Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System
Abstract
:1. Introduction
2. Methods Workflow
2.1. Materials and Reagents
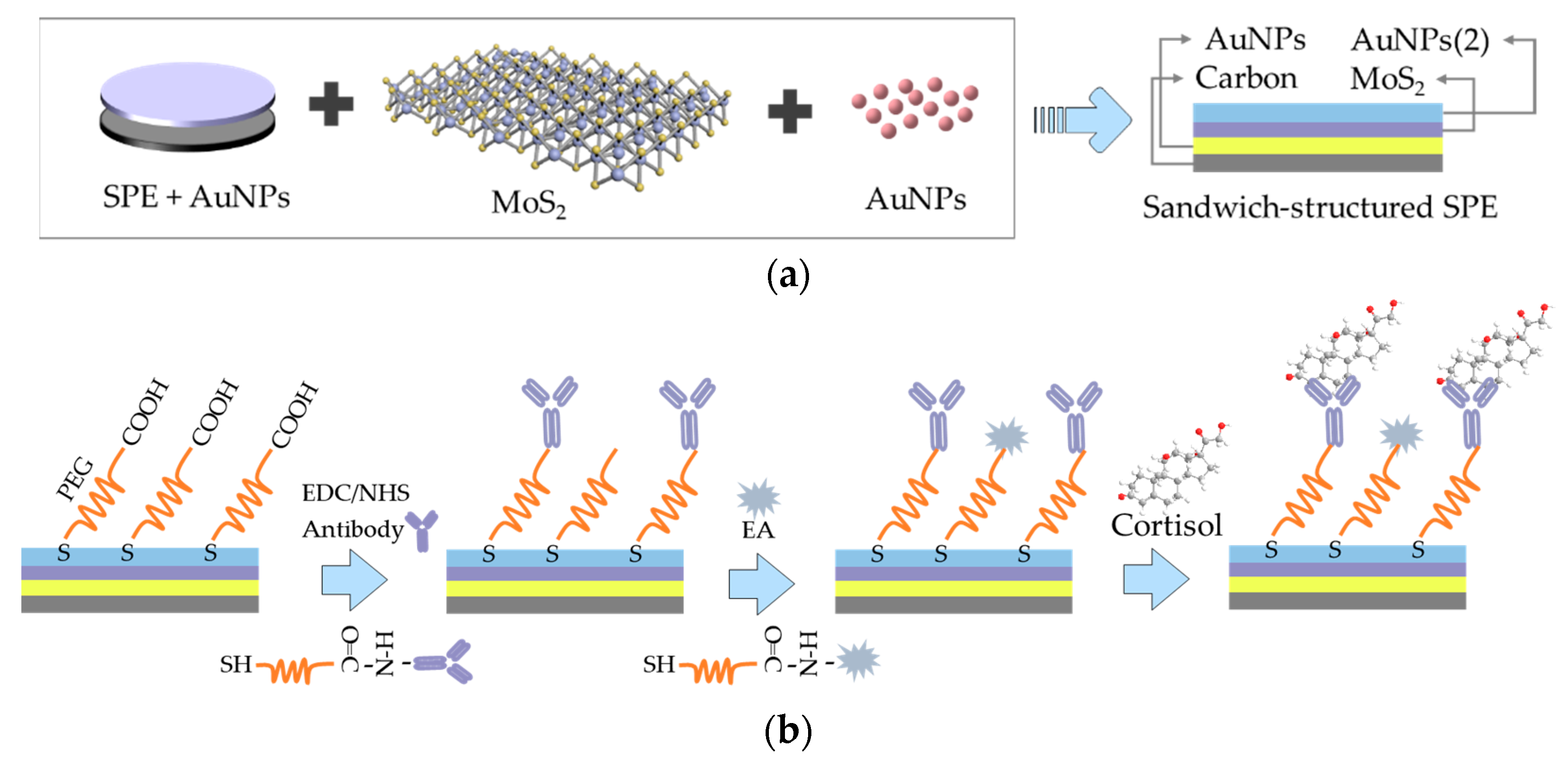
2.2. Fabrication of the Sandwich-Structured Immunosensor for Cortisol Detection
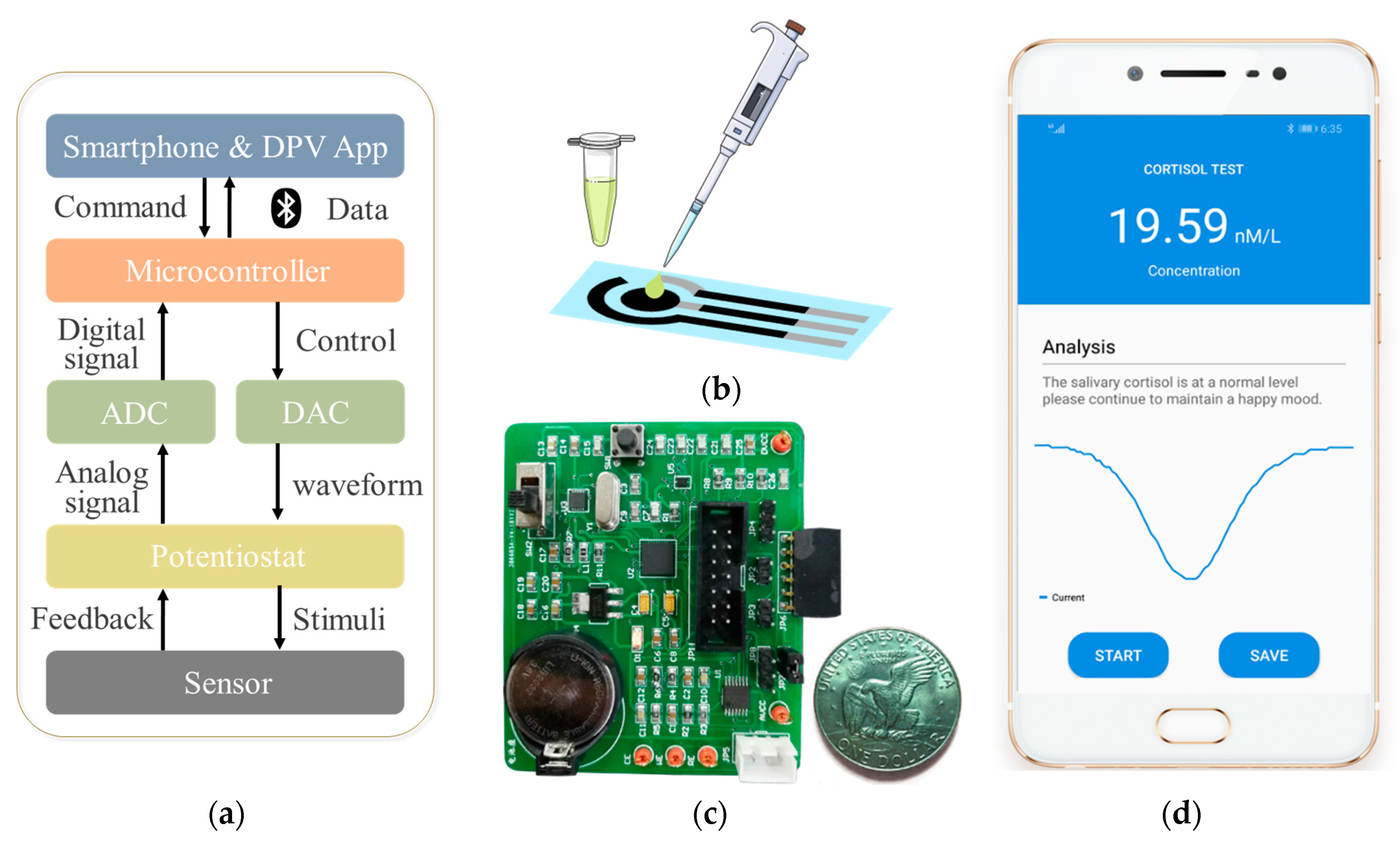
2.3. Architecture of the Smartphone-Controlled Detection Circuit and App
2.4. Electrochemical Detection of Cortisol
3. Results and Discussion
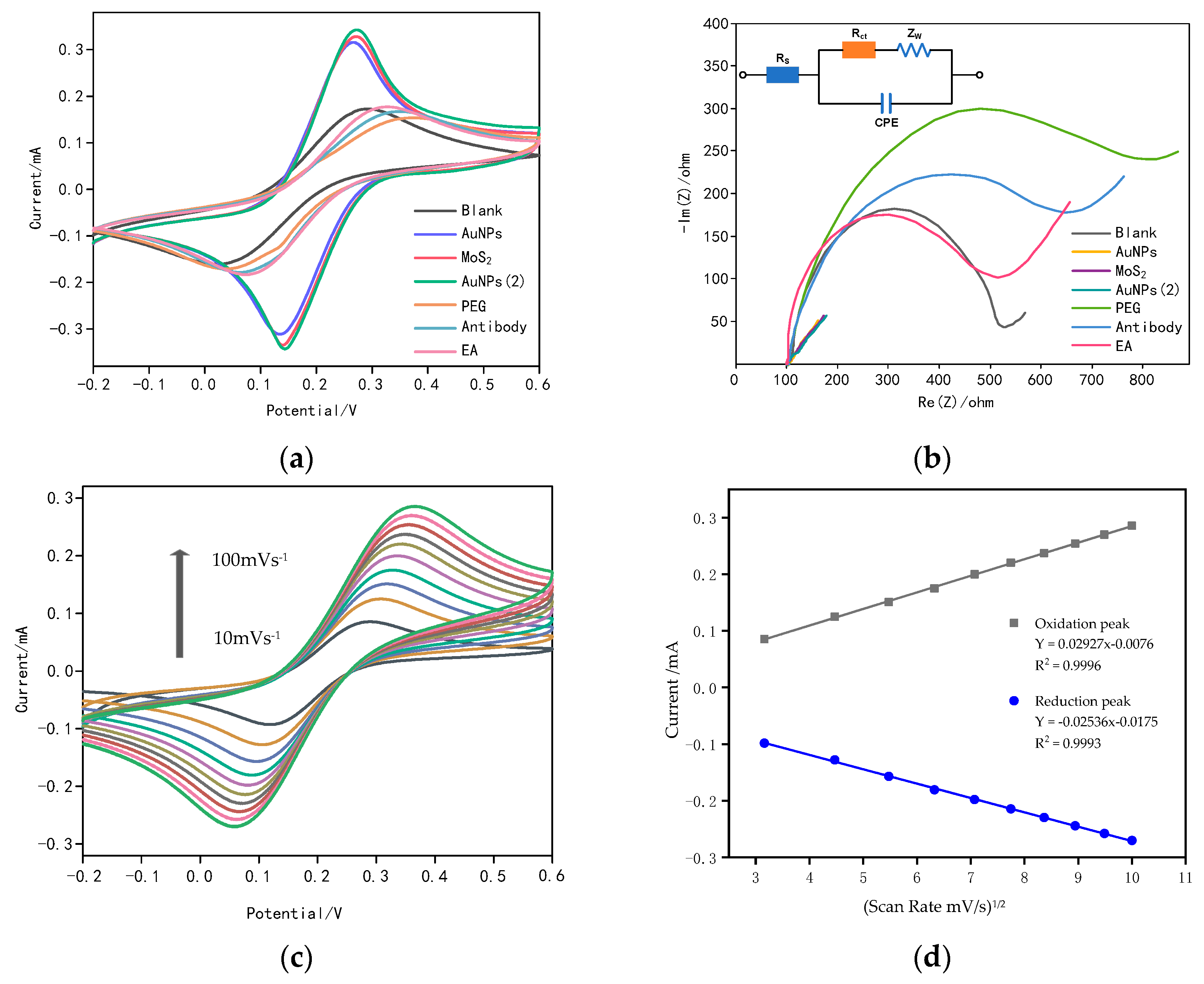
3.1. Characterization of the Gold Nanoparticles/Molybdenum Disulfide/Gold Nanoparticles (AuNPs/MoS2/AuNPs) Sandwich-Structured Immunosensor
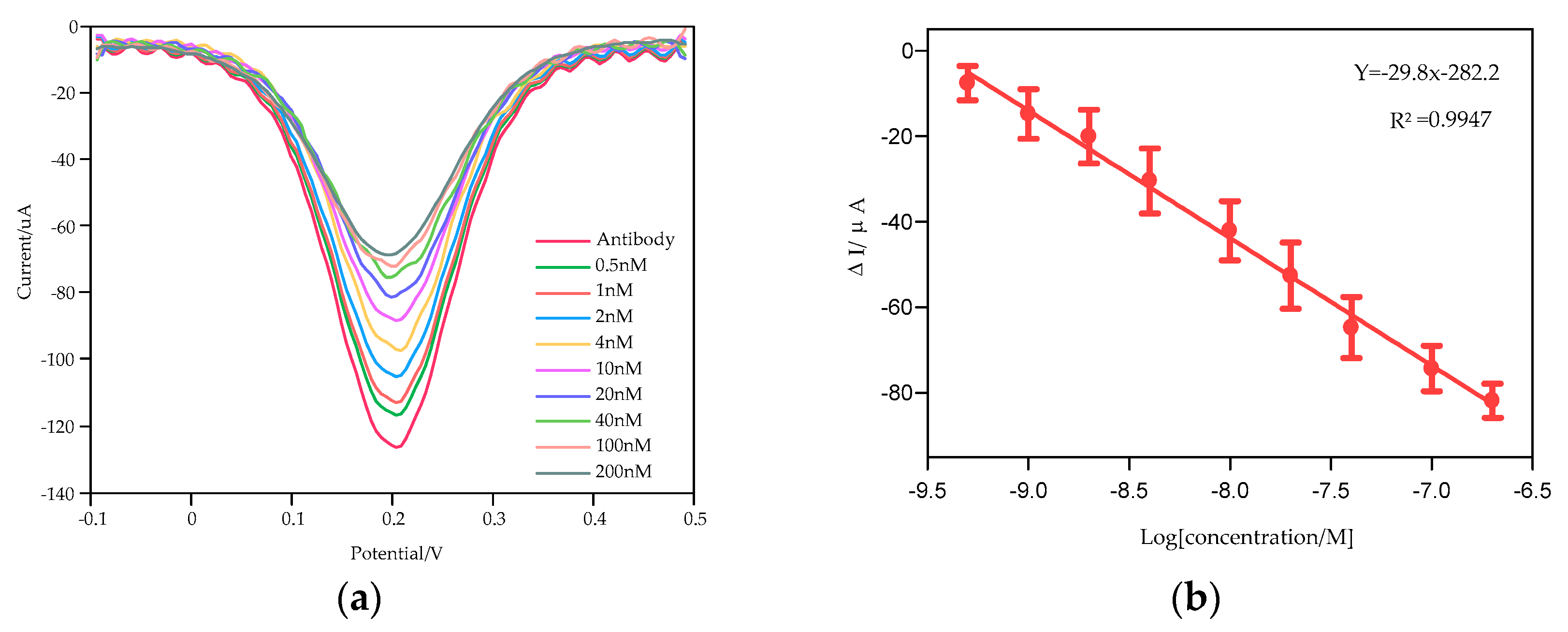
3.2. Cortisol Detection Based on the Smartphone System
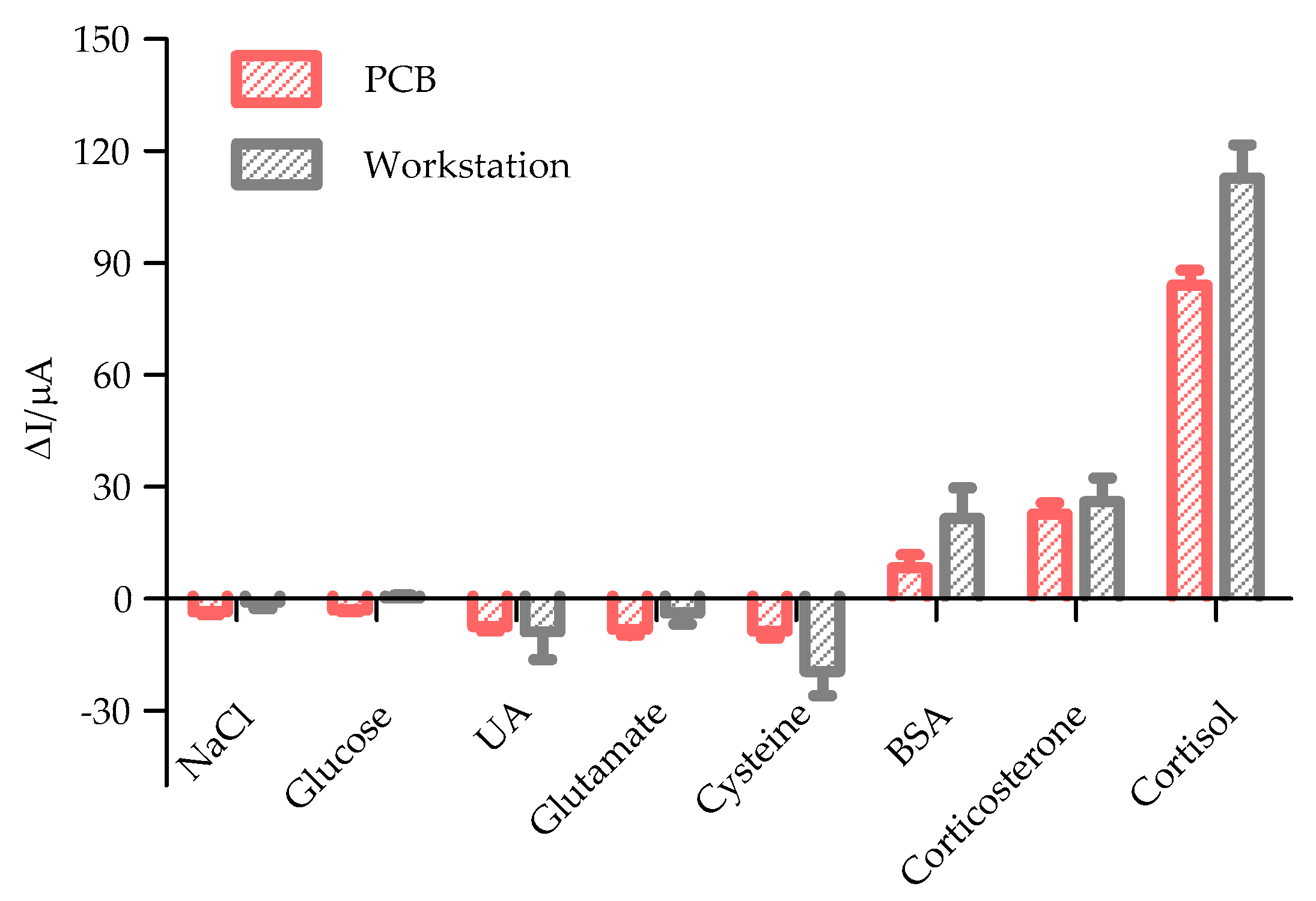
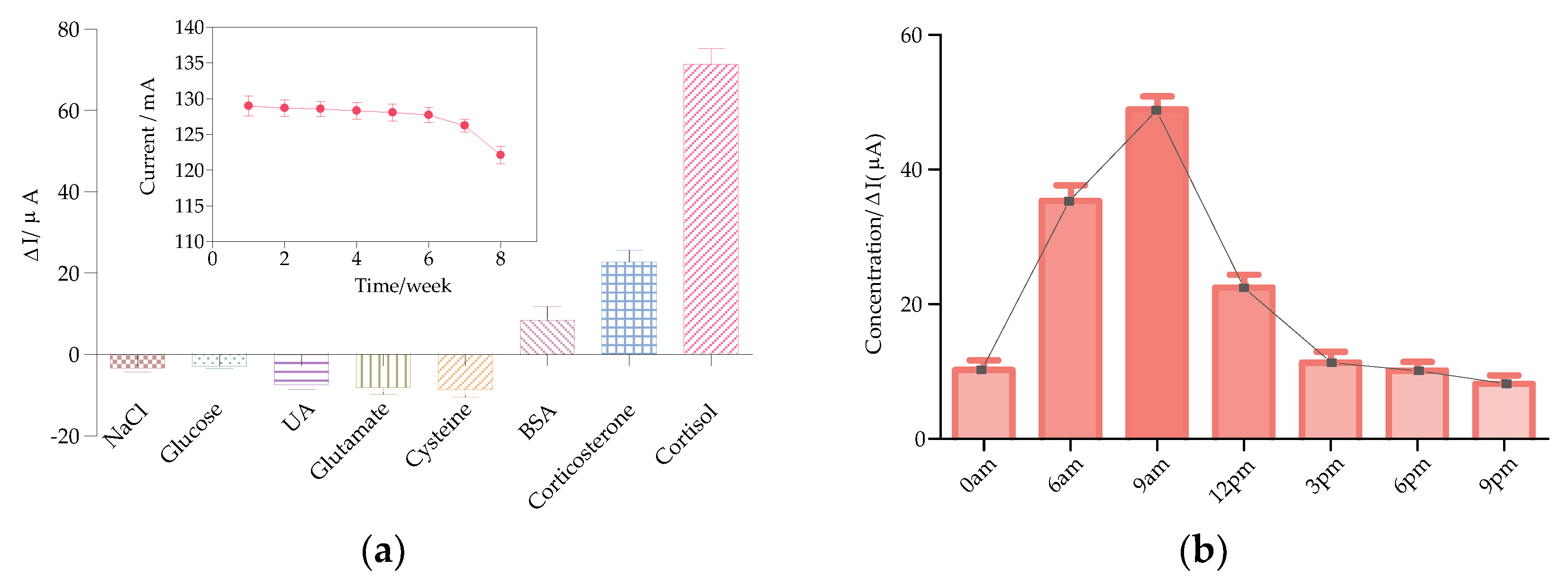
3.3. Specificity and Stability Study
3.4. Human Saliva Test Using the Smartphone-Controlled Differential Pulse Voltammetry (DPV) System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S. Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steudte-Schmiedgen, S.; Stalder, T.; Schönfeld, S. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 2015, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Descalzi, G.; Ikegami, D.; Ushijima, T. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015, 38, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurol. 2005, 6, 463. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007, 133, 25. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.D.; Ben-Shlomo, Y.; Beswick, A. Cortisol, testosterone, and coronary heart disease: Prospective evidence from the Caerphilly study. Circulation 2005, 112, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.S.; Varghese, F.P.; McEwen, B.S. Association of depression with medical illness: Does cortisol play a role? Biol. Psychiatry 2004, 55, 1–9. [Google Scholar] [CrossRef]
- Starkman, M.N.; Giordani, B.; Gebarski, S.S. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol. Psychiatry 1999, 46, 1595–1602. [Google Scholar] [CrossRef]
- Løvås, K.; Husebye, E.S. Continuous subcutaneous hydrocortisone infusion in Addison’s disease. J. Endocrinol. 2007, 157, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Sephton, S.E.; Sapolsky, R.M.; Kraemer, H.C. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Corbalán-Tutau, D.; Madrid, J.A.; Nicolás, F. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 2014, 123, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.Z.; Liu, Z.X.; Liu, L. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Xu, N.; Liu, Z. Smartphone-based differential pulse amperometry system for real-time monitoring of levodopa with carbon nanotubes and gold nanoparticles modified screen-printing electrodes. Biosens. Bioelectron. 2019, 129, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Q.; Lu, Y. Passive and wireless near field communication tag sensors for biochemical sensing with smartphone. Sens. Actuators B Chem. 2017, 246, 748–755. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Zhang, Q. Smartphone-based sensing system using ZnO and graphene modified electrodes for VOCs detection. Biosens. Bioelectron. 2017, 93, 94–101. [Google Scholar] [CrossRef]
- Kinnamon, D.; Ghanta, R.; Lin, K.-C. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.; Vasudev, A.; Arya, S.K. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Pinto, V.; Sousa, P.; Catarino, S.O. Microfluidic immunosensor for rapid and highly-sensitive salivary cortisol quantification. Biosens. Bioelectron. 2017, 90, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Matsuda, Y.; Sasaki, S. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosens. Bioelectron. 2013, 41, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Abraham, G.E.; Buster, J.E.; Teller, R.C. Radioimmunoassay of plasma cortisol. Anal. Lett. 1972, 5, 757–765. [Google Scholar] [CrossRef]
- Vieira, J.G.H.; Nakamura, O.H.; Carvalho, V.M. Determination of cortisol and cortisone in human saliva by a liquid chromatography-tandem mass spectrometry method. Arch. Endocrinol. Metab. 2014, 58, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, N.; Taniguchi, S.; Takano, E. A molecularly imprinted nanocavity-based fluorescence polarization assay platform for cortisol sensing. J. Mater. Chem. B 2016, 4, 1770–1777. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, K.; Jung, H. Direct immune-detection of cortisol by chemiresistor graphene oxide sensor. Biosens. Bioelectron. 2017, 98, 473–477. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Tomizawa, Y. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators B Chem. 2013, 182, 139–146. [Google Scholar] [CrossRef]
- Arya, S.K.; Dey, A.; Bhansali, S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens. Bioelectron. 2011, 28, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Myung, N.V.; Shetty, V. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef]
- Arya, S.K.; Chornokur, G.; Venugopal, M. Dithiobis (succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisol. Biosens. Bioelectron. 2010, 25, 2296–2301. [Google Scholar] [CrossRef] [Green Version]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive label-free detection of cortisol and lactate using graphene embedded screen-printed electrode. Nanomicro Lett. 2018, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectron. 2016, 78, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Munje, R.D.; Muthukumar, S.; Selvam, A.P. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Sci. Rep. 2015, 5, 14586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Zhai, J.; Wang, D. Two-dimensional graphene bridges enhanced photoinduced charge transport in dye-sensitized solar cells. ACS Nano 2010, 4, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-layer MoS2: A promising layered semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805–136808. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lu, Y.; Wu, Z. Two-dimensional molybdenum disulfide (MoS2) with gold nanoparticles for biosensing of explosives by optical spectroscopy. Sens. Actuators B Chem. 2018, 261, 279–287. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Shavanova, K.; Bakakina, Y.; Burkova, I. Application of 2D non-graphene materials and 2D oxide nanostructures for biosensing technology. Sensors 2016, 16, 223. [Google Scholar] [CrossRef]
- Sreeprasad, T.; Nguyen, P.; Kim, N. Controlled, defect-guided, metal-nanoparticle incorporation onto MoS2 via chemical and microwave routes: Electrical, thermal, and structural properties. Nano Lett. 2013, 13, 4434–4441. [Google Scholar] [CrossRef]
- Su, S.; Sun, H.; Cao, W. Dual-target electrochemical biosensing based on DNA structural switching on gold nanoparticle-decorated MoS2 nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Yao, Y.; Zhang, Q. Olfactory biosensor for insect semiochemicals analysis by impedance sensing of odorant-binding proteins on interdigitated electrodes. Biosens. Bioelectron. 2015, 67, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Herrwerth, S.; Rosendahl, T.; Feng, C. Covalent coupling of antibodies to self-assembled monolayers of carboxy-functionalized poly (ethylene glycol): Protein resistance and specific binding of biomolecules. Langmuir 2003, 19, 1880–1887. [Google Scholar] [CrossRef]
- Anne, A.; Demaille, C.; Moiroux, J. Terminal attachment of polyethylene glycol (PEG) chains to a gold electrode surface. Cyclic voltammetry applied to the quantitative characterization of the flexibility of the attached PEG chains and of their penetration by mobile PEG chains. Macromolecules 2002, 35, 5578–5586. [Google Scholar] [CrossRef]
- Pang, P.; Teng, X.; Chen, M. Ultrasensitive enzyme-free electrochemical immunosensor for microcystin-LR using molybdenum disulfide/gold nanoclusters nanocomposites as platform and Au@Pt core-shell nanoparticles as signal enhancer. Sens. Actuators B Chem. 2018, 266, 400–407. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K. Mediator and label free estimation of stress biomarker using electrophoretically deposited Ag@AgO–polyaniline hybrid nanocomposite. Biosens. Bioelectron. 2013, 50, 35–41. [Google Scholar] [CrossRef]

| Samples | Added (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|
| Sample 1 | 0 | 19.59 | - |
| 10 | 29.72 | 101.29 | |
| 20 | 39.21 | 98.08 | |
| 40 | 61.39 | 104.51 | |
| Sample 2 | 0 | 8.20 | - |
| 10 | 18.03 | 98.23 | |
| 20 | 28.17 | 99.84 | |
| 40 | 50.46 | 105.64 | |
| Sample 3 | 0 | 14.33 | - |
| 10 | 24.57 | 102.43 | |
| 20 | 33.59 | 96.30 | |
| 40 | 54.29 | 99.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, N.; Men, H.; Li, S.; Lu, Y.; Low, S.S.; Li, X.; Zhu, L.; Cheng, C.; Xu, G.; et al. Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System. Sensors 2020, 20, 1422. https://doi.org/10.3390/s20051422
Liu J, Xu N, Men H, Li S, Lu Y, Low SS, Li X, Zhu L, Cheng C, Xu G, et al. Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System. Sensors. 2020; 20(5):1422. https://doi.org/10.3390/s20051422
Chicago/Turabian StyleLiu, Jingjing, Ning Xu, Hong Men, Shuang Li, Yanli Lu, Sze Shin Low, Xin Li, Lihang Zhu, Chen Cheng, Gang Xu, and et al. 2020. "Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System" Sensors 20, no. 5: 1422. https://doi.org/10.3390/s20051422
APA StyleLiu, J., Xu, N., Men, H., Li, S., Lu, Y., Low, S. S., Li, X., Zhu, L., Cheng, C., Xu, G., & Liu, Q. (2020). Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System. Sensors, 20(5), 1422. https://doi.org/10.3390/s20051422










