng. 2012, 112, 218–226. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Nondestructive Measurement of Fresh Tomato Lycopene Content and Other Physicochemical Characteristics Using Visible−NIR Spectroscopy. J. Agric. Food Chem. 2008, 56, 9813–9818. [Google Scholar] [CrossRef]
- Ohnishi, M.; Kusakawa, N.; Masaki, S.; Honda, K.; Shimada, Y.; Fujimoto, I.; Hirao, K. Investigation on deep layer measurements in the cerebral cortex within the adult head by near infrared spectroscopy using an absorbance difference technique. J. Near Infrared Spectrosc. 2003, 11, 27–38. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Development of a multichannel hyperspectral imaging probe for property and quality assessment of horticultural products. Postharvest Biol. Technol. 2017, 133, 88–97. [Google Scholar] [CrossRef]
- Do Trong, N.N.; Erkinbaev, C.; Tsuta, M.; De Baerdemaeker, J.; Nicolaï, B.; Saeys, W. Spatially resolved diffuse reflectance in the visible and near-infrared wavelength range for non-destructive quality assessment of ‘Braeburn’apples. Postharvest Biol. Technol. 2014, 91, 39–48. [Google Scholar] [CrossRef]
- Xia, J.; Weaver, A.; Gerrard, D.E.; Yao, G. Distribution of optical scattering properties in four beef muscles. Sens. Instrum. Food Qual. Saf. 2008, 2, 75–81. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Assessment of Tomato Soluble Solids Content and pH by Spatially-Resolved and Conventional Vis/NIR Spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Hu, D.; Chen, K. Quality assessment of tomato fruit by optical absorption and scattering properties. Postharvest Biol. Technol. 2018, 143, 78–85. [Google Scholar] [CrossRef]
- USDA. U.S. Standards for Grades of Fresh Tomatoes. United States Department of Agriculture; Agricultural Marketing Service: Washington, DC, USA, 1991.
- Huang, Y.; Yang, Y.; Sun, Y.; Zhou, H.; Chen, K. Identification of Apple Varieties Using a Multichannel Hyperspectral Imaging System. Sensors 2020, 20, 5120. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Detection of internal defect of apples by a multichannel Vis/NIR spectroscopic system. Postharvest Biol. Technol. 2020, 161, 111065. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Prediction of Firmness Parameters of Tomatoes by Portable Visible and Near-Infrared Spectroscopy. J. Food Eng. 2018, 222, 185–198. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Xu, Y.; Chen, K. Prediction of tomato firmness using spatially-resolved spectroscopy. Postharvest Biol. Technol. 2018, 140, 18–26. [Google Scholar] [CrossRef]
- Wang, A.; Wei, X. A sequential method for estimating the optical properties of two-layer agro-products from spatially-resolved diffuse reflectance: Simulation. Artif. Intell. Agric. 2019, 3, 69–78. [Google Scholar] [CrossRef]
- Lu, R. Overview of Light Interaction with Food and Biological Materials; CRC Press: Abingdon, UK, 2016; pp. 19–42. [Google Scholar]
- Tiwari, G.; Slaughter, D.C.; Cantwell, M. Nondestructive maturity determination in green tomatoes using a handheld visible and near infrared instrument. Postharvest Biol. Technol. 2013, 86, 221–229. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Multivariate approach to the measurement of tomato maturity and gustatory attributes and their rapid assessment by Vis−NIR Spectroscopy. J. Agric. Food Chem. 2008, 56, 1538–1544. [Google Scholar] [CrossRef] [PubMed]


| Maturity | AF | CS | PF | SSC | pH |
|---|---|---|---|---|---|
| Green | 9.03 ± 1.88 a | 34.57 ± 7.30 a | 17.52 ± 2.13 a | 4.42 ± 0.34 a | 3.98 ± 0.22 a |
| Breaker | 5.75 ± 1.33 b | 18.95 ± 3.86 b | 15.08 ± 2.16 b | 4.61 ± 0.43 b | 4.02 ± 0.10 b |
| Turning | 5.54 ± 1.37 b | 16.04 ± 3.31 c | 13.89 ± 2.16 c | 4.80 ± 0.47 c | 4.10 ± 0.10 c |
| Pink | 5.38 ± 1.48 b | 13.91 ± 2.78 d | 11.69 ± 1.73 d | 5.12 ± 0.44 d | 4.14 ± 0.09 d |
| Light Red | 4.66 ± 1.36 c | 10.54 ± 1.67 e | 8.72 ± 1.46 e | 5.37 ± 0.50 e | 4.24 ± 0.11 e |
| Red | 4.04 ± 1.36 d | 8.20 ± 2.17 f | 7.12 ± 1.54 f | 5.73 ± 0.56 f | 4.34 ± 0.12 f |
| Type of Spectra | Training Set | Test Set | |
|---|---|---|---|
| Cal (%) | CV (%) | Pred (%) | |
| Mean_SR 1 | 92.5 | 85.8 | 83.3 |
| Mean_SR 2 | 94.7 | 90.6 | 82.9 |
| Mean_SR 3 | 98.9 | 93.9 | 90.0 |
| Mean_SR 4 | 100 | 96.4 | 91.3 |
| Mean_SR 5 | 98.9 | 95.0 | 92.9 |
| Mean_SR 6 | 100 | 95.3 | 96.7 |
| Mean_SR 7 | 98.9 | 95.8 | 93.8 |
| Mean_SR 8 | 100 | 95.0 | 93.8 |
| Mean_SR 9 | 100 | 93.9 | 95.4 |
| Mean_SR 10 | 100 | 98.1 | 97.9 |
| Mean_SR 11 | 100 | 97.2 | 97.5 |
| Mean_SR 12 | 100 | 99.2 | 97.5 |
| Mean_SR 13 | 100 | 97.2 | 95.4 |
| Mean_SR 14 | 100 | 96.1 | 97.1 |
| Mean_SR 15 | 100 | 96.9 | 98.3 |
| Mean_all_SR Spectra | 98.6 | 93.1 | 91.3 |
| Type of Spectra | Maturity | Training Set/% | Test Set/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G | B | T | P | L | R | Accuracy | |||
| Mean_SR 15 | G | 100 | 42 | 1 | 0 | 0 | 0 | 0 | 100 |
| B | 100 | 0 | 35 | 1 | 0 | 0 | 0 | 94.6 | |
| T | 100 | 0 | 0 | 39 | 0 | 0 | 0 | 97.5 | |
| P | 100 | 0 | 1 | 0 | 40 | 0 | 0 | 100 | |
| L | 100 | 0 | 0 | 0 | 0 | 46 | 1 | 100 | |
| R | 100 | 0 | 0 | 0 | 0 | 0 | 34 | 97.1 | |
| Mean_all_SR_Spectra | G | 98.3 | 42 | 0 | 0 | 0 | 0 | 0 | 100 |
| B | 98.4 | 0 | 35 | 5 | 0 | 0 | 0 | 94.6 | |
| T | 96.7 | 0 | 2 | 30 | 2 | 0 | 0 | 75.0 | |
| P | 98.3 | 0 | 0 | 5 | 38 | 0 | 1 | 95.0 | |
| L | 100 | 0 | 0 | 0 | 0 | 41 | 1 | 89.1 | |
| R | 100 | 0 | 0 | 0 | 0 | 5 | 33 | 94.3 | |
| Parameters | Spectral Type | G | B | T | P | L | R |
|---|---|---|---|---|---|---|---|
| Sensitivity | Mean_SR 15 | 1.000 | 0.946 | 0.975 | 1.000 | 1.000 | 0.971 |
| Mean_All_SR_Spectra | 1.000 | 0.946 | 0.750 | 0.950 | 0.891 | 0.943 | |
| Specificity | Mean_SR 15 | 0.995 | 0.995 | 1.000 | 0.995 | 0.995 | 1.000 |
| Mean_All_SR_Spectra | 1.000 | 0.975 | 0.980 | 0.970 | 0.995 | 0.976 |
| Type of Spectra | Maturity | Training Set/% | Test Set/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G | B | T | P | L | R | Accuracy | |||
| New_mean_SR 15 | G | 98.3 | 40 | 5 | 0 | 0 | 0 | 0 | 95.2 |
| B | 88.9 | 2 | 28 | 0 | 0 | 0 | 0 | 75.7 | |
| T | 88.3 | 0 | 3 | 33 | 4 | 0 | 0 | 82.5 | |
| P | 86.7 | 0 | 1 | 7 | 35 | 2 | 1 | 87.5 | |
| L | 94.4 | 0 | 0 | 0 | 1 | 34 | 4 | 73.9 | |
| R | 98.4 | 0 | 0 | 0 | 0 | 10 | 30 | 85.7 | |
| New_mean_all_SR_Spectra | G | 100 | 42 | 1 | 0 | 0 | 0 | 0 | 100 |
| B | 98.4 | 0 | 35 | 5 | 0 | 0 | 0 | 94.6 | |
| T | 96.7 | 0 | 1 | 29 | 2 | 0 | 0 | 72.5 | |
| P | 100 | 0 | 0 | 5 | 38 | 1 | 1 | 95.0 | |
| L | 100 | 0 | 0 | 1 | 0 | 41 | 1 | 89.1 | |
| R | 100 | 0 | 0 | 0 | 0 | 4 | 33 | 94.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Si, W.; Chen, K.; Sun, Y. Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy. Sensors 2020, 20, 7229. https://doi.org/10.3390/s20247229
Huang Y, Si W, Chen K, Sun Y. Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy. Sensors. 2020; 20(24):7229. https://doi.org/10.3390/s20247229
Chicago/Turabian StyleHuang, Yuping, Wan Si, Kunjie Chen, and Ye Sun. 2020. "Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy" Sensors 20, no. 24: 7229. https://doi.org/10.3390/s20247229
APA StyleHuang, Y., Si, W., Chen, K., & Sun, Y. (2020). Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy. Sensors, 20(24), 7229. https://doi.org/10.3390/s20247229





