Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications
Abstract
:1. Introduction
2. Plasmonics for Enhanced Chiroptical Effects
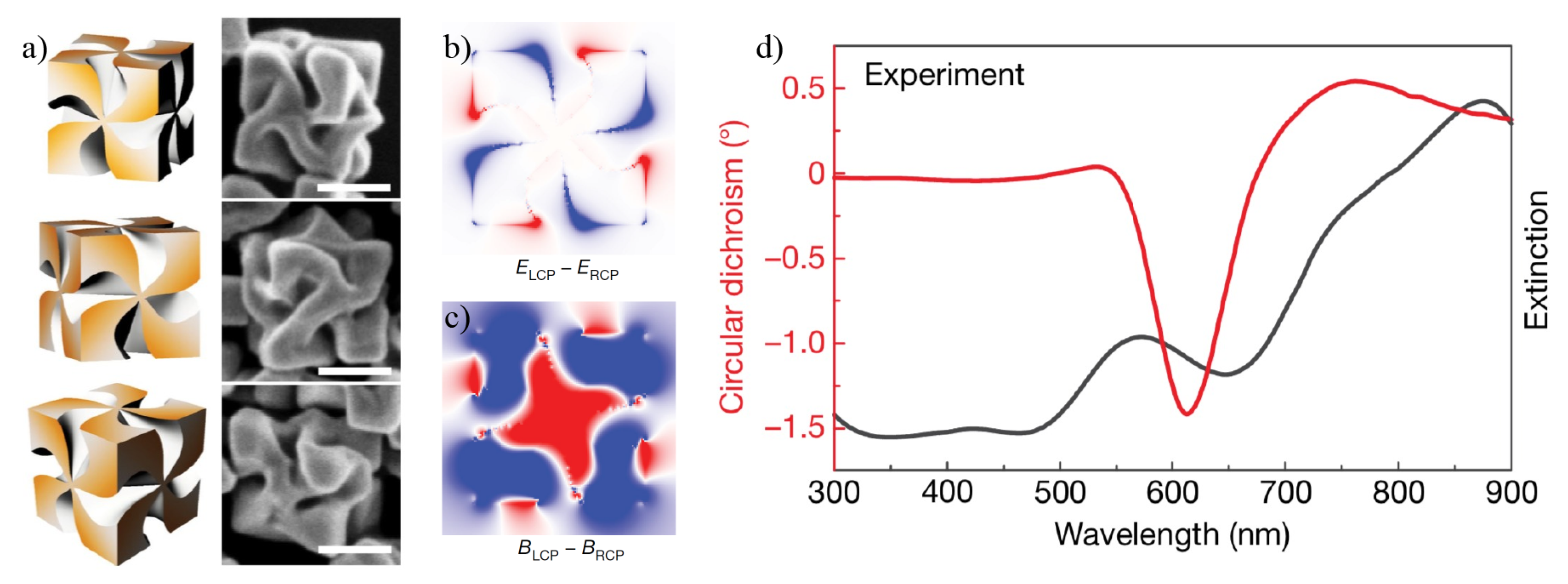
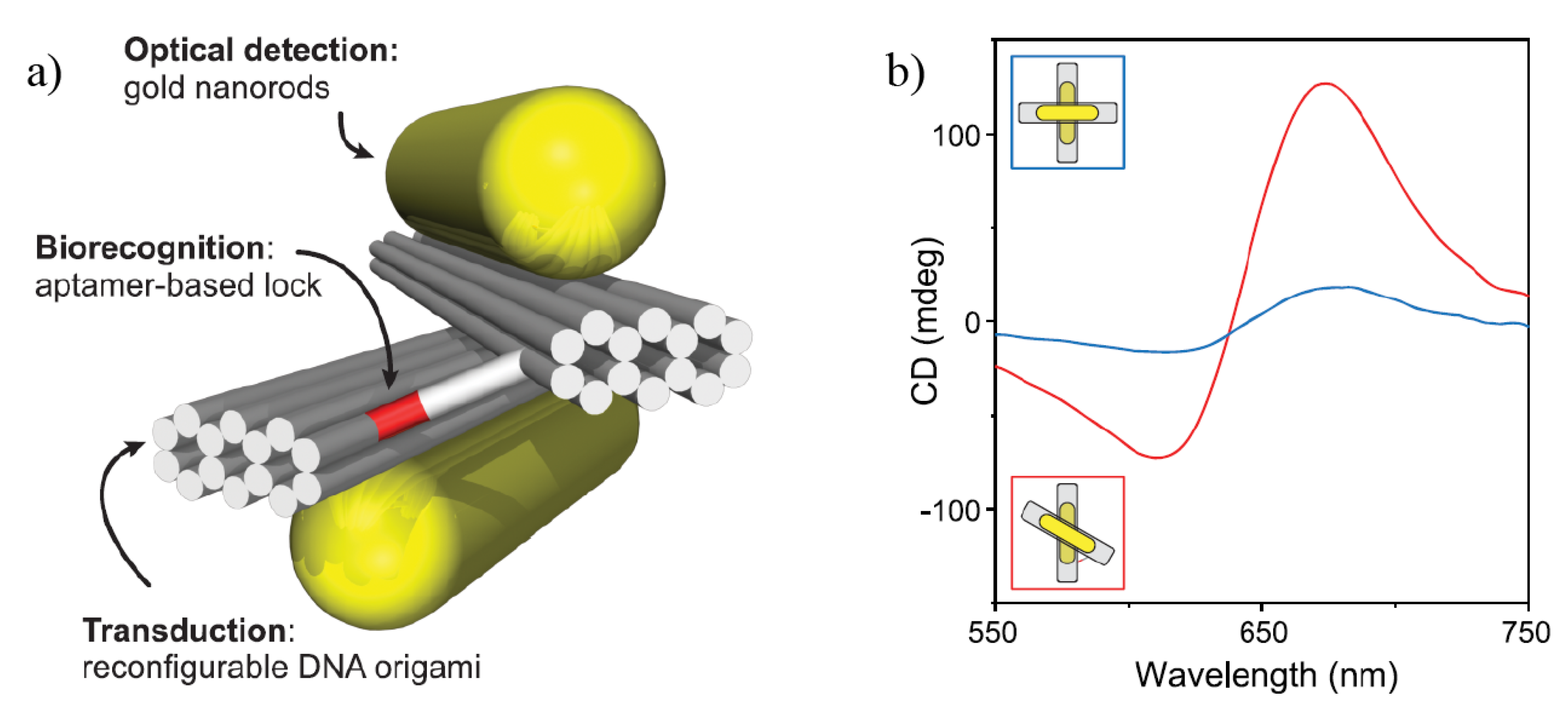
3. Chiral Plasmonic Nanoparticles
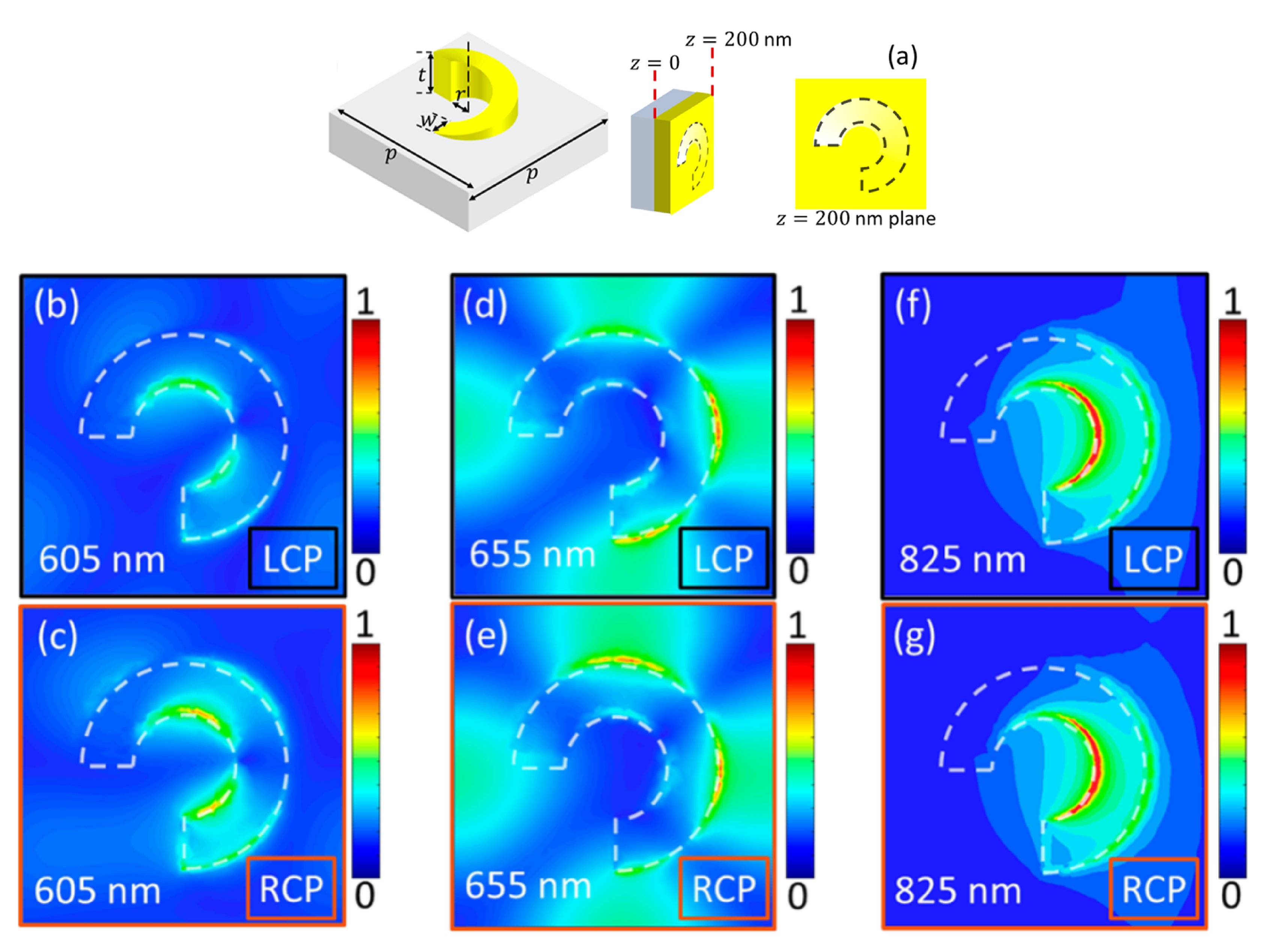
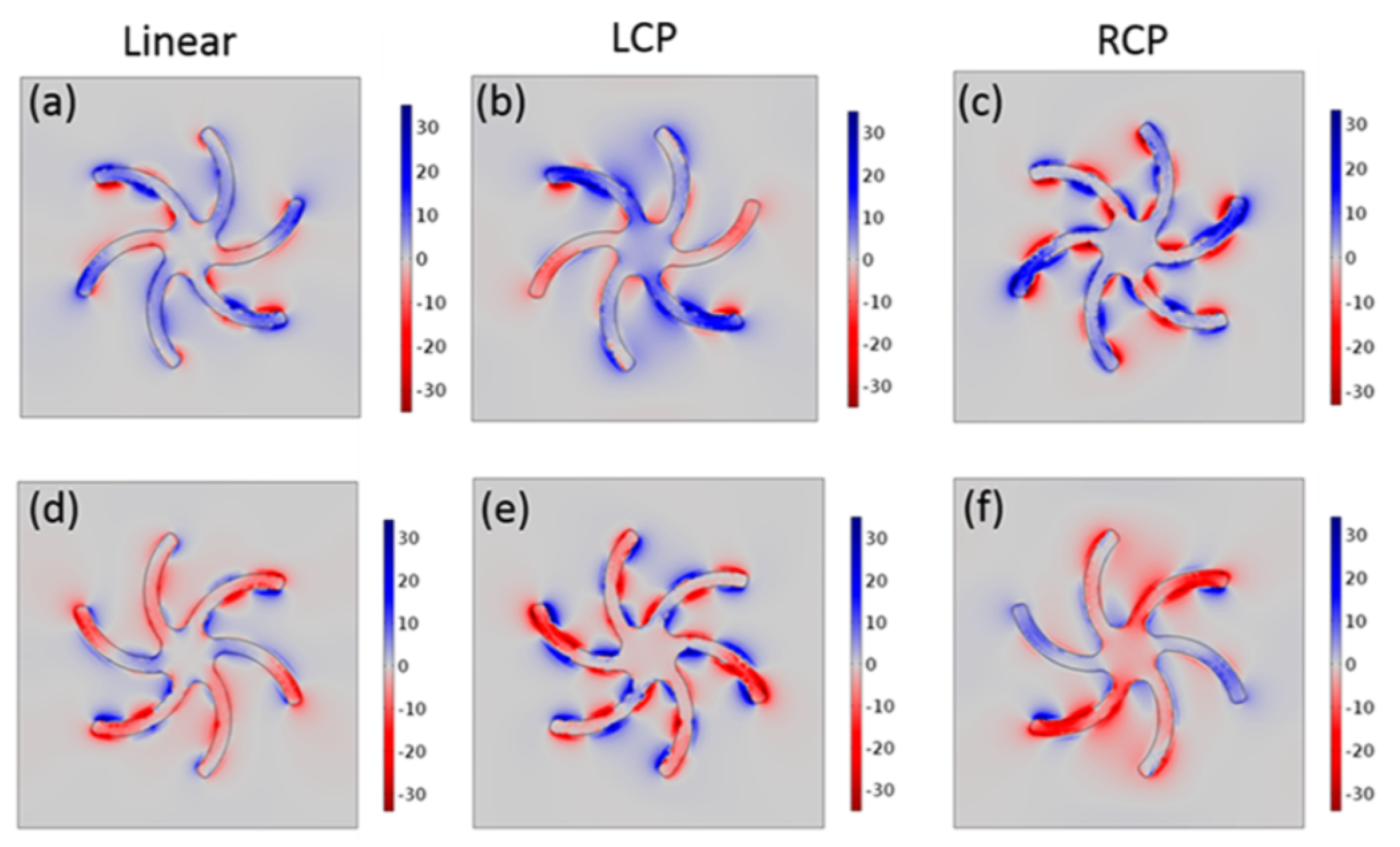
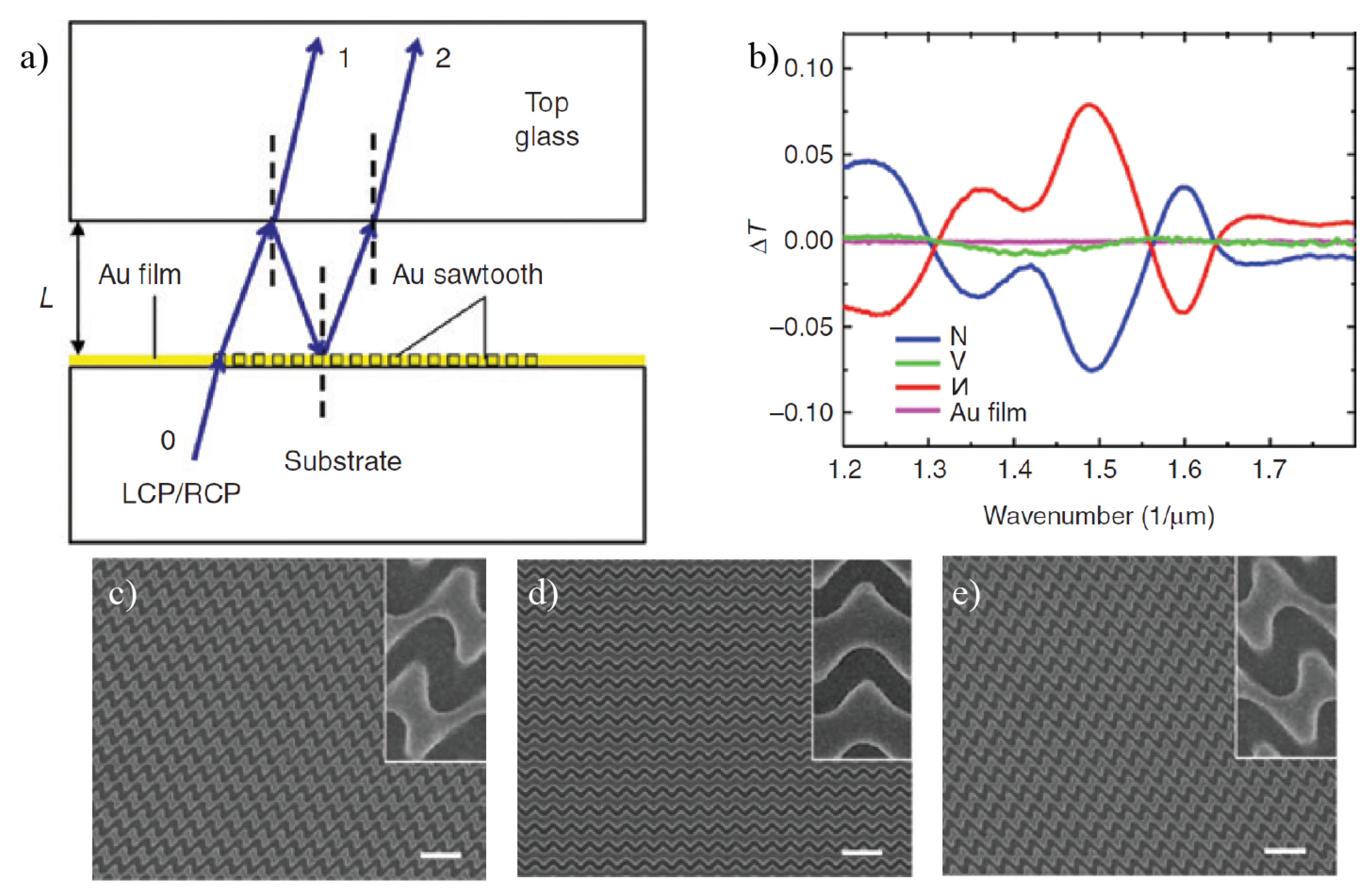
4. Chiral Plasmonic Metasurfaces
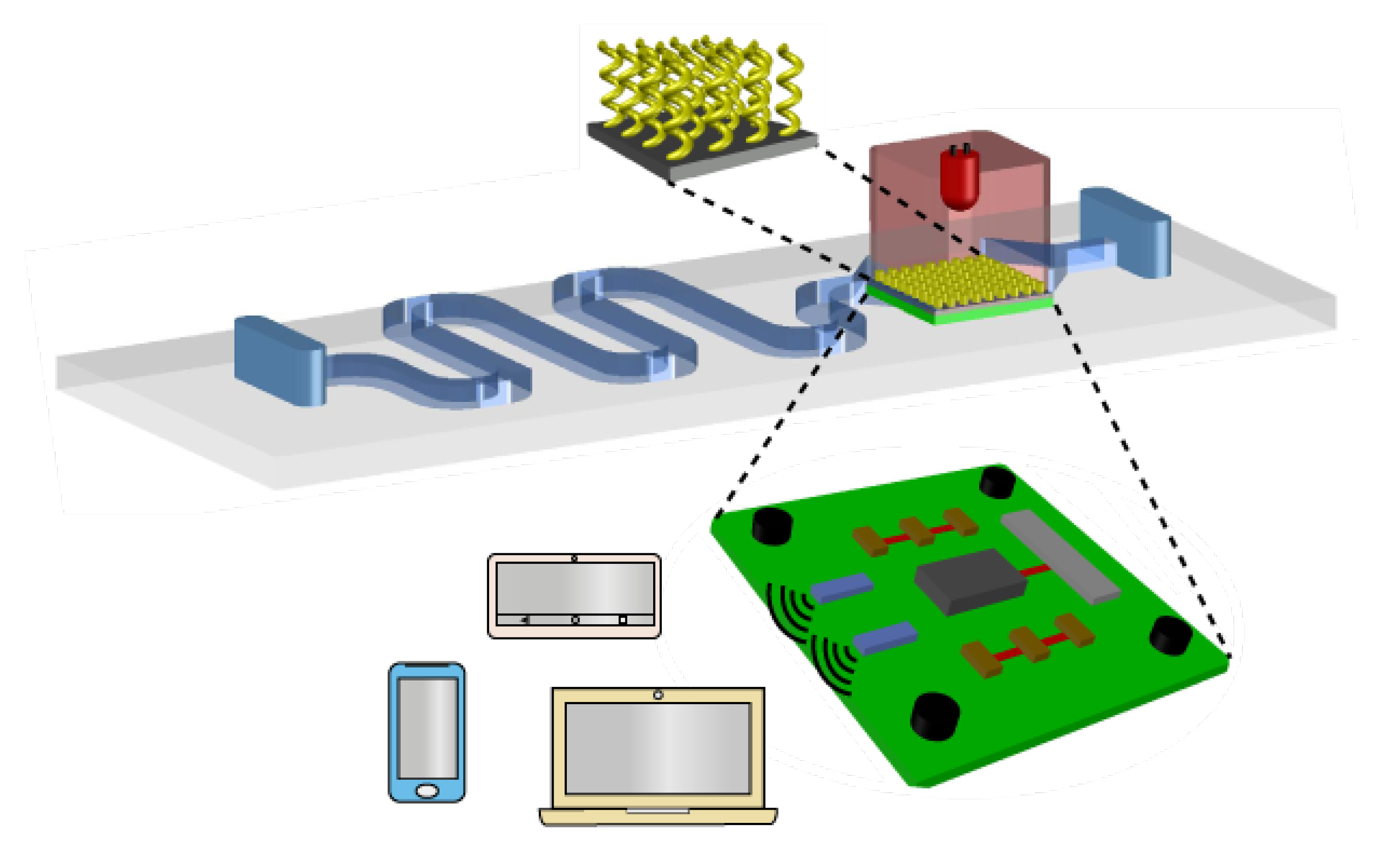
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- McConathy, J.; Owens, M.J. Stereochemistry in Drug Action. Primary Care Companion J. Clin. Psychiatry 2013, 5, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Shakya, P.; Kumar, A.; Alok, S.; Kamal, M.; Singh, S.P. Stereochemistry and its role in drug design. Int. J. Pharm. Sci. Res. 2014, 5, 4644–4659. [Google Scholar]
- Singh, R.; Nalwa, H.S. Medical Applications of Nanoparticles in Biological Imaging, Cell Labeling, Antimicrobial Agents and Anticancer Nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterization of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta 2014, 1839, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Zhou, J.; Wang, J.; Zhou, R.; Xu, B. Chirality Controls Reaction-Diffusion of Nanoparticles for Inhibiting Cancer Cells. Chem. Nano Mat. 2016, 3, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazayeria, M.H.; Aghaieb, T.; Avanc, A.; Vatankhahd, A.; Ghaffarid, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Hyu, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing...Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein Folding and Misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to Study Proteins by Circular Dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar]
- Lieberman, I.; Shemer, G.; Fried, T.; Kosower, E.M.; Markovich, G. Plasmon-Resonance-Enhanced Absorption and Circular Dichroism. Angew. Chem. Int. Ed. 2008, 47, 4855–4857. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.; Carpy, T.; Johnston, J.; Popland, M.; Mikhaylovskiy, R.V.; Lapthorn, A.J.; Kelly, S.M.; Barron, L.D.; Gadegaard, N.; Kadodwala, M. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 2010, 5, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, A.G.; Gibbs, J.G.; Lee, T.; Fischer, P. Hybrid nanocolloids with programmed three-dimensional shape and material composition. Nat. Mater. 2013, 12, 802–807. [Google Scholar] [CrossRef]
- Esposito, M.; Tasco, V.; Cucuna, M.; Todisco, F.; Benedetti, A.; Tarantini, I.; Giorgi, M.; Sanvitto, D.; Passaseo, A. Nanoscale 3D Chiral Plasmonic Helices with Circular Dichroism at Visible Frequencies. ACS Photonics 2014, 2, 105–114. [Google Scholar] [CrossRef]
- Nair, G.; Singh, H.J.; Paria, D.; Venkatapathi, M.; Ghosh, A. Plasmonic Interactions at Close Proximity in Chiral Geometries: Route toward Broadband Chiroptical Response and Giant Enantiomeric Sensitivity. J. Phys. Chem. C 2014, 118, 4991–4997. [Google Scholar] [CrossRef]
- Gutsche, P.; Mäusle, R.; Burger, S. Locally Enhanced and Tunable Optical Chirality in Helical Metamaterials. Photonics 2016, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Zu, S.; Bao, Y.; Fang, Z. Planar plasmonic chiral nanostructures. Nanoscale 2016, 8, 3900–3905. [Google Scholar] [CrossRef]
- Schäferling, M.; Yin, X.; Engheta, N.; Giessen, H. Helical Plasmonic Nanostructures as Prototypical Chiral Near-Field Sources. ACS Photonics 2014, 1, 530–537. [Google Scholar] [CrossRef]
- Schäferling, M.; Engheta, N.; Giessen, H.; Weiss, T. Reducing the Complexity: Enantioselective Chiral Near-Fields by Diagonal Slit and Mirror Configuration. ACS Photonics 2016, 3, 1076–1084. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Pan, L.; Fang, Y. Analyzing Intrinsic Plasmonic Chirality by Tracking the Interplay of Electric and Magnetic Dipole Modes. Sci. Rep. 2017, 7, 11151. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Oliveira, O.N., Jr. Plasmonic Biosensing. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Bochenkov, V.E.; Shabatina, T.I. Chiral Plasmonic Biosensors. Biosensors 2018, 8, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Xu, L.; Wang, L.; Xu, C.; Kuang, H. Chirality-Based Biosensors. Adv. Funct. Mater. 2019, 29, 1805512. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Deng, Z.; Ding, B.; Yan, H.; Liu, Y. DNA-Origami-Direct Self-Assembly of Discrete Silver-Nanoparticle Architectures. Angew. Chem. Int. 2010, 49, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Yin, X.; Schäferling, M.; Zhao, J.; Hein, S.M.; Braun, P.V.; Giessen, H. Large-Area 3D Chiral Plasmonic Structures. ACS Nano 2013, 7, 6321–6329. [Google Scholar] [CrossRef]
- Kühler, P.; Roller, E.M.; Schreiber, R.; Liedl, T.; Lohmüller, F.J. Plasmonic DNA-Origami Nanonantennas for Surface-Enhanced Raman Spectroscopy. Nano Lett. 2014, 14, 2914–2919. [Google Scholar] [CrossRef]
- Song, J.C.W.; Rudner, M.S. Chiral plasmons without magnetic field. Proc. Natl. Acad. Sci. USA 2016, 113, 4658–4663. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jia, H.; Yao, K.; Cai, W.; Chen, H.; Liu, Y. Circular Dichroism Metamirrors with Near-Perfect Extinction. ACS Photonics 2016, 3, 2096–2101. [Google Scholar] [CrossRef]
- Schnell, M.; Sarriugarte, P.; Neuman, T.; Khanikaev, A.B.; Shvets, G.; Aizpurua, J.; Hillenbrand, R. Real-Space Mapping of the Chiral Near-Field Distributions in Spiral Antennas and Planar Metasurfaces. Nano Lett. 2016, 16, 663–670. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Wang, G.; Fu, T.; Qu, Y.; Zhang, Z. Plasmonic chirality of L-shaped nanostructure composed of two slices with different thickness. Opt. Express 2016, 24, 2307–2317. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Yang, X.; Gao, J. All-metal structural color printing based on aluminum plasmonic metasurfaces. Opt. Express 2016, 24, 20472–20480. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chi, C.; Jiang, M.; Li, R.; Zu, S.; Li, Y.; Fang, Z. Plasmonic Chiral Nanostructures: Chiroptical Effects and Applications. Adv. Opt. Mater. 2017, 5, 1700040. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alú, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Tsakmakidis, K.L.; Askarpour, A.N.; Dehkhoda, P.; Tavakoli, A.; Altug, H. Nanophotonic Platforms for Enhanced Chiral Sensing. ACS Photonics 2018, 5, 2669–2675. [Google Scholar] [CrossRef]
- Woźniak, P.; De Leon, I.; Höflich, K.; Haverkamp, C.; Christiansen, S.; Leuchs, G.; Banzer, P. Chiroptical response of a single plasmonic nanohelix. Opt. Express 2018, 15, 19275–19293. [Google Scholar] [CrossRef]
- Kong, X.T.; Khorashad, L.K.; Wnag, Z. Photothermal Circular Dichroism Induced by Plasmon Resonances in Chiral Metamaterial Absorbers and Bolometers. Nano Lett. 2018, 18, 2001–2008. [Google Scholar] [CrossRef]
- Kaniewska, M.; Trojanowicz, M. Chiral Biosensors and Immunosensors. In Biosensors: Emerging Materials and Applications; In Tech: Rijeka, Croatia, 2011; Volume 7. [Google Scholar]
- Murugkar, S.; Leon, I.D.; Horton, M.; Qassin, H.; Leach, J.; Boyd, R.W. Planar chiral metamaterials for biosensing applications. Plasmonics Biol. Med. X 2013, 8597, 85970Y. [Google Scholar]
- Wu, T.; Ren, J.; Wang, R.; Zhang, X. Competition of Chiroptical Effect Caused by Nanostructures and Chiral Molecules. J. Phys. Chem. C 2014, 118, 20529–20537. [Google Scholar] [CrossRef] [Green Version]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological samples. TrAC Trends Anal. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhang, H.F.; Li, M.; Ng, S.W.; Feng, J.H.; Mao, G.G. Chiroptical Activity from an Achiral Biological Meta-Organic Framework. J. Am. Chem. Soc. 2018, 140, 11569–11572. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cohen, A.E. Optical Chirality and Its Interaction with Matter. Phys. Rev. Lett. 2010, 104, 163901. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A. On the Optical Rotary Dispersion of Polymers. J. Chem. Phys. 1965, 43, 959. [Google Scholar] [CrossRef]
- Lipkin, D.M. Existence of a New Conservation Law in Electromagnetic Theory. J. Math. Phys. 1964, 5, 696. [Google Scholar] [CrossRef]
- Poulikakos, L.V.; Thureja, P.; Stollmann, A.; de Leo, E.; Norris, D.J. Chiral Light Design and Detection Inspired by Optical Antenna Theory. Nano Lett. 2018, 18, 4633–4640. [Google Scholar] [CrossRef]
- Pham, A.; Zhao, A.; Genet, C.; Drezet, A. Optical Chirality Density and Flux Measured in the Local Density of States of Spiral Plasmonic Structures. Phys. Rev. A 2018, 98, 013837. [Google Scholar] [CrossRef]
- Aba, T.; Qu, Y.; Abudukelimu, A.; Ullah, H.; Zhang, Z. Chiral Response of a Metasurface Composed of Nanoholes and Tilted Nanorods. Appl. Opt. 2019, 58, 5936–5941. [Google Scholar] [CrossRef]
- Rajaei, M.; Zeng, J.; Albooyeh, M.; Kamandi, M.; Hanifeh, M.; Capolino, F.; Wickramasinghe, H.K. Giant Circular Dichroism at Visible Frequencies Enabled by Plasmonic Ramp-Shaped Nanostructures. ACS Photonics 2019, 6, 924–931. [Google Scholar] [CrossRef]
- Gilroy, C.; Hashiyada, S.; Endo, K.; Karimullah, A.S.; Barron, L.D.; Okamoto, H.; Togawa, Y.; Kadodwala, M. Roles of Superchirality and Interference in Chiral Plasmonic Biodetection. J. Phys. Chem. C 2019, 123, 15195–15203. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, J.; Yang, X. Chiral Grayscale Imaging with Plasmonic Metasurfaces of Stepped Nanoapertures. Adv. Opt. Mater. 2019, 7, 1801467. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Rong, X.; Luo, Y.; Chen, H.; Zheng, L.; Lin, F.; Shen, B.; Gong, Y.; Zhang, S.; et al. Tailoring MoS2 Valley-Polarized Photoluminescence with Super Chiral Near-Field. Adv. Mater. 2018, 30, 1801908. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.L.; Lin, Z.-H.; Kuo, H.Y.; Huang, T.-T.; Huang, Y.T.; Chung, T.L.; Chu, C.H.; Huang, J.-S.; Tsai, D.P. Stress-Induced 3D Chiral Fractal Metasurface for Enhanced and Stabilized Broadband Near-Field Optical Chirality. Adv. Opt. Mater. 2019, 7, 1900617. [Google Scholar] [CrossRef]
- Champi, H.A.A.; Bustamante, R.H.; Salcedo, W.J. Optical Enantioseparation of Chiral Molecules Using Asymmetric Plasmonic Nanoapertures. Opt. Mater. Exp. 2019, 9, 1763. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Adamo, G.; Teh, B.H.; Wu, Q.Y.S.; Teng, J.; Sun, H. A Novel Chiral Metasurface with Controllable Circular Dichroism Induced by Coupling Localized and Propagating Modes. Adv. Opt. Mater. 2016, 4, 883–888. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, L.; Wang, L.; Zhang, Z. Giant circular dichroism induced by tunable resonance in twisted Z-shaped nanostructure. Opt. Exp. 2017, 25, 5480. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Khorashad, L.K.; Gadegaard, N.; Barron, L.D.; Govorov, A.O.; Karimullah, A.S.; Kadodwala, M. Controlling Metamaterial Transparency with Superchiral Fields. ACS Photonics 2018, 5, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Huang, Y.; Qv, L.; Fang, Y. Electromagnetic Energy Redistribution in Coupled Chiral Particle Chain-Film System. Nanoscale Res. Lett. 2018, 13, 194. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Gao, J. Spin-Controlled Wavefront Shaping with Plasmonic Chiral Geometric Metasurfaces. Light Sci. Appl. 2018, 7, 84. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, W.; Zhang, J.; Zhang, L.; Xue, T.; Liu, M.; Meng, C.; Mao, D.; Mei, T. Dynamic manipulation of optical chirality for gammadion nanostructures. Appl. Phys. Exp. 2019, 12, 072015. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Wang, Y.; Xiao, L. Tunable atom-trapping based on a plasmonic chiral metamaterial. Nanophotonics 2019, 8, 1739–1745. [Google Scholar] [CrossRef]
- Yin, S.; Ji, W.; Xiao, D.; Li, Y.; Li, K.; Yin, Z.; Jiang, S.; Shao, L.; Luo, D.; Liu, Y.J. Intrinsically or extrinsically reconfigurable chirality in plasmonic chiral metasurfaces. Opt. Commun. 2019, 448, 10–14. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Gao, J. 3D Janus Plasmonic Helical Nanoapertures for Polarization-Encrypted Data Storage. Light Sci. Appl. 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, J.; Feng, J.; Qian, Q.; Zhou, Y. Reversible modulation of plasmonic chiral signals of achiral gold nanorods using a chiral supramolecular template. Chem. Commun. 2019, 55, 11378–11381. [Google Scholar] [CrossRef] [PubMed]
- Hashiyada, S.; Narushima, T.; Okamoto, H. Active Control of Chiral Optical near Fields on a Single Metal Nanorod. ACS Photonics 2019, 6, 677–683. [Google Scholar] [CrossRef]
- Horrer, A.; Zhang, Y.; Gérard, D.; Béal, J.; Kociak, M.; Plain, J.; Bachelot, R. Local Optical Chirality Induced by Near-Field Mode Interference in Achiral Plasmonic Metamolecules. Nano Lett. 2020, 20, 509–516. [Google Scholar] [CrossRef]
- Reyntjens, S.; Puers, R. Focused ion beam induced deposition: Fabrication of three-dimensional microstructures and Young’s modulus of the deposited material. J. Micromech. Microeng. 2000, 10, 181–188. [Google Scholar] [CrossRef]
- Huth, M.; Porrati, F.; Dobrovolskiy, O.V. Focused electron beam induced deposition meets materials science. Microelectron. Eng. 2018, 185, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Schneider, F.; Möritz, N.; Dietz, H. The sequence of events during folding of a DNA origami. Sci. Adv. 2019, 5, eaaw1412. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Duan, X.; Liu, N. A plasmonic nanorod that walks on DNA origami. Nat. Commun. 2015, 6, 8102. [Google Scholar] [CrossRef]
- Huang, Y.; Nguyen, M.; Natarajan, A.K.; Nguyen, V.H. A DNA Origami-Based Chiral Plasmonic Sensing Device. Appl. Mater. Interfaces 2018, 10, 44221–44225. [Google Scholar] [CrossRef] [Green Version]
- Shemer, G.; Krichevski, O.; Markovich, G.; Molotsky, T.; Lubitz, T.; Kotyar, A.B. Chirality of Silver Nanoparticles Synthesized on DNA. J. Am. Chem. Soc. 2006, 128, 11006–11007. [Google Scholar] [CrossRef]
- Swasey, S.M.; Karimova, M.; Aikens, C.M.; Schultz, D.E.; Simon, A.J.; Gwinn, E.G. Chiral Electronic Transitions in Fluorescent Silver Cluster Stabilized by DNA. ACS Nano 2014, 8, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Luong, N.; Fan, Z.; Kuzyk, A.; Nickels, P.C.; Zhang, T.; Smith, D.M.; Yurke, B.; Kuang, W.; Govorov, A.O.; et al. Chiral plasmonic DNA nanostructures with switchable circular dichroism. Nat. Commun. 2013, 4, 2948. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Govorov, A.O. Helical Metal Nanoparticle Assemblies with Defects: Plasmonic Chirality and Circular Dichroism. J. Phys. Chem. 2011, 115, 13254–13261. [Google Scholar] [CrossRef]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Kar, S.; Anupriya, E.S.; Somasundaran, S.M.; Das, A.D.; Sissa, C.; Painelli, A.; Thomas, K.G. Chiral Plasmons: Au Nanoparticle Assemblies on Thermoresponsive Organic Template. ACS Nano 2019, 13, 4392–4401. [Google Scholar] [CrossRef]
- Kumar, J.; Eraña, H.; López-Martínez, E.; Claes, N.; Martín, V.F.; Solís, D.M.; Bals, S.; Cortajarena, A.L.; Castilla, J.; Liz-Marzán, L.M. Detection of Amyloid Fibrils in Parkinson’s Disease Using Plasmonic Chirality. Proc. Natl. Acad. Sci. USA 2018, 115, 3225–3230. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Tatsuma, T. Chiral Plasmonic Nanostructures Fabricated by Circularly Polarized Light. Nano Lett. 2018, 18, 3209–3212. [Google Scholar] [CrossRef]
- Hentschel, M.; Schäferling, M.; Duan, X.; Giessen, H.; Liu, N. Chiral plasmonics. Sci. Adv. 2017, 3, e1602735. [Google Scholar] [CrossRef] [Green Version]
- Schäferling, M.; Dregely, D.; Hentschel, M.; Giessen, H. Tailoring Enhanced Optical Chirality: Design Principles for Chiral Plasmonic Nanostructure. Phys. Rev. X 2012, 2, 031010. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Saleh, A.A.E.; Dionne, J.A. Enantioselective Optical Trapping of Chiral Nanoparticles with Plasmonic Tweezers. ACS Photonics 2016, 3, 304–309. [Google Scholar] [CrossRef]
- Chen, H.T.; Taylor, A.J.; Yu, N. A review of metasurfaces: Physics and applications. Rep. Prog. Phys. 2016, 79, 076401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glybovski, S.B.; Tretyakov, S.A.; Belov, P.A.; Kivshar, Y.S.; Simovski, C.R. Metasurfaces: From Microwaves to Visible. Phys. Rep. 2016, 634, 1–72. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Gao, J.; Yang, X. Near-infrared chiral plasmonic metasurface absorbers. Opt. Express 2018, 26, 31484–31489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yao, Z.; Wang, Q.; Hu, F.; Xu, X. Coupling Tai Chi Chiral Metamaterials with Strong Optical Activity in Terahertz Region. Plasmonics 2015, 10, 1005–1011. [Google Scholar] [CrossRef]
- Sezen, M. Focused Ion Beam (FIB)—Novel Methodologies and Recent Applications for Multidisciplinary Sciences. Mod. Electron Microsc. Phys. Life Sci. 2016, 18, 121–140. [Google Scholar]
- Chen, Y.; Gao, J.; Yang, X. Chiral Metamaterials of Plasmonic Slanted Nanoapertures with Symmetry Breaking. Nano Lett. 2018, 18, 520–527. [Google Scholar] [CrossRef]
- Ndao, A.; Belkhir, A.; Salut, R.; Baida, F.I. Slanted annular aperture arrays as enhanced-transmission metamaterials: Excitation of the plasmonic transverse electromagnetic guided mode. Appl. Phys. Lett. 2013, 103, 211901. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Khoo, E.H.; Leong, E.S.P.; Hu, L.; Jiang, X.; Li, Y.; Luo, D.; Si, G.; Liu, Y.J. Maskless fabrication of slanted annular aperture arrays. Nanotechnology 2017, 28, 225302. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Q.; Wang, X.; Gao, W.; Li, J.; Tam, W.Y. Measuring circular phase-dichroism of chiral metasurface. Nanophotonics 2019, 8, 909–920. [Google Scholar] [CrossRef]
- Ji, R.; Wang, S.-W.; Liu, X.; Guo, H.; Lu, W. Hybrid helical metamaterials for giant and ultra-wide circular dichroism. ACS Photonics 2016, 12, 2368–2374. [Google Scholar] [CrossRef]
- Wang, Z.; Wnag, Y.; Adamo, G.; Teng, J.; Sun, H. Induced Optical Chirality and Circularly Polarized Emission from Achiral CdSe/ZnS Quantum Dots via Resonantly Coupling with Plasmonic Chiral Metasurfaces. Laser Photonics Rev. 2019, 13, 1800276. [Google Scholar] [CrossRef]
- Fang, Y.; Verre, R.; Shao, L.; Nordlander, P.; Käll, M. Hot Electron Generation and Cathodoluminescence Nanoscopy of Chiral Split Ring Resonators. Nano Lett. 2016, 16, 5183–5190. [Google Scholar] [CrossRef]
- Liu, H.; Shang, Z.; Wu, X.; Dou, C.; Zhang, J. Tunable circular dichroism of bilayer b-type chiral nanostructure. Opt. Commun. 2019, 448, 76–81. [Google Scholar] [CrossRef]
- Rifat, A.A.; Rahmani, M.; Xu, L.; Miroshnichenko, E. Hybrid Metasurface Based Tunable Near-Perfect Absorber and Plasmonic Sensor. Materials 2018, 11, 1091. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wei, H.; Bao, K.; Håkanson, U.; Halas, N.J.; Nordlander, P.; Xu, H. Chiral Surface Plasmon Polaritons on Metallic Nanowires. Phys. Rev. Lett. 2011, 107, 096801. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Yang, X.; Gao, J. Realizing structural color generation with aluminum plasmonic V-groove metasurface. Opt. Express 2017, 25, 20454–20465. [Google Scholar] [CrossRef]
- St. John, A.; Price, C.P. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar]
- Shaw, L.V. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chen, Z.; Cheng, F.; Zhang, Y.; Chen, L. Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic-like reaction mediated etching of gold nanorods. Biosens. Bioelectron. 2017, 89, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.M.; Venner, A.A.; Shea, J.; Fuezery, A.; Huang, Y.; Massicote, L.; Tetreault, N.; Tomalty, C.; Shaw, J.L.V. Point-of-care testing: A position statement from the Canadian Society of Clinical Chemists. Clin. Biochem. 2018, 53, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Baek, S.H.; Nguyen, T.P.; Park, K.Y.; Kim, E.B.; Kim, J.G.; Park, J.P.; Kailasa, S.K.; Kim, H.J.; Chung, C.; et al. Gold-copper nanoshell dot-blot immunoassay for naked-eye sensitive detection of tuberculosis specific CFP-10 antigen. Biosens. Bioelectron. 2018, 121, 111–117. [Google Scholar]
- Goel, S. From waste to watts in micro-devices: Review on development of Membranedand Membraneless Microfluidic Microbial Fuel Cell. Appl. Mater. Today 2018, 11, 270–279. [Google Scholar] [CrossRef]
- Mazzotta, F.; Höök, F.; Jonsson, M.P. High throughput fabrication of plasmonic nanostructures in nanofluidic pores for biosensing applications. Nanotechnology 2012, 23, 415304. [Google Scholar] [CrossRef]
- Gomez, F.A. The future of microfluidic point-of-care diagnostic devices. Bioanalysis 2013, 5, 1–3. [Google Scholar] [CrossRef]
- Oh, B.R.; Huang, N.T.; Chen, W.; Seo, J.H.; Chen, P.; Cornell, T.T.; Shanley, T.P.; Fu, J.; Kurabayashi, K. Integrated Nanoplasmonic Sensing for Cellular Functional Immunoanalysis Using Human Blood. ACS Nano 2014, 8, 2667–2676. [Google Scholar] [CrossRef]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosters, L.; Buclin, T.; Guiducci, C. Label-Free Detection of Tobramycin in Serum by Transmission-Localized Surface Plasmon Resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Li, J. Construction of Plasmonic Nano-Biosensor-Based Devices for Point-of-Care Testing. Small Methods 2017, 1, 1700197. [Google Scholar] [CrossRef]
- Wang, L.J.; Chang, Y.C.; Sun, R.; Li, L. A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Werable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing-xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Vilela, P.H.; Rodrigues, J.J.P.C.; Solic, P.S.; Saleem, K.; Furtado, V. Performance evaluation of a Fog-assisted IoT solution for e-Health applications. Future Gener. Comput. Syst. 2019, 97, 379–386. [Google Scholar] [CrossRef]
- Sacha, G.M.; Varona, P. Artificial intelligence in nanotechnology. Nanotechnology 2013, 24, 452002. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Cheng, F.; Liu, Y. Deep-Learning-Enable On-Demand Design of Chiral Metamaterials. ACS Nano 2018, 12, 6326–6334. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An Emerging Era in the Management of Parkinson’s Disease: Wearable Technologies and the Internet of Things. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Klímová, B.; Kucča, K. Internet of Things in the Assesment, Diagnostics and Treatment of Parkinson’s Disease. Health Technol. 2019, 9, 87–91. [Google Scholar] [CrossRef]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, M.; Woolley, T. Point of Care Testing. Surgery 2016, 34, 91–93. [Google Scholar] [CrossRef]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- King, K.R.; Grazette, L.P.; Paltoo, D.N.; MCDevitt, J.T.; Sia, S.K.; Barrett, P.M.; Apple, F.S.; Gurbel, P.A.; Weissleder, R.; Leeds, H.; et al. Point-of-Care Technologies for Precision Cardiovascular Care and Clinical Research. JACC Basic Transl. Sci. 2016, 1, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.; Sridhara, A.; Melo, R.; Richer, L.; Chee, N.H.; Kim, J.; Linder, V.; Steinmiller, D.; Sia, S.K.; Gomes-Solecki, M. Microfluidics-based point-of-care test for serodiagnosis of Lyme Disease. Sci. Rep. 2016, 6, 35069. [Google Scholar] [CrossRef]
- Darwish, N.T.; Sekaran, S.D.; Khor, S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators B 2018, 255, 3316–3331. [Google Scholar] [CrossRef]
- Loubier, S.; Moatti, J.P. Economic evaluation of point-of-care diagnostic technologies for infectious diseases. Clin. Microbiol. Infect. 2010, 16, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.J.; You, P.; Fridley, G.; Mabey, D.; Peeling, R. Point-of-care diagnostic tests for low-resource settings. The Lancet Glob. Health 2015, 3, 257–258. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.A. Clinical Pharmacogenomics: Opportunities and Challenges at Point of Care. Clin. Pharmacol. Ther. 2013, 93, 33–35. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Microfluidics 2016, 10, 024119. [Google Scholar] [CrossRef] [PubMed]
- Rebouda, J.; Xub, G.; Garretta, A.; Adrikoc, M.; Yanga, Z.; Tukahebwac, E.M.; Rowellc, C.; Coopera, M. Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc. Natl. Acad. Sci. USA 2019, 116, 4834–4842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.A.G.; Materon, E.M.; Melendez, M.E.; Carvalho, A.L.; Faria, R.C. Disposable Microfluidic Immunoarray Device for Sensitive Breast Cancer Biomarker Detection. ACS Appl. Mater. Interfaces 2017, 9, 27433–27440. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Paiva-Marques, W.; Reyes Gómez, F.; N. Oliveira, O., Jr.; Mejía-Salazar, J.R. Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors 2020, 20, 944. https://doi.org/10.3390/s20030944
A. Paiva-Marques W, Reyes Gómez F, N. Oliveira O Jr., Mejía-Salazar JR. Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors. 2020; 20(3):944. https://doi.org/10.3390/s20030944
Chicago/Turabian StyleA. Paiva-Marques, Willian, Faustino Reyes Gómez, Osvaldo N. Oliveira, Jr., and J. Ricardo Mejía-Salazar. 2020. "Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications" Sensors 20, no. 3: 944. https://doi.org/10.3390/s20030944
APA StyleA. Paiva-Marques, W., Reyes Gómez, F., N. Oliveira, O., Jr., & Mejía-Salazar, J. R. (2020). Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors, 20(3), 944. https://doi.org/10.3390/s20030944





