Simulation Study for Designing a Dedicated Cardiac TOF-PET System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geometry System Description
2.2. Monte Carlo Simulations
2.2.1. Scanner Simulations with Different TOF Values
2.2.2. Simulations for Sensitivity Evaluation
2.2.3. Simulations for Spatial Resolution Evaluation
2.3. Image Quality Indicators
3. Results
3.1. Monte Carlo Simulation Results
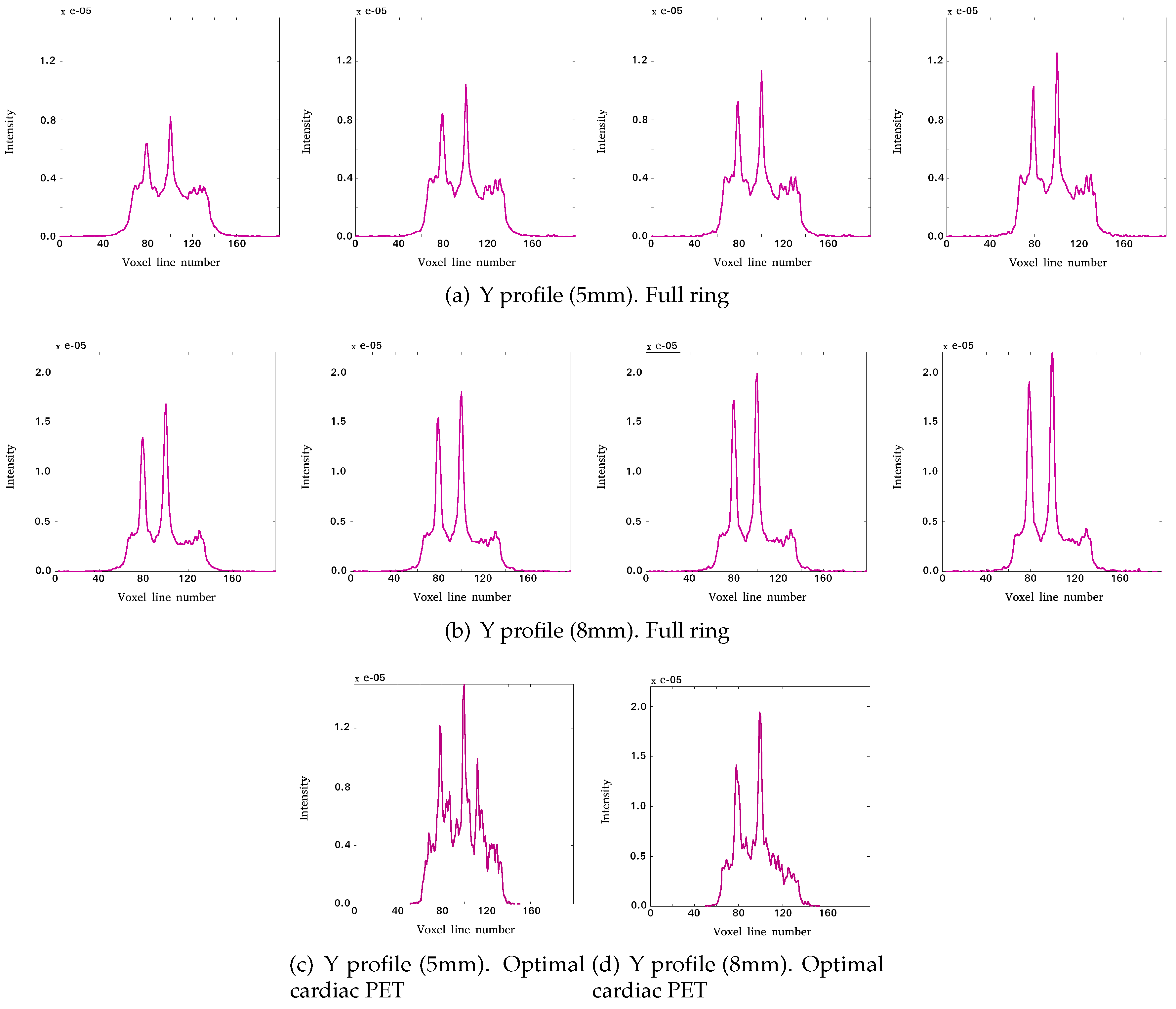
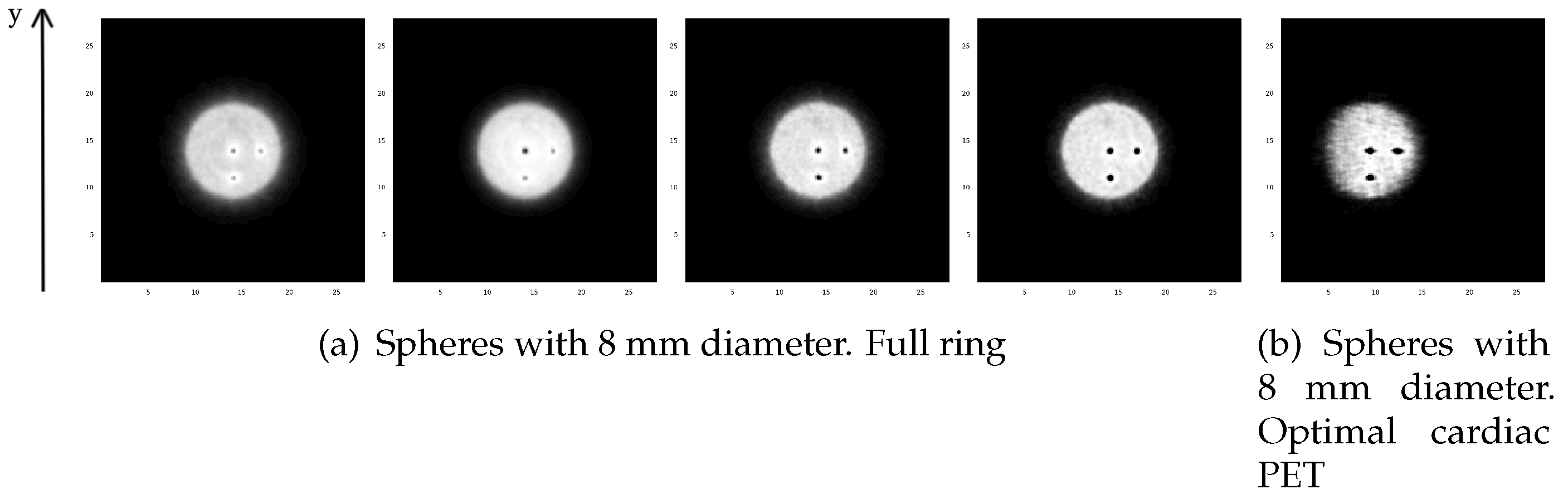
3.1.1. Reconstructed Images for Different TOF Value Simulations
3.1.2. Sensitivity Evaluation Results
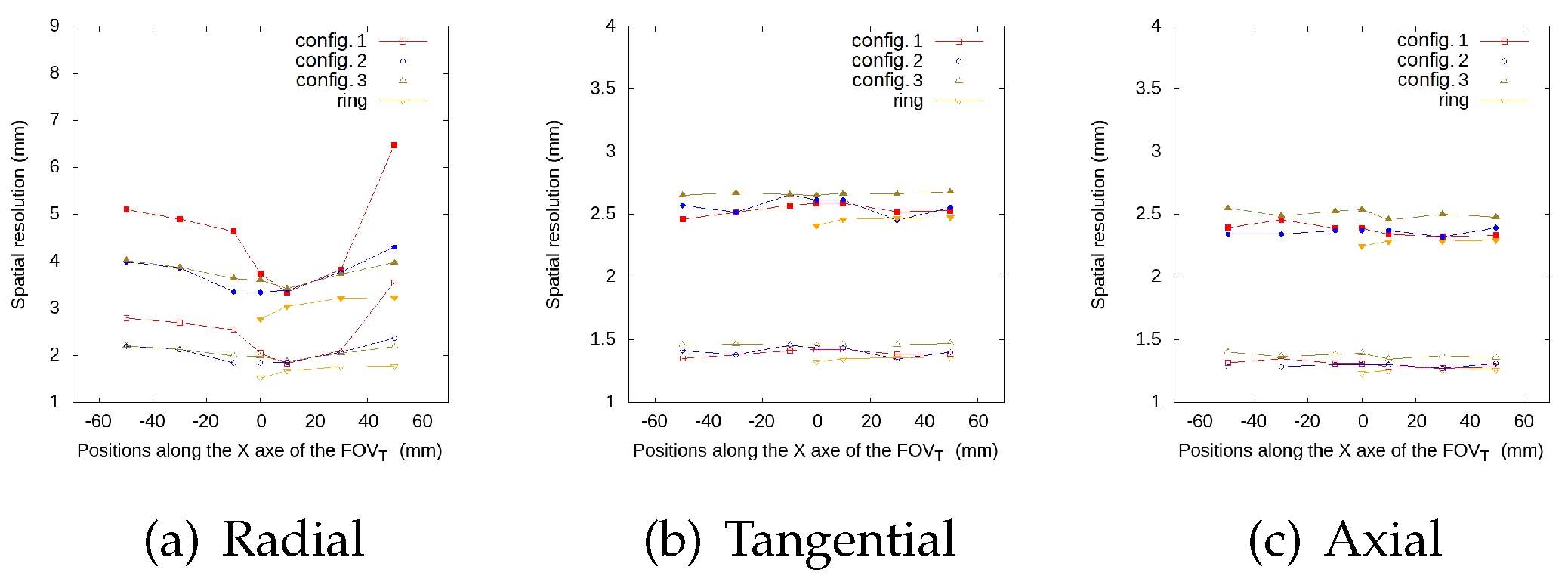
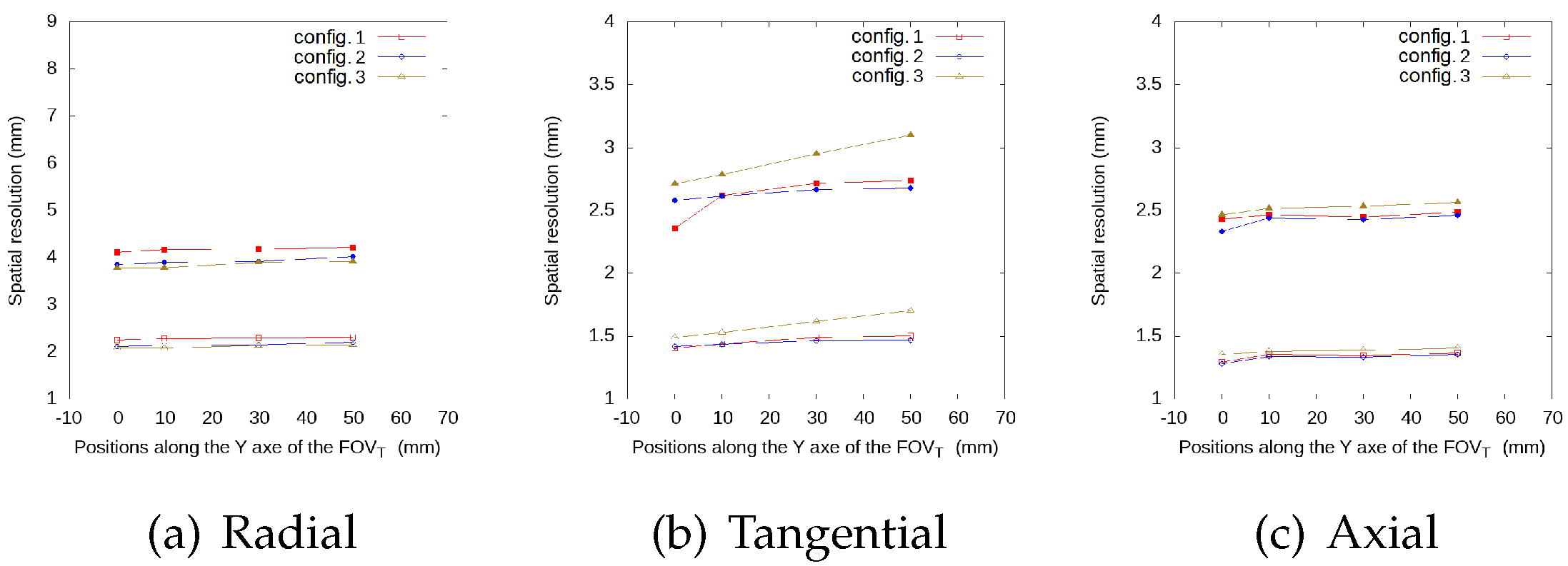
3.1.3. Spatial Resolution Evaluation Results
3.2. Impact of TOF in Image Quality Indicators
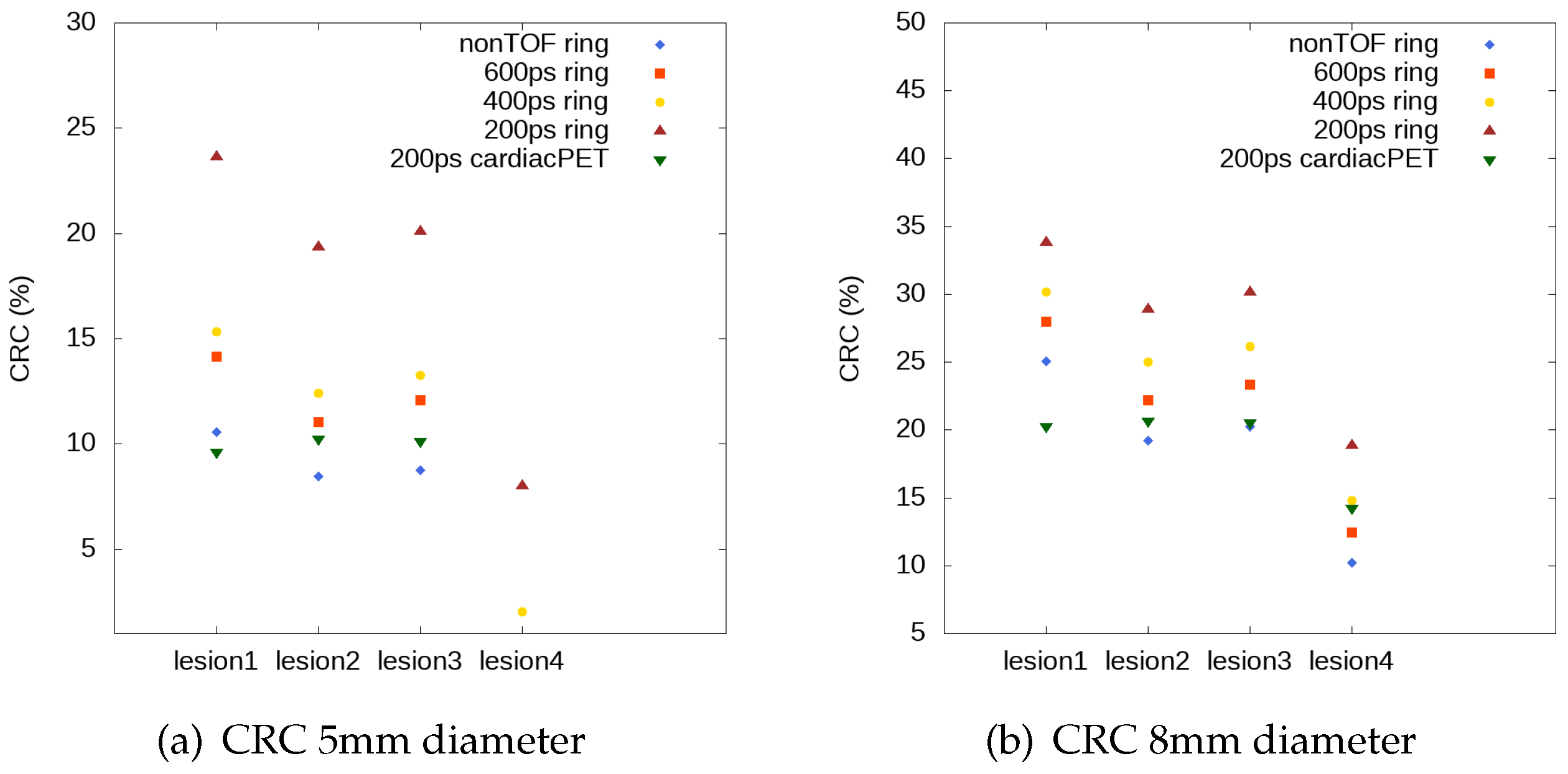
3.2.1. Contrast Recovery Coefficient Evaluation
4. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Gaemperli, O.; Kaufmann, P.A. PET and PET/CT in cardiovascular disease. Ann. N.Y. Acad. Sci. 2011, 1228, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bengel, F.M. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia. JACC Cardiovasc. Imaging 2018, 11, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gupte, A.; Zhang, A.; Hamilton, D.J. Pet imaging and its application in cardiovascular diseases. Methodist Debakey Cardiovasc J. 2017, 1, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Orozco, L.E.; Tio, R.A.; Alexanderson, E.; Dweck, M.; Vliegenthart, R.; El Moumni, M.; Prakken, N.; Gonzalez-Godinez, I.; Slart, R.H. Quantitative myocardial perfusion evaluation with positron emission tomography and the risk of cardiovascular events in patients with coronary artery disease: A systematic review of prognostic studies. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Schelbert, H.R. Quantification of myocardial blood flow: What is the clinical role? Cardiol. Clinics 2009, 27, 277–289. [Google Scholar] [CrossRef]
- Knuuti, J.; Kajander, S.; Mäki, M.; Ukkonen, H. Quantification of myocardial blood flow will reform the detection of CAD. J. Nucl. Cardiol. 2009, 16, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Peng, H. Design study of a cardiac-dedicated PET system. Nucl. Instrum. Methods Phys. Res. Sect. A 2015, 779, 39–46. [Google Scholar] [CrossRef]
- González, A.J.; Sánchez, F.; Benlloch, J.M. Organ-dedicated molecular imaging systems. Trans. Radiat. Plasma Med. Sci. 2018, 2, 388–403. [Google Scholar] [CrossRef]
- Moliner, L.; Rodríguez-Alvarez, M.J.; Catret, J.V.; González, A.; Ilisie, V.; Benlloch, J.M. NEMA performance evaluation of caremibrain dedicated brain PET and comparison with the whole-body and dedicated brain PET systems. Sci. Rep. 2019, 9, 15484. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.M.; Tashima, H.; Yoshida, E.; Nishikido, F.; Yamaya, T. Simulation study comparing the helmet-chin PET with a cylindrical PET of the same number of detectors. Phys. Med. Biol. 2017, 62, 4541. [Google Scholar] [CrossRef]
- Cho, Z.H.; Son, Y.D.; Kim, H.K.; Kwon, D.H.; Joo, Y.H.; Ra, J.B.; Choi, Y.; Kim, Y.B. Development of positron emission tomography with wobbling and zooming for high sensitivity and high-resolution molecular imaging. IEEE Trans. Med. Imaging 2019, 38, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Surti, S.; Karp, J.S. Design considerations for a limited-angle, dedicated breast, TOF PET scanner. Phys. Med. Biol. 2008, 53, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Surti, S.; Karp, J.S. Advances in time-of-flight PET. Physica Med. 2016, 32, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, C.S.; Deller, T.W.; Delso, G.; Khalighi, M.M.; Maramraju, S.H.; Grant, A.M. NEMA NU 2-2012 performance studies for the SiPM-based ToF-PET component of the GE SIGNA PET/MR system. Med. Phys. 2016, 43, 2334–2343. [Google Scholar]
- Van Sluis, J.; De Jong, J.; Schaar, J.; Noordzij, W.; Van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance characteristics of the digital biograph vision PET/CT system. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef]
- Ito, M.; Lee, M.S.; Lee, J.S. Continuous depth-of-interaction measurement in a single-layer pixelated crystal array using a single-ended readout. Phys. Med. Biol. 2013, 58, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- Bugalho, R.; Di Francesco, A.; Ferramacho, L.; Leong, C.; Niknejad, T.; Oliveira, L.; Pacher, L.; Da Rocha Rolo, M.; Rivetti, A.; Silveira, M.; et al. Experimental results with TOFPET2 ASIC for time-of-flight applications. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 912, 195–198. [Google Scholar] [CrossRef]
- Lecoq, P.; Gundacker, S.; Auffray, E.; Frisch, B.; Jarron, P.; Knapitsch, A.; Meyer, T.; Pizzichemi, M. Time of flight positron emission tomography towards 100ps resolution with L(Y)SO: An experimental and theoretical analysis. J. Instrum. 2013, 8, P07014. [Google Scholar]
- Salvat, F.; PENELOPE. A Code System for Monte Carlo Simulation of Electron and Photon Transport. Available online: http://www.oecd-nea.org/lists/penelope.html (accessed on 26 July 2019).
- Strydhorst, J.; Buvat, I. Redesign of the GATE PET coincidence sorter. Phys. Med. Biol. 2016, 61, N522–N531. [Google Scholar] [CrossRef]
- Baró, J.; Sempau, J.; Fernández-Varea, J.M.; Salvat, F. Penelope: An algorithm for Monte Carlo simulation of the penetration and energy loss of electrons and positrons in matter. Nucl. Instrum. Meth. 1995, 100, 31–46. [Google Scholar] [CrossRef]
- Sempau, J.; Acosta, E. An algorithm for Monte Carlo simulation of coupled electron-photon showers. Nucl. Instrum. Methods 1997, 132, 377. [Google Scholar] [CrossRef]
- Sempau, J.; Fernández-Varea, J.M.; Acosta, E.; Salvat, F. Experimental benchmarks of the Monte Carlo code penelope. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 207, 107–123. [Google Scholar] [CrossRef]
- Reader, A.; Zhao, S.; Walledge, R.; Ally, S.; Zweit, J.; Hastings, D.; Jeavons, A.; Julyan, P.; Manavaki, R.; Bakatselos, F. One-pass list-mode EM algorithm for high-resolution 3-D PET image reconstruction into large arrays. IEEE Trans. Nucl. Sci. 2002, 49, 693–699. [Google Scholar] [CrossRef]
- Spanoudaki, V.C.; Levin, C.S. Photo-detectors for time of flight positron emission tomography (ToF-PET). Sensors (Switzerland) 2010, 10, 10484–10505. [Google Scholar] [CrossRef]
- Jacobs, F.; Sundermann, E.; De Sutter, B.; Christiaens, M.; Lemahieu, I. A fast algorithm to calculate the exact radiological path through a pixel or voxel space. J. Comput. Inf. Technol. 1998, 1, 89–94. [Google Scholar]
- Siddon, R.L. Fast calculation of the exact radiological path for a three-dimensional CT array. Med. Phys. 1985, 12, 252–255. [Google Scholar] [CrossRef]
- Vandenberghe, S.; Daube-Witherspoon, M.E.; Lewitt, R.M.; Karp, J.S. Fast reconstruction of 3D time-of-flight PET data by axial rebinning and transverse mashing. Phys. Med. Biol. 2006, 51, 1603–1621. [Google Scholar] [CrossRef]
- NEMA Standards Publication NU 2-2012. Performance Measurements of Positron Emission Tomographs. Available online: https://www.nema.org/Standards/ComplimentaryDocuments/Contents%20and%20Scope%20NEMA%20NU%202%202012.pdf (accessed on 26 July 2019).
- Yu, W.; Zeng, L. A novel weighted total difference based image reconstruction algorithm for few-view computed tomography. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Ravindranath, B.; Junnarkar, S.S.; Purschke, M.L.; Maramraju, S.H.; Hong, X.; Tomasi, D.; Bennett, D.; Cheng, K.; Southekal, S.S.; Stoll, S.P.; et al. Results from Prototype II of the BNL Simultaneous PET-MRI Dedicated Breast Scanner. In Proceedings of the IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009. [Google Scholar]
- Tashima, H.; Yamaya, T.; Yoshida, E.; Kinouchi, S.; Watanabe, M.; Tanaka, E. A single-ring OpenPET enabling PET imaging during radiotherapy. Phys. Med. Biol. 2012, 57, 4705. [Google Scholar] [CrossRef]
- Yamaya, T.; Inaniwa, T.; Minohara, S.; Yoshida, E.; Inadama, N.; Nishikido, F.; Shibuya, K.; Lam, C.F.; Murayama, H. A proposal of an open PET geometry. Phys. Med. Biol. 2008, 53, 757–773. [Google Scholar] [CrossRef]
- Miyake, K.K.; Matsumoto, K.; Inoue, M.; Nakamoto, Y.; Kanao, S.; Oishi, T.; Kawase, S.; Kitamura, K.; Yamakawa, Y.; Akazawa, A.; et al. Performance Evaluation of a New Dedicated Breast PET Scanner Using NEMA NU4-2008 Standards. J. Nucl. Med. 2014, 55, 1198–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yu, W.; Xie, S. A Dedicated PET System for Human Brain and Head/Neck Imaging. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference, Seoul, Korea, 27 October–2 November 2013. [Google Scholar]
- Yamamoto, S.; Honda, M.; Oohashi, T.; Shimizu, K.; Senda, M. Development of a brain PET system, PET-Hat: A wearable PET system for brain research. IEEE Trans. Nucl. Sci. 2011, 58, 668–673. [Google Scholar] [CrossRef]
- Zorraquino, C.; Bugalho, R.; Rolo, M.; Silva, J.C.; Veckalns, V.; Silva, R.; Ortigão, C.; Neves, J.; Tavernier, S.; Varela, J. EndoTOFPET-US DAQ, Designing the Data Acquisition System of a High Resolution Endoscopic PET-US Detector. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference, Seoul, Korea, 27 October–2 November 2013. [Google Scholar]
- Garibaldi, F.; Capuani, S.; Colilli, S.; Cosentino, L.; Cusanno, F.; De Leo, R.; Finocchiaro, P.; Foresta, M.; Giove, F.; Giuliani, F.; et al. TOPEM: A PET-TOF endorectal probe, compatible with MRI for diagnosis and follow up of prostate cancer. Nucl. Instrum. Methods Phys. Res. Sect. A 2013, 702, 13–15. [Google Scholar] [CrossRef]
- González-Montoro, A.; Sánchez, F.; Martí, R.; Hernández, L.; Aguilar, A.; Barberá, J.; Catret, J.V.; Cañizares, G.; Conde, P.; Lamprou, E.; et al. Detector block performance based on a monolithic LYSO crystal using a novel signal multiplexing method. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 912, 372–377. [Google Scholar] [CrossRef]
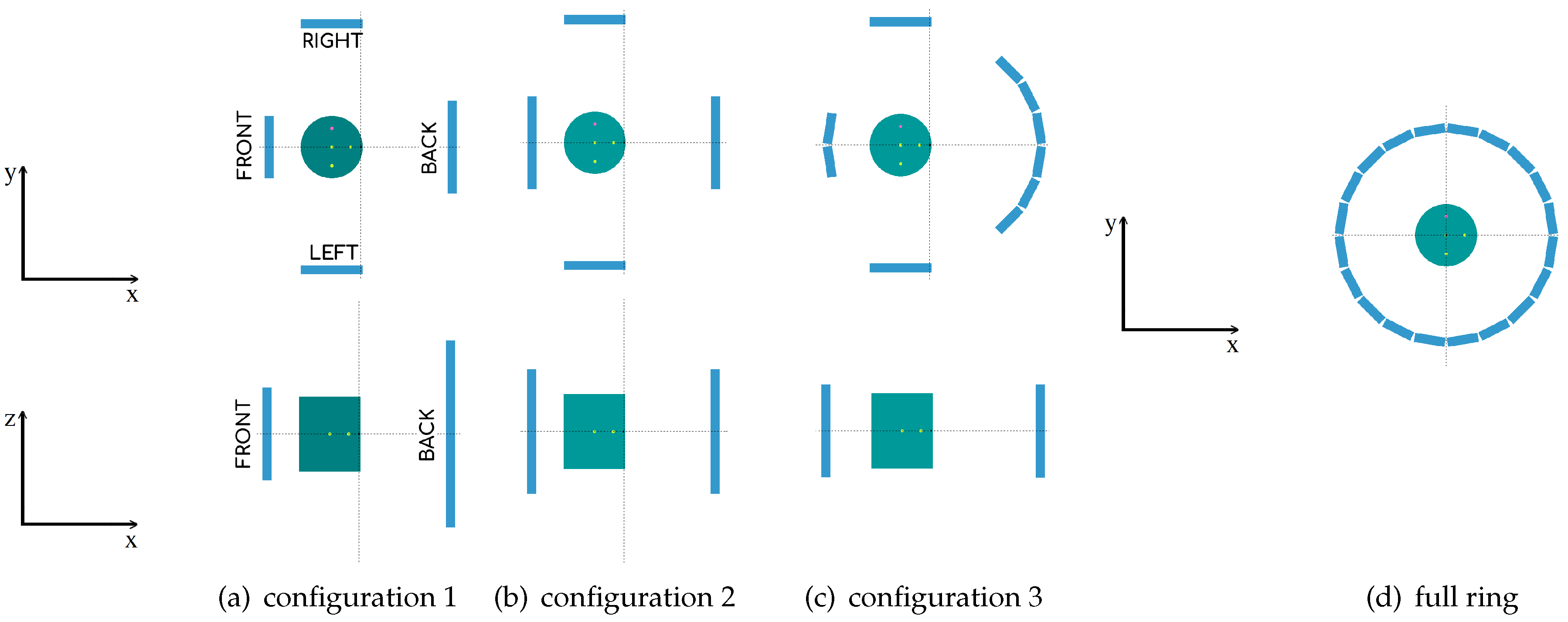
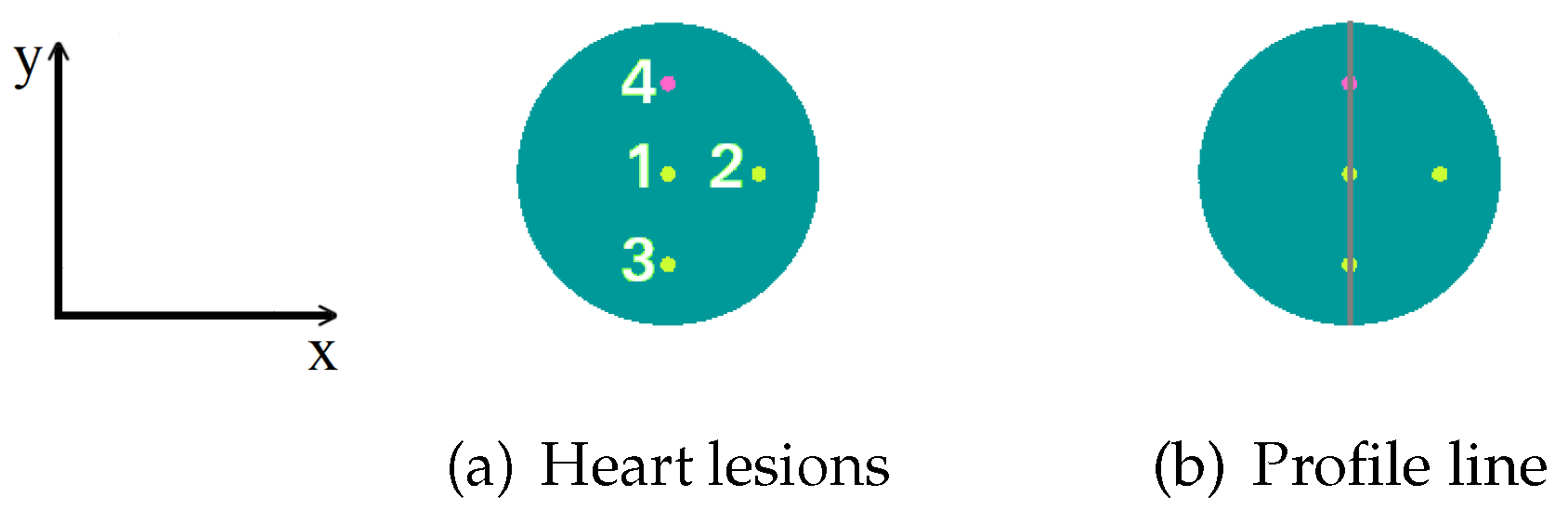





| Geometry | Matrix Modules | |
|---|---|---|
| Front−Back | Left−Right | |
| Configuration 1 | 2 × 3 − 3 × 6 | 2 × 3 − 2 × 3 |
| Configuration 2 | 3 × 4 − 3 × 4 | 2 × 3 − 2 × 3 |
| Configuration 3 | 2 × 3 − 6 × 3 | 2 × 3 − 2 × 3 |
| Geometry | (cps/kBq) |
|---|---|
| Configuration 1 | 3.87 |
| Configuration 2 | 3.81 |
| Configuration 3 | 2.17 |
| Full ring | 5.86 |
| Radial | Configuration 1 | Configuration 2 | Configuration 3 | Full Ring | |||||
|---|---|---|---|---|---|---|---|---|---|
| Offset (mm) | Center | 3/8 | Center | 3/8 | Center | 3/8 | Center | 3/8 | |
| −50 | FWHM | 5.08 | 5.48 | 3.98 | 4.34 | 4.50 | 4.75 | - | - |
| −50 | FWTM | 30.72 | 33.14 | 24.08 | 26.28 | 27.22 | 28.76 | - | - |
| −30 | FWHM | 5.01 | 5.21 | 3.77 | 4.10 | 4.25 | 4.64 | - | - |
| −30 | FWTM | 30.30 | 31.56 | 22.80 | 24.80 | 25.73 | 28.10 | - | - |
| −10 | FWHM | 4.72 | 4.83 | 3.49 | 4.07 | 4.02 | 4.42 | - | - |
| −10 | FWTM | 28.55 | 29.24 | 21.10 | 24.65 | 24.36 | 26.77 | - | - |
| 0 | FWHM | 3.82 | 4.10 | 3.43 | 3.84 | 4.01 | 4.18 | 2.55 | 2.59 |
| 0 | FWTM | 23.10 | 24.84 | 20.75 | 23.22 | 24.25 | 25.31 | 15.43 | 15.71 |
| +10 | FWHM | 3.35 | 3.46 | 3.47 | 3.75 | 3.69 | 4.09 | 2.92 | 2.98 |
| +10 | FWTM | 20.27 | 20.94 | 20.99 | 22.68 | 22.34 | 24.75 | 17.7 | 18.04 |
| +30 | FWHM | 3.70 | 3.87 | 3.55 | 4.09 | 4.10 | 4.50 | 3.04 | 3.14 |
| +30 | FWTM | 22.40 | 23.41 | 21.49 | 24.75 | 24.83 | 27.26 | 18.43 | 19.00 |
| +50 | FWHM | 6.32 | 6.42 | 4.35 | 4.53 | 4.36 | 4.53 | 3.07 | 3.17 |
| +50 | FWTM | 38.23 | 38.87 | 26.35 | 27.40 | 26.38 | 27.42 | 18.61 | 19.18 |
| Tangential | Configuration 1 | Configuration 2 | Configuration 3 | ||||
|---|---|---|---|---|---|---|---|
| Offset (mm) | Center | 3/8 | Center | 3/8 | Center | 3/8 | |
| 0 | FWHM | 3.82 | 4.10 | 3.43 | 3.84 | 4.01 | 4.18 |
| 0 | FWTM | 23.10 | 24.84 | 20.75 | 23.22 | 24.25 | 25.31 |
| +10 | FWHM | 4.02 | 4.45 | 4.01 | 4.10 | 4.22 | 4.38 |
| +10 | FWTM | 24.35 | 26.92 | 24.30 | 24.84 | 25.57 | 26.53 |
| +30 | FWHM | 4.09 | 4.60 | 4.09 | 4.20 | 4.71 | 4.81 |
| +30 | FWTM | 24.78 | 27.82 | 24.78 | 25.42 | 28.54 | 29.13 |
| +50 | FWHM | 4.29 | 4.74 | 4.22 | 4.38 | 5.07 | 5.15 |
| +50 | FWTM | 25.94 | 28.70 | 25.55 | 26.52 | 30.66 | 31.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver, S.; Moliner, L.; Ilisie, V.; Benlloch, J.M.; Rodríguez-Álvarez, M.J. Simulation Study for Designing a Dedicated Cardiac TOF-PET System. Sensors 2020, 20, 1311. https://doi.org/10.3390/s20051311
Oliver S, Moliner L, Ilisie V, Benlloch JM, Rodríguez-Álvarez MJ. Simulation Study for Designing a Dedicated Cardiac TOF-PET System. Sensors. 2020; 20(5):1311. https://doi.org/10.3390/s20051311
Chicago/Turabian StyleOliver, Sandra, Laura Moliner, Víctor Ilisie, J.M. Benlloch, and M.J. Rodríguez-Álvarez. 2020. "Simulation Study for Designing a Dedicated Cardiac TOF-PET System" Sensors 20, no. 5: 1311. https://doi.org/10.3390/s20051311





