3.1. New Ion-Channel Sensing Working Model
With regard to electrochemical gene sensor array chips based on ion-channel sensing, Aoki et al. [
17,
18] have discussed the working principle of a sensor with oligonucleotide targets and PNA probes. Before PNA/DNA hybridization, the negatively charged markers can access the electrode and transfer electrons to the WE. After hybridization, the electrostatic repulsion between the negatively charged marker and the PNA/DNA duplexes on the WE surface hinders the redox reaction in the marker. We term the abovementioned mechanism an ordinary sensor working model: this mechanism is depicted in
Figure 2a. This working model was established based on the assumption that PNA/DNA duplexes are uniformly spread over the WE.
We assessed the patterns of the hybridized PNA/DNA duplexes over the WE modified by antiparallel PNA (N1) and 6-HHT using fluorescently labeled cDNA (D1, carboxytetramethylrhodamine (TAMRA)). The electrode diameter was ∅300 μm, and the target cDNA concentration was 100 nM.
Figure 2b shows the fluorescence hybridization images. The PNA/DNA duplexes were not uniformly spread over the WE but rather exhibited a one-dimensional swirl-like pattern. This fluorescence pattern was considered to be a modified PNA (N1) pattern in which the fluorescently labeled cDNA (D1) was hybridized. The self-assembled 6-HHT monolayer had high orientation and stability [
21]. Therefore, the 6-HHT modification after PNA modification may have helped align the PNAs in a swirl-like pattern because PNA is electrically neutral. A large area of the WE did not contain the PNA/DNA duplex. Therefore, the previously mentioned working principle does not explain the mechanism governing the hindrance of the redox reaction over the entire WE.
Eley and Spivey [
22] have reported on the conductivity of dsDNA and proposed a conductivity model for transferring π electrons through the π–π stacks of the base pairs. After this, numerous controversial studies have investigated whether dsDNA is conductive [
23,
24,
25,
26,
27,
28,
29]. Guo et al. [
30] assessed dsDNA conductivity by attaching carbon nanotubes to dsDNA and reported that dsDNA without mismatches was as conductive as graphite, while the mismatched dsDNA had 300-fold greater resistivity. Therefore, the matched PNA/DNA and PNA/miRNA duplexes should be conductive through their π–π stacks.
The new sensor working model is presented in
Figure 2c. Prior to PNA/DNA hybridization, negatively charged markers can access the electrode and transfer electrons to the WE, similarly to the original sensor working model. However, the modified PNA pattern after 6-HHT modification is a one-dimensional swirl-like pattern and is not uniformly spread over the WE. After hybridization, the negative charge in the backbone of the hybridized oligonucleotides is electrically connected to the WE through the π–π stacks of the hybridized base pairs. As the surface of the WE is a gold layer, this electrically connected negative charge generates a uniform negative potential over the entire WE and hinders the redox reaction of the marker.
3.2. ΔE Potential Shift
The total negative charge of the hybridized oligonucleotides,
ΔQ, leads to a potential difference
ΔE in the electric double layer capacitance
C when measuring the CV as follows:
The diagrams show the differences in the electronic potential between the reference electrode (RE) and the WE pre- and posthybridization. During prehybridization, the electronic potential between the RE and WE that is required to yield the current (
im) is shown in
Figure 3a, where the external bias can be expressed as follows:
After hybridization, the electronic potential between the RE and WE decreases by
ΔE due to the negative charge on the hybridized oligonucleotide,
ΔQ, as shown in
Figure 3b. There will be a reduction in the current if the external bias is identical to
V0. To retrieve the identical current (
im), the external bias
V1 must be increased by
ΔE to yield the same potential difference between the RE and WE, as shown in
Figure 3c and expressed by Equation (3):
where
V0: the external bias to yield (
im) in prehybridization;
V1: the external bias to retrieve (
im) in posthybridization;
ΔE: the potential difference caused by the hybridized charge
(ΔQ);
ΔΦ0r: the potential difference between the RE and the aqueous solution (spontaneous potential);
ΔΦb: the potential difference in the bulk aqueous solution (generally close to zero);
ΔΦ0w: the potential difference between the WE and the aqueous solution (spontaneous potential); and
ΔVb0: the potential difference controlled by external bias (
V0).
Similar equations have been proposed with regard to DNA target detection sensitivity using ion-sensitive field effect transistors (IS-FETs) [
31] and potentiometry [
32]. Ohtake [
31] investigated the effect of using DNA or PNA probes with IS-FET electrodes on target DNA hybridization. He calculated the increase in the quantity of electricity per gate area using Equation (4), where
ΔVT is the threshold voltage shift of the IS-FET caused by hybridization and
L and
W are the gate length and width, respectively. Here,
C is the capacitance of the gate fabricated using SiO
2 (100 nm), Si
3N
4 (100 nm), and Ta
2O
5 (40 nm) thin films and not the electric double-layer capacitance, as expressed in Equation (1):
Goda et al. [
32] have investigated the sensitivity of potentiometric DNA detection performed using PNA and DNA probes. Using Equation (5), they discussed the potential shift caused by hybridization. Here,
CDL is the electric double-layer capacitance, as in Equation (1). However,
ΔQ is the negative charge of the captured DNA within the electrical double layer. Goda et al. insisted that the DNA located outside of the electrical double layer fails to generate an interfacial potential as a result of the charge-screening effect caused by the external electrolytes in the buffer solution:
Apart from the differences between the equations used in this study and Equations (4) and (5) in terms of parameter definition, our new model is different from existing models because our objective is to understand the complete mechanism governing the influence of the negative charge of the captured DNA on the potential shift of gold WEs through electric double-layer capacitance. Therefore, discovering the one-dimensional swirl-like pattern of PNAs and the mechanism of the electrical conductivity caused by the π electrons in the PNA/DNA duplexes is important. For example, Goda et al. [
32] discussed the average surface density of immobilized PNAs; however, the PNA pattern was not considered. Additionally, their discussion with regard to Equation (5) was based on an analogy of the IS-FET model. However, as long as the electric double-layer capacitance is used instead of the gate capacitance,
ΔQ should exist on the gold electrode (on which the PNA or DNA probes are modified) and will not be confined to the electrical double layer itself, as is known from basic electromagnetic theory.
3.3. ΔE Evaluation
The potential shift
ΔE was investigated and compared to the conventional evaluation parameter [
15] of hybridization in the CV measurements, that is, the changes in the oxidation current. Five blocks were modified using the PNA probe (N3) and 6-HHT. After the prehybridization CV measurements, miRNA (R3) hybridization was performed at 40 °C for 40 min with an aqueous solution of 1 × saline sodium citrate (SSC) and 20% dimethyl sulfoxide (DMSO). Thereafter, we performed posthybridization CV measurements.
Figure 4a compares the curves between the pre- and posthybridization CVs. The prehybridization curve is indicated by the solid line, and the posthybridization curves are indicated by the dotted lines, where the short dots denote 240 nM and the long dots denote 4 nM. In the conventional evaluation method, the sensor response is expressed as the ratio of posthybridization current reduction (
i0 –
i) to the prehybridization peak current (
i0), where (
i) is measured at potential (
Ep), determining the peak current (
i0). When the target miRNA levels were markedly high, that is, up to 240 nM, the posthybridization CV current at
Ep was suppressed to a low value (
i’) in accordance with the hybridized negative charge of the target miRNA. Therefore, the conventional evaluation parameter, that is,
(i0 - i)/i0 or
i/i0, can be useful in evaluating the degree of hybridization.
However, evaluations based on this parameter have several disadvantages. First, in the prehybridization CV measurements, we could not explicitly set the peak current voltage Ep due to gradual changes in the oxidation current around the peak current. When the oxidation current approached its peak value, there was a decrease in the concentration of the reductant marker [Fe(CN)6]4–, whereas the concentration of the oxidant [Fe(CN)6]3– increased. At peak current, the concentration gradient of the reductant perpendicular to that of the WE peaked, which yielded the peak current value. Along with this gradual change in the gradient, the oxidation current also gradually changed, which resulted in errors when setting the Ep value and evaluating (i0 - i)/i0 or i/i0.
Another difficulty occurred when the amount of the hybridized target was as low as 4 nM. The current i, at the Ep on the posthybridization CV, was approximately identical to that of the i0 on the prehybridization CV. Thus, the evaluation parameter i/i0 remained constant at approximately one.
Compared to the prehybridization CV, the posthybridization CV exhibited a shift to a higher potential value, as expressed by Equation (3), and maintained a similar oxidation curve. Therefore, the voltage shift value ΔE at an identical measurement current (im) is a potentially good evaluation parameter for the amount of hybridized target miRNA, because it is based on the simple physical model of Equation (1).
Figure 4b shows a 74-pc correlation chart of
i/i0 and
ΔE when the concentration of the complementary target miRNA (R3) was 4 nM. Because the target miRNA concentration was low, the majority of the
i/i0 plots tending toward one remained unchanged and exhibited poor detection sensitivity for the hybridized target miRNA. However, the
ΔE plots had a wide spread from 0 to 90 mV. Compared to the conventional
i/i0 evaluation parameter,
ΔE had higher sensitivity and was better suited to the detection of hybridized miRNA at a low concentration.
3.4. New Hybridization System for Ultrasmall Aqueous Solution Amounts
To detect the small amount of miRNA (1.7 amol or less) in the serum through
ΔE measurements, the volume of the aqueous solution for hybridization must be limited to 425 pL or less to maintain the concentration at 4 nM.
Figure 5 shows a comparison between (a) an ordinary hybridization system and (b) a new hybridization system, where a small well caused by an opening in the epoxy resin and Teflon plate limits the volume of the aqueous solution. This sandwich structure ensures a constant small well volume over each WE and allows for comparing the hybridization results between the WEs. Moreover, the Teflon plate suppresses the evaporation of the aqueous solution during hybridization and prevents the aqueous solution from being removed from each well through capillary forces due to its hydrophobicity property when pressed to the array chip by a metal weight.
In the new hybridization system, the size of the WE must be considered from the point of view of sensitivity. Assuming two differently sized WEs, e.g., WE
1 and WE
2, are used to detect the same minimal potential shift
ΔE in Equation (1), the following Equation (6) can be derived:
where
ΔQ1: the charge required to achieve the minimal potential shift
ΔE in
WE1;
ΔQ2: the charge required to achieve the minimal potential shift
ΔE in
WE2;
C1: the electric double-layer capacitance of
WE1; and
C2: the electric double-layer capacitance of
WE2.
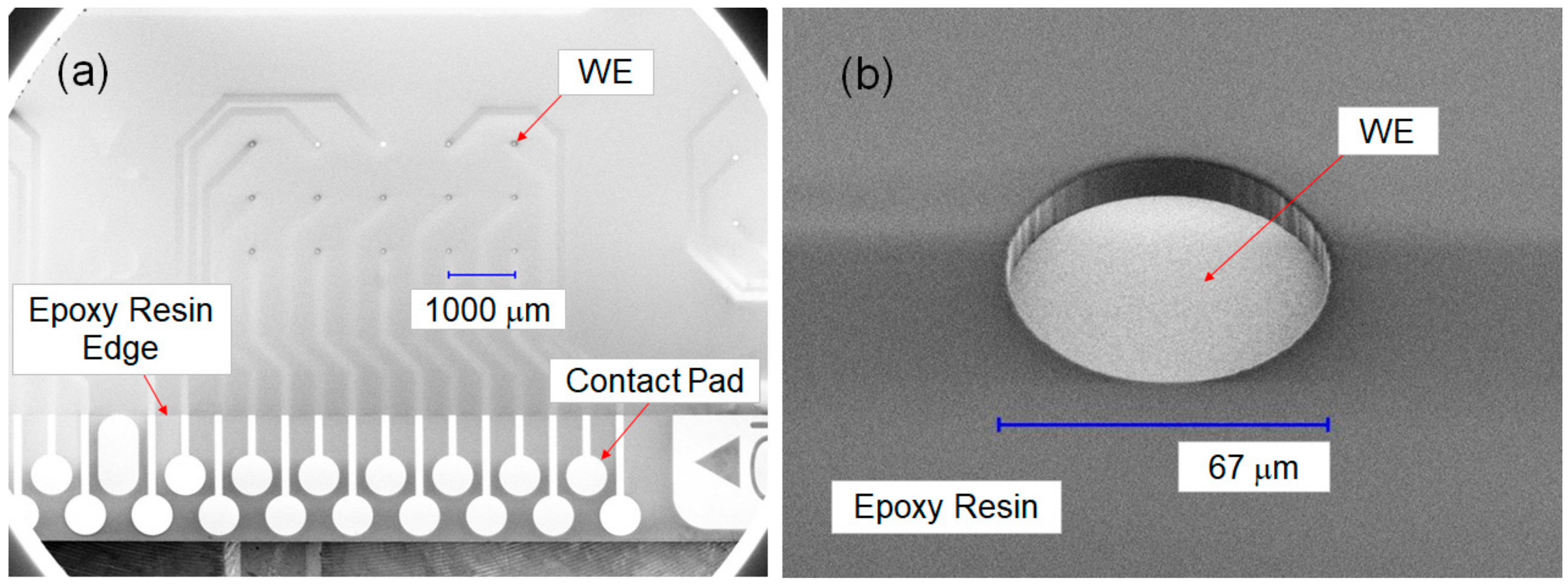
If the area of WE1 is less than that of WE2, the electric double-layer capacitance C1 is less than C2, because the capacitance is proportional to the area of the WE. Therefore, the charge required to achieve the same minimal potential shift ΔE becomes smaller in ΔQ1 compared to ΔQ2. To confirm this, two well-sized array chips were prepared in the new hybridization system by changing the well sizes to ∅67 μm/t10 μm and ∅95 μm/t10 μm: the respective well volumes were 35 pL and 71 pL. Both of the well volumes were significantly smaller than the target well volume (less than 425 pL). Here, ∅67 μm was selected, considering the ease of processing the photosensitive epoxy resin to obtain a clean WE surface without etching debris, and ∅95 μm was selected to make the area of the WE approximately twice of ∅67 μm (to conform to Equation (6)).
3.5. MiRNA Detection Sensitivity
Five of the eight blocks in the array chip were modified using a PNA probe (N3) for complementary miRNA hybridization. Two more blocks were modified by PNA (N1) and (N16) for noncomplementary hybridization. The remaining block was not modified by PNA to check whether there were any target miRNA chemisorbs and remained on the 6-HHT-modified working electrodes. The
ΔE detection sensitivity data are box-plotted in
Figure 6a (35 pL (∅67 μm)) and
Figure 6b (71 pL (∅95 μm)) at various target miRNA molar amounts. In each box plot, (N3/R3) indicates 75 pcs of data of a complementary system, (N1/R3) and (N16/R3) each refer to 15 pcs of data of noncomplementary systems, and (PNA-less/R3) indicates 15 pcs of data of a reference system (to identify the occurrence of the chemisorption of miRNA onto 6-HHT).
In
Figure 7a, the median values of the complementary system (N3/R3) in
Figure 6a,b are plotted on a linear scale as a function of the molar amount
N. The smaller well volume of 35 pL had the highest
ΔE detection sensitivity at 140 zmol. The sensitivity increased with the decrease in the WE size, as predicted in
Section 3.4. When
ΔE was replotted against the target molar concentration
D, the sensitivity to the well volume varied, as shown in
Figure 7b. The sensitivity was the highest at 4 nM for the well volume of 35 pL (∅67 μm). In terms of molar concentration, the sensitivity appeared to depend less on the size of the WE, while the sensitivity appeared to depend more on the size of the WE in terms of the molar amount.
Equation (1) can be modified to Equation (7), where
N is the target miRNA molar amount, and
α (0 ≤
α ≤ 1) is the hybridization efficiency representing the hybridized miRNA molar amount divided by the total miRNA molar amount originally preserved inside the well:
where
q: the charge of the electrons;
m: the base length of target miRNA;
NA: Avogadro’s number;
d0: the thickness of the electric double-layer capacitance;
ε: the permittivity of the electric double-layer capacitance;
ε0: the permittivity of the vacuum;
α: the hybridization efficiency (0≤
α ≤1);
S: the area of the WE; and
N: the target miRNA molar amount in the well.
To achieve a higher detection sensitivity in the molar amount, the gradient of Equation (7) should be increased. Therefore, decreasing
S, that is, the area of the WE, and increasing the hybridization efficiency,
α, are both effective. The nonlinear curve in
Figure 7a means that
α has nonlinear characteristics. This may be the reason for the tangential line around the lowest molar amount not crossing the origin. In
Figure 7a, the gradient of Equation (7) at the lowest molar amount is calculated as 5.86 × 10
16 (V/mol) for ∅67 μm and 8.58 × 10
15 (V/mol) for ∅95 μm. By taking the ratio of these gradients, the hybridization efficiency ratio of the two differently sized WE can be calculated, as expressed in Equation (8). As expected, decreasing
S by decreasing the diameter from ∅95 μm to ∅67 μm contributes to an increase in sensitivity. However, the ratio of
S is two, whereas the ratio of
α is 3.4 and 1.7 times higher than the ratio of
S. This means that the hybridization efficiency
α is strongly dependent on the WE area:
Equation (7) can be modified to Equation (9) using the molar concentration
D, where
t is the thickness of the well. Notably, Equation (9) expresses that decreasing the WE size does not directly increase the molar concentration sensitivity; rather, only the hybridization efficiency
α does:
where
t is the thickness of the well, and
D is the target miRNA molar concentration in the well.
The increase in the hybridization efficiency α due to the decreasing WE size is probably caused by the differences in the PNA modification pattern. The one-dimensional swirl-like PNA pattern may be more congested in WEs of a smaller size. This is plausible because the congestion degree of the PNA pattern directly dictates the interaction probability between the PNA probe and the target miRNA. In other words, as the congestion degree becomes higher, the interaction probability increases.
Unfortunately, the congested one-dimensional PNA pattern is not as effective for hybridization as the PNA probes uniformly spread over the WEs. However, the realization of the latter remains unclear. Using DNA probes may be an effective solution; however, this decreases the hybridization with negatively charged miRNA. If the PNA modification pattern can be changed from one-dimensional to two-dimensional while keeping a reasonable distance between the PNA probes, a remarkable sensitivity improvement will occur in terms of molar concentration.
3.6. Statistical Analysis
We statistically analyzed the
ΔE data obtained from all tests. To specify the highest
ΔE detection sensitivity of this incubation system from a statistical perspective, we performed an unpaired test (two-tailed) between the 75 pcs of complementary data (N3/R3) and the other systems (N1/R3, 15 pcs; N16/R3, 15 pcs; PNA-less/R3, 15 pcs), followed by determining the
p-value. To prevent a type 1 error in this multiple comparison, we performed Dunnett’s test. Equation (10) yields the
t-value using the
VE expressed by the unbiased variances in Equation (11), as follows:
The
p-value (based on Dunnett’s test) only indicates a rare risk where there are no differences between the mean values. To determine the degree of separation between the mean values, the effect size was evaluated using Cohen’s
d-value (defined in Equation (12)), where the unbiased variances in Equation (13) express
ud as follows:
Figure 8a,b shows the calculated Dunnett’s
p-value and Cohen’s
d-value. Three groups were used for statistical analysis: (N3/R3) versus (N1/R3), (N3/R3) versus (N16/R3), and (N3/R3) versus (PNA-less/R3) for the 35-pL and 71-pL hybridization.
Dunnett’s test revealed that in the 35-pL hybridization system, if the miRNA levels were 140 zmol or greater, the mean value of the complementary and noncomplementary systems was not equal at a 95% significance level. Moreover, Cohen’s
d-value revealed that, under this condition, the mean value of the complementary and noncomplementary systems had good separation that exceeded the medium (≥0.5) or large (≥0.8) effect size criteria.
Figure 8 shows that the possibility of miRNA chemisorption to 6-HHT was low.
These statistical analyses indicate that in the 35-pL system, the maximum
ΔE detection sensitivity was 140 zmol. This new hybridization methodology exhibited good sensitivity against our initial target value of 830 zmol to 1.7 amol. Compared to the calculated sensitivity of 1 nmol (discussed in
Section 1), we achieved a 10
10-fold improvement for the molar sensitivity, which implies that in an ordinary incubation system, most miRNA targets are wasted, while only the targets close to the WE contribute to the hybridization results.
3.7. Two Aspects of Sensitivity
Sensitivity in a hybridization system has two aspects. One is the measurement sensitivity itself, which is associated with the molar amount of the target. The ultrasmall amount of the incubation system in the aqueous solution revealed that the novel ΔE measurement and smaller WE could detect miRNA amounts as low as 140 zmol with a ∅67-μm WE with adequate sensitivity.
The second aspect is associated with the target molar concentration.
Figure 7b shows that the highest sensitivity for a molar concentration with a ∅67-μm WE was 4 nM when the incubation condition was 40 °C for 40 min. For successful hybridization, there should initially be a reasonable interaction probability between the PNA and the miRNA. Thereafter, hydrogen bonding occurs between the bases, followed by π–π stacking to complete the hybridization process. A reasonably high molar concentration of miRNA ensures the occurrence of this interaction. Even if 1.7 amol of miRNA were present in the 10 mL of serum, the molar concentration was 0.17 fM, which is much lower than 4 nM and does not yield a reasonable interaction probability.
To accomplish the direct detection of miRNA in serum, these two aspects of sensitivity must be fulfilled simultaneously. From this viewpoint, increasing the sensitivity in terms of the molar concentration becomes an issue. One approach is to investigate a method of changing the PNA modification pattern from one-dimensional to two-dimensional to dramatically increase the hybridization efficiency α. The other approach is to extract and concentrate the miRNA from the serum and deliver it to a small incubation well for hybridization.