Human Vital Signs Detection Methods and Potential Using Radars: A Review
Abstract
:1. Introduction
2. Conventional Contact-Based Vital Signs Acquisition
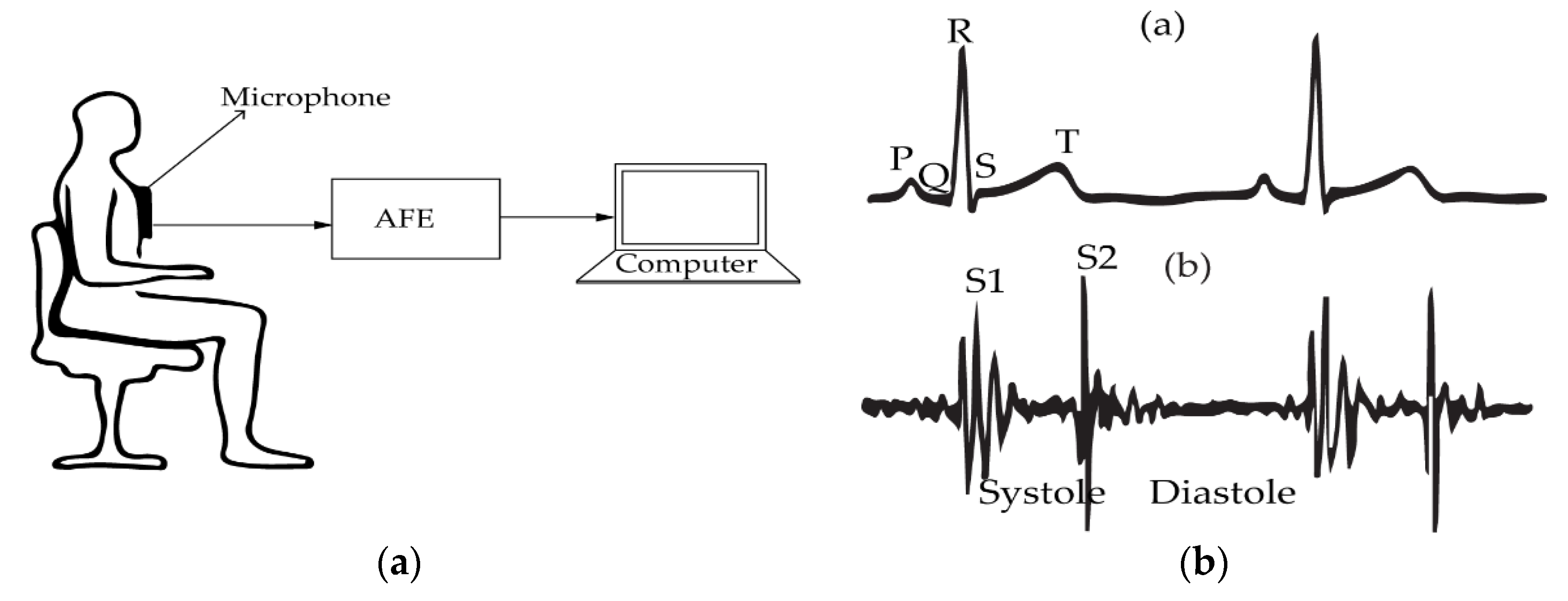
2.1. Electrocardiography
2.2. Photoplethysmography
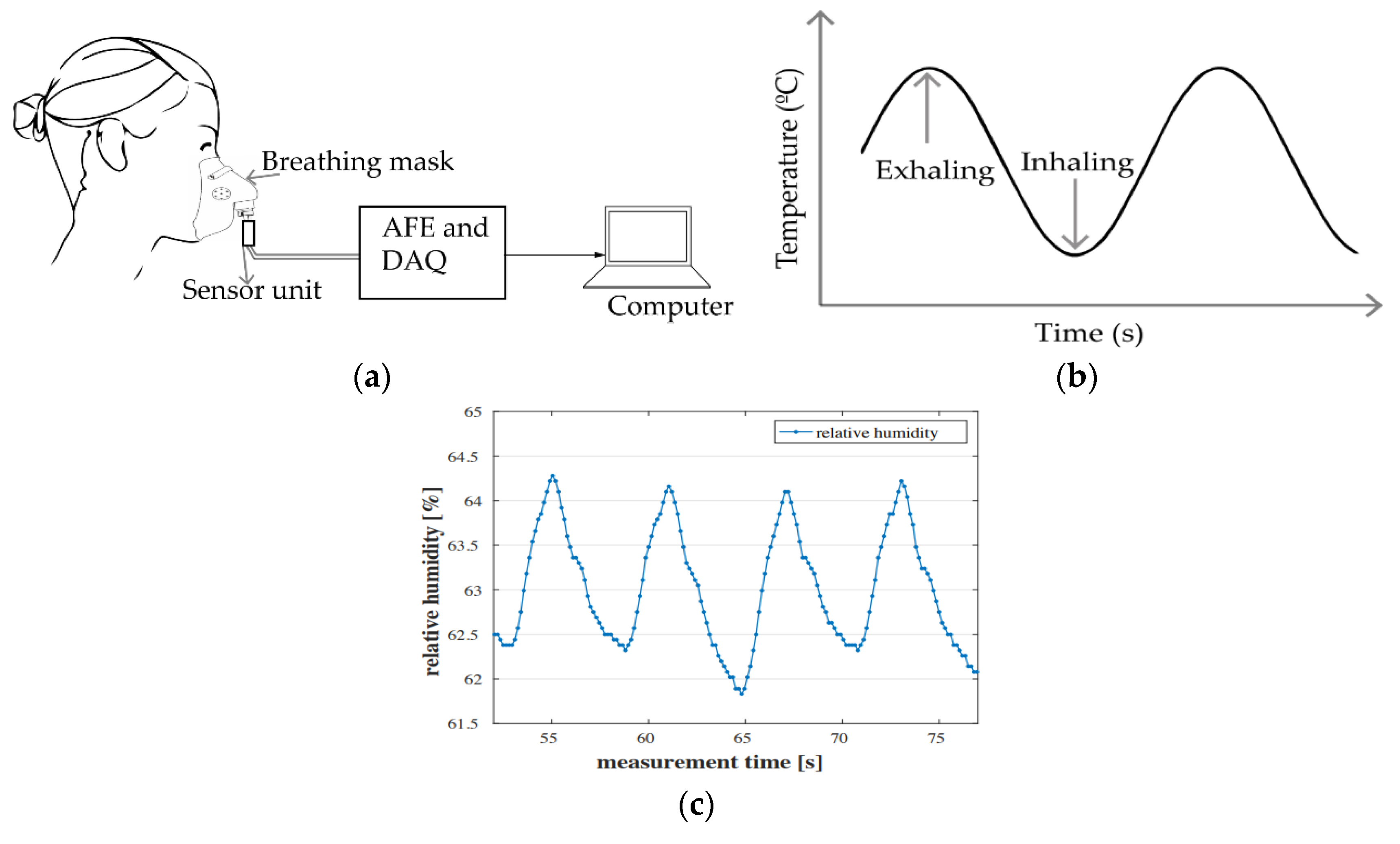
2.3. Air Components-, Temperature- and Humidity-Based Methods
2.3.1. Air Components
2.3.2. Air Temperature
2.3.3. Air Humidity
2.4. Chest-Wall Mechanical Displacement Sensing and Blood Pressure-Sensing Methods
2.4.1. Chest-Wall Displacement Sensing
2.4.2. Blood Pressure Sensing
2.5. Phonocardiography (PCG)
3. Contactless Vital Signs Detection Using Radar Techniques
3.1. Continuous-Wave Radars
3.1.1. Operation Principle
3.1.2. Algorithms and Signal Processing
3.1.3. Biomedical Practice
3.1.4. Challenges
3.2. Frequency-Modulated Continuous-Wave (FMCW) Radars
3.2.1. Operation Principle
3.2.2. Algorithms and Signal Processing
3.2.3. Biomedical Practice
3.2.4. Challenges
3.3. Stepped-Frequency Continuous-Wave (SFCW) Radars
3.3.1. Operation Principle
3.3.2. Algorithms and Signal Processing
3.3.3. Biomedical Practice
3.3.4. Challenges
3.4. Ultra-Wideband (UWB) Pulse-Based Radars
3.4.1. Operation Principle
3.4.2. Algorithms and Signal Processing
3.4.3. Biomedical Practice
3.4.4. Challenges
3.5. Random Body Movement (RBM) Cancellation Techniques in Doppler Radars
3.6. Heart Rate Variability Assessment Using Vital Signs Radar
3.7. Effect of Frequency on the Detection Accuracy of Vital Signs Radars
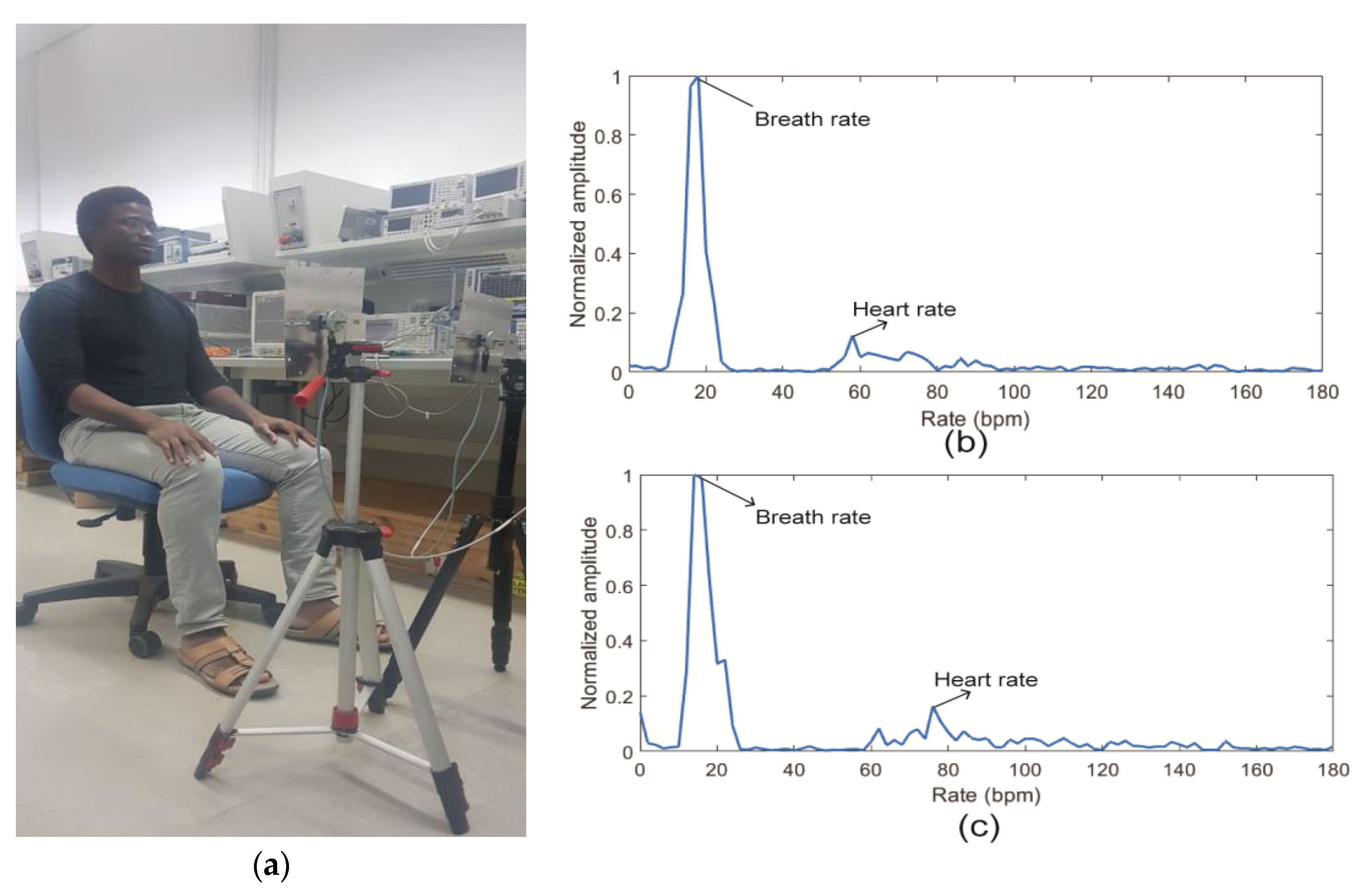
4. Experiment for Measuring Human Cardio-Respiratory Rates Using a Continuous-Wave (CW) Doppler Radar
4.1. Experiment Setup
4.2. Data Processing
4.3. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BR | Breath rate |
| HR | Hearth rate |
| BP | Blood pressure |
| OSAS | Obstructive sleep apnea syndrome |
| SIDS | Sudden infant death syndrome |
| SUID | Sudden unexpected infant death |
| WHO | World health organization |
| COPD | Cognitive obstructive pulmonary disease |
| HRV | Hearth rate variability |
| ECG | Electrocardiogram |
| EDR | ECG-driven respiration |
| AFE | Analog front-end |
| DSP | Digital signal processing |
| MA | Motion artifact |
| PPG | Photoplethysmogram |
| LED | Light-emitting-diode |
| IR | Infrared |
| DC | Direct-current |
| VPG | Video-plethysmography |
| RGB | Red-green-blue |
| ROI | Region-of-interest |
| SAW | Surface acoustic wave |
| FBG | Fiber Bragg grating |
| AC | Alternative-current |
| MEMS | Microelectromechanical systems |
| 3D | three-dimensional |
| PCG | Phonocardiography |
| CW | Continuous-wave |
| FPGA | Field-programmable gate array |
| FMCW | Frequency-modulated Continuous-wave |
| SFCW | Stepped-frequency continuous-wave |
| Rx | Receiver |
| Tx | Transmitter |
| LO | Local oscillator |
| IF | intermediate frequency |
| SSB | Single sideband |
| DSB | Double sideband |
| AD | Arctangent Demodulation |
| CSD | Complex signal demodulation |
| I | In-phase |
| Q | Quadrature |
| RBM | Random body movement |
| EMD | Empirical mode decomposition |
| VCO | Voltage-controlled oscillator |
| PLL | Phase-locked-loop |
| VNA | Vector network analyzer |
| UWB | Ultra-wideband |
| IR-UWB | Impulse radio ultra-wideband |
| FFT | Fast Fourier transform |
| IFFT | Inverse Fast-Fourier transform |
| FCC | Federal communication committee |
| ECC | European electronic communication commission |
| SUT | Subject-under-test |
| ADC | Analog-to-digital converter |
| VGA | Variable-gain amplifier |
References
- Barrett, K.E.; Barman, S.M.; Brooks, H.L.; Yuan, J.X.-J.; Ganong, W.F. Ganongs Review of Medical Physiology; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- What Should My Heart Rate Be? Available online: https://www.medicalnewstoday.com/articles/235710.php (accessed on 26 January 2020).
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 26 January 2020).
- Sudden Unexpected Infant Death and Sudden Infant Death. Available online: https://www.cdc.gov/sids/data.htm (accessed on 1 February 2020).
- Benjafield, A.; Valentine, K.; Ayas, N.; Eastwood, P.R.; Heinzer, R.C.; Ip, M.S.; Patel, S.R.; Peppard, P.E.; Sinha, S.; Tufik, S.; et al. Global Prevalence of Obstructive Sleep Apnea in Adults: Estimation Using Currently Available Data. In Proceedings of the American Thoracic Society 2018 International Conference, San Diego, CA, USA, 21 May 2018. [Google Scholar]
- Helfenbein, E.; Firoozabadi, R.; Chien, S.; Carlson, E.; Babaeizadeh, S. Development of three methods for extracting respiration from the surface ECG: A review. J. Electrocardiol. 2014, 47, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Rantonen, T.; Jalonen, J.; Grönlund, J.; Antila, K.; Southall, D.; Välimäki, I. Increased amplitude modulation of continuous respiration precedes sudden infant death syndrome. Early Hum. Dev. 1998, 53, 53–63. [Google Scholar] [CrossRef]
- Hodgetts, T.J.; Kenward, G.; Vlachonikolis, I.G.; Payne, S.; Castle, N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation 2002, 54, 125–131. [Google Scholar] [CrossRef]
- Ichwana, D.; Ikhlas, R.Z.; Ekariani, S. Heart Rate Monitoring System During Physical Exercise for Fatigue Warning Using Non-invasive Wearable Sensor. In Proceedings of the 2018 International Conference on Information Technology Systems and Innovation (ICITSI), Bandung, Padang, Indonesia, 22–26 October 2018; pp. 497–502. [Google Scholar]
- Puente-Maestu, L.; Pedro, J.G.D.; Martínez-Abad, Y.; Oña, J.M.R.D.; Llorente, D.; Cubillo, J.M. Dyspnea, Ventilatory Pattern, and Changes in Dynamic Hyperinflation Related to the Intensity of Constant Work Rate Exercise in COPD. Chest 2005, 128, 651–656. [Google Scholar] [CrossRef]
- Gagnon, P.; Bussières, J.S.; Ribeiro, F.; Gagnon, S.L.; Saey, D.; Gagné, N.; Provencher, S.; Maltais, F. Influences of Spinal Anesthesia on Exercise Tolerance in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Resp. Crit. Care Med. 2012, 186, 606–615. [Google Scholar] [CrossRef]
- Coca, A.; Roberge, R.J.; Williams, W.J.; Landsittel, D.P.; Powell, J.B.; Palmiero, A. Physiological Monitoring in Firefighter Ensembles: Wearable Plethysmographic Sensor Vest versus Standard Equipment. J. Occup. Environ. Hyg. 2009, 7, 109–114. [Google Scholar] [CrossRef]
- Paradiso, R.; Faetti, T.; Werner, S. Wearable monitoring systems for psychological and physiological state assessment in a naturalistic environment. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2250–2253. [Google Scholar]
- Atlef, J.L. Principles of Clinical Electrocardiography. Anesthesiology 1980, 52, 195. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lin, B.-S.; He, K.-Y.; Lin, B.-S. Design of a Wearable 12-Lead Noncontact Electrocardiogram Monitoring System. Sensors 2019, 19, 1509. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.N.; Das, R.; Pal, S. Automated Real-Time Processing of Single Lead Electrocardiogram for Simultaneous Heart Rate and Respiratory Rate Monitoring. J. Med. Devices 2017, 11. [Google Scholar] [CrossRef]
- Schmidt, M.; Schumann, A.; Müller, J.; Bär, K.-J.; Rose, G. ECG derived respiration: Comparison of time-domain approaches and application to altered breathing patterns of patients with schizophrenia. Physiol. Meas. 2017, 38, 601–615. [Google Scholar] [CrossRef]
- Hallfors, N.; Jaoude, M.A.; Liao, K.; Ismail, M.; Isakovic, A. Graphene oxide—Nylon ECG sensors for wearable IoT healthcare. In Proceedings of the 2017 Sensors Networks Smart and Emerging Technologies (SENSET), Beirut, Lebanon, 12–14 September 2017. [Google Scholar]
- Hsieh, J.-C.; Hsu, M.-W. A cloud computing based 12-lead ECG telemedicine service. BMC Med. Inform. Decis. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 12-Lead ECG Systems in Clinical Trials. Available online: https://www.clinicaltrialsarena.com/analysis/12-lead-ecg-systems-in-clinical-trials/ (accessed on 20 December 2019).
- Majumder, S.; Chen, L.; Marinov, O.; Chen, C.-H.; Mondal, T.; Deen, M.J. Noncontact Wearable Wireless ECG Systems for Long-Term Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Bayasi, N.; Tekeste, T.; Saleh, H.; Mohammad, B.; Khandoker, A.; Ismail, M. Low-Power ECG-Based Processor for Predicting Ventricular Arrhythmia. IEEE Trans. Very Large Scale Integr. {VLSI) Syst. 2016, 24, 1962–1974. [Google Scholar] [CrossRef]
- Yasin, M.; Tekeste, T.; Saleh, H.; Mohammad, B.; Sinanoglu, O.; Ismail, M. Ultra-Low Power, Secure IoT Platform for Predicting Cardiovascular Diseases. IEEE Trans. Circuits Syst. I Reg. Papers 2017, 64, 2624–2637. [Google Scholar] [CrossRef]
- Sinha, R. An Approach for Classifying ECG Arrhythmia Based on Features Extracted from EMD and Wavelet Packet Domains. Master Dissertation. 2012. Available online: https://www.researchgate.net/publication/287200946_An_Approach_for_Classifying_ECG_Arrhythmia_Based_on_Features_Extracted_from_EMD_and_Wavelet_Packet_Domains. (accessed on 3 March 2020).
- Stingeni, L.; Cerulli, E.; Spalletti, A.; Mazzoli, A.; Rigano, L.; Bianchi, L.; Hansel, K. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermat. 2015, 73, 44–48. [Google Scholar] [CrossRef]
- Özkaya, E.; Bozkurt, P.K. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: A rare case with concomitant roles of nickel and acrylates. Contact Dermat. 2014, 70, 121–123. [Google Scholar] [CrossRef]
- Deswysen, A.-C.; Zimerson, E.; Goossens, A.; Bruze, M.; Baeck, M. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes in an infant. Contact Dermat. 2013, 69, 379–381. [Google Scholar] [CrossRef]
- Nemati, E.; Deen, M.; Mondal, T. A wireless wearable ECG sensor for long-term applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Arcelus, A.; Sardar, M.; Mihailidis, A. Design of a capacitive ECG sensor for unobtrusive heart rate measurements. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 13 July 2013. [Google Scholar]
- Khayatzadeh, M.; Zhang, X.; Tan, J.; Liew, W.S.; Lian, Y. A 0.7-V 17.4-µW 3-lead wireless ECG SoC. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 583–592. [Google Scholar] [CrossRef]
- Jevon, P. Procedure for recording a standard 12-lead electrocardiogram. Br. J. Nurs. 2010, 19, 649–651. [Google Scholar] [CrossRef]
- Charlton, P.H.; Birrenkott, D.A.; Bonnici, T.; Pimentel, M.A.; Johnson, A.E.; Alastruey, J.; Tarassenko, L.; Watkinson, P.J.; Beale, R.; Clifton, D.A. Breathing Rate Estimation From the Electrocardiogram and Photoplethysmogram: A Review. IEEE Rev. Biomed. Eng. 2018, 11, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailón, R.; Sörnmo, L.; Laguna, P. Advanced Methods and Tools for ECG Data Analysis. Artech House: Lond. 2006, 1, 215–243. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaroni, C.; Nicolò, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Shao, D.; Liu, C.; Tsow, F.; Yang, Y.; Du, Z.; Iriya, R.; Yu, H.; Tao, N. Noncontact Monitoring of Blood Oxygen Saturation Using Camera and Dual-Wavelength Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 1091–1098. [Google Scholar] [CrossRef]
- Humphreys, K.; Ward, T.; Markham, C. Noncontact simultaneous dual wavelength photoplethysmography: A further step toward noncontact pulse oximetry. Rev. Sci. Instrum. 2007, 78, 044304. [Google Scholar] [CrossRef]
- Gastel, M.V.; Verkruysse, W.; Haan, G.D. Data-Driven Calibration Estimation for Robust Remote Pulse-Oximetry. Appl. Sci. 2019, 9, 3857. [Google Scholar] [CrossRef] [Green Version]
- Meredith, D.J.; Clifton, D.; Charlton, P.; Brooks, J.; Pugh, C.W.; Tarassenko, L. Photoplethysmographic derivation of respiratory rate: A review of relevant physiology. J. Med. Eng. Technol. 2011, 36, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Reisner, A.; Shaltis, P.A.; Mccombie, D.; Asada, H.H. Utility of the Photoplethysmogram in Circulatory Monitoring. Anesthesiology 2008, 108, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [PubMed] [Green Version]
- Fletcher, R.R.; Chamberlain, D.; Paggi, N.; Deng, X. Implementation of smart phone video plethysmography and dependence on lighting parameters. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3747–3750. [Google Scholar]
- Guzman, J.H.; Couderc, J.-P.; Tsouri, G.R. Accurate Hemodynamic Sensing using Video Plethysmography with High Quality Cameras. In Proceedings of the 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), Oslo, Norway, 8–10 May 2019; pp. 1–6. [Google Scholar]
- Kwon, S.; Kim, J.; Lee, D.; Park, K. ROI analysis for remote photoplethysmography on facial video. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4938–4941. [Google Scholar]
- Singh, O.P.; Howe, T.A.; Malarvili, M. Real-time human respiration carbon dioxide measurement device for cardiorespiratory assessment. J. Breath Res. 2018, 12, 026003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Venkateshan, S. Measurements of Temperature. In Mechanical Measurements, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 103–182. [Google Scholar]
- Herwaarden, A.V.; Sarro, P. Thermal sensors based on the seebeck effect. Sens. Actuators 1986, 10, 321–346. [Google Scholar] [CrossRef]
- Huang, Y.P.; Young, M.S.; Tai, C.C. Noninvasive respiratory monitoring system based on the piezoceramic transducer’s pyroelectric effect. Rev. Sci. Instrum. 2008, 79, 035103. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Huang, K.-N. Monitoring of breathing rate by a piezofilm sensor using pyroelectric effect. In Proceedings of the 2013 1st International Conference on Orange Technologies (ICOT), Tainan, Taiwan, 12–16 March 2013; pp. 99–102. [Google Scholar]
- Liang, Y.; Mazzolini, A.P.; Stoddart, P.R. Fibre Bragg grating sensor for respiratory monitoring. In Proceedings of the ACOFT/AOS 2006—Australian Conference on Optical Fibre Technology/Australian Optical Society, Melbourne, VIC, Australia, 10–13 July 2006; pp. 75–77. [Google Scholar]
- Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.A.; Damato, R.; Schena, E. Fiber Bragg Grating Probe for Relative Humidity and Respiratory Frequency Estimation: Assessment During Mechanical Ventilation. IEEE Sens. J. 2018, 18, 2125–2130. [Google Scholar] [CrossRef]
- Kano, S.; Dobashi, Y.; Fujii, M. Silica Nanoparticle-Based Portable Respiration Sensor for Analysis of Respiration Rate, Pattern, and Phase During Exercise. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Sun, Y.E.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Iacoponi, S.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.; Damato, R.; Schena, E. Polymer-coated fiber optic probe for the monitoring of breathing pattern and respiratory rate. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1616–1619. [Google Scholar]
- Tang, Y.; Li, Z.; Ma, J.; Wang, L.; Yang, J.; Du, B.; Yu, Q.; Zu, X. Highly sensitive surface acoustic wave (SAW) humidity sensors based on sol–gel SiO2 films: Investigations on the sensing property and mechanism. Sens. Actuators B-Chem. 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Scholz, R.; Bracio, B.R.; Brutscheck, M.; Trommler, P. Non-invasive respiratory rate detection in spontaneous respiration by humidity measurement. In Proceedings of the 2017 28th Irish Signals and Systems Conference (ISSC), Killarney, Ireland, 20–21 June 2017; pp. 1–6. [Google Scholar]
- Moll, J.M.; Wright, V. An objective clinical study of chest expansion. Ann. Rheum. Dis. 1972, 31, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorillo, A.; Critello, C.; Pullano, S. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators A-Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Rossi, D.D.; Carpi, F.; Lorussi, F.; Scilingo, E.P.; Tognetti, A. Electroactive fabrics and wearable man–machine interfaces. Wearable Electronics and Photonics, 1st ed.; Tao, X., Ed.; Woodhead Publishing: Cambridge, UK, 2005; pp. 59–80. [Google Scholar]
- Egami, Y.; Suzuki, K.; Tanaka, T.; Yasuhara, T.; Higuchi, E.; Inoue, H. Preparation and characterization of conductive fabrics coated uniformly with polypyrrole nanoparticles. Synth. Met. 2011, 161, 219–224. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Ostadabbas, S.; Guenterberg, E.; Pantelopoulos, A. Wireless Medical-Embedded Systems: A Review of Signal-Processing Techniques for Classification. IEEE Sens. J. 2013, 13, 423–437. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A Wearable Capacitive Sensor for Monitoring Human Respiratory Rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05. [Google Scholar] [CrossRef]
- Retory, Y.; Niedzialkowski, P.; Picciotto, C.D.; Bonay, M.; Petitjean, M. New Respiratory Inductive Plethysmography (RIP) Method for Evaluating Ventilatory Adaptation during Mild Physical Activities. PLoS ONE 2016, 11, e0151983. [Google Scholar] [CrossRef] [Green Version]
- Collop, N.A.; Tracy, S.L.; Kapur, V.; Mehra, R.; Kuhlmann, D.; Fleishman, S.A.; Ojile, J.M. Obstructive Sleep Apnea Devices for Out-Of-Center (OOC) Testing: Technology Evaluation. J. Clin. Sleep Med. 2011, 7, 531–548. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, V.; Zhang, J.; Russi, E.; Bloch, K. Detection of inspiratory flow limitation during sleep by computer assisted respiratory inductive plethysmography. Eur. Respir. J. 2000, 15, 570. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Quandt, B.M.; Scherer, L.J.; Boesel, L.F.; Wolf, M.; Bona, G.-L.; Rossi, R.M. Body-Monitoring and Health Supervision by Means of Optical Fiber-Based Sensing Systems in Medical Textiles. Adv. Healthc. Mater. 2014, 4, 330–355. [Google Scholar] [CrossRef]
- Chethana, K.; Prasad, A.S.G.; Omkar, S.N.; Asokan, S. Fiber bragg grating sensor based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2016, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, N.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Tomaso, G.D.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart Vest for Respiratory Rate Monitoring of COPD Patients Based on Non-Contact Capacitive Sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [Green Version]
- Clarenbach, C.F.; Senn, O.; Brack, T.; Kohler, M.; Bloch, K.E. Monitoring of Ventilation During Exercise by a Portable Respiratory Inductive Plethysmograph. Chest 2005, 128, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.; Polimadei, A.; Formica, D.; Schena, E. Smart Textile Based on Fiber Bragg Grating Sensors for Respiratory Monitoring: Design and Preliminary Trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K. Respiration Rate Measurement Based on Impedance Pneumography. In Application Report SBAA181; Texas Instruments: Dallas, TX, USA, 2011. [Google Scholar]
- Chen, R.; Chen, K.; Dai, Y.; Zhang, S. Utility of transthoracic impedance and novel algorithm for sleep apnea screening in pacemaker patient. Sleep Breath. 2018, 23, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, A.S.; Lenahan, J.L.; Izadnegahdar, R.; Ansermino, J.M. A Systematic Review of Tools to Measure Respiratory Rate in Order to Identify Childhood Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-T.; Chan, H.-L.; Wang, C.-L.; Jian, H.-M.; Lin, S.-H. Instantaneous Respiratory Estimation from Thoracic Impedance by Empirical Mode Decomposition. Sensors 2015, 15, 16372–16387. [Google Scholar] [CrossRef] [PubMed]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllyla, R. Measurement of respiratory rate with high-resolution accelerometer and emfit pressure sensor. In Proceedings of the Proceedings of the 2006 IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Phan, D.H.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Thi, N.Y.P. Estimation of respiratory waveform and heart rate using an accelerometer. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 4916–4919. [Google Scholar]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D. Respiratory Rate and Flow Waveform Estimation from Tri-axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Liu, G.-Z.; Guo, Y.-W.; Zhu, Q.-S.; Huang, B.-Y.; Wang, L. Estimation of Respiration Rate from Three-Dimensional Acceleration Data Based on Body Sensor Network. TELEMED. E-HEALTH 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Passaro, V.M.N.; Cuccovillo, A.; Vaiani, L.; Carlo, M.D.; Campanella, C.E. Gyroscope Technology and Applications: A Review in the Industrial Perspective. Sensors 2017, 17, 2284. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Jung, S. Gyro sensor drift compensation by Kalman filter to control a mobile inverted pendulum robot system. In Proceedings of the 2009 IEEE International Conference on Industrial Technology, Gippsland, VIC, Australia, 10–13 February 2009; pp. 1–6. [Google Scholar]
- Yoon, J.-W.; Noh, Y.-S.; Kwon, Y.-S.; Kim, W.-K.; Yoon, H.-R. Improvement of Dynamic Respiration Monitoring Through Sensor Fusion of Accelerometer and Gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Milici, S.; Lazaro, A.; Villarino, R.; Girbau, D.; Magnarosa, M. Wireless Wearable Magnetometer-Based Sensor for Sleep Quality Monitoring. IEEE Sens. J. 2018, 18, 2145–2152. [Google Scholar] [CrossRef]
- Yu, H.-W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.-M.; Kang, T.J.; Kim, Y.H. Self-Powered Humidity Sensor Based on Graphene Oxide Composite Film Intercalated by Poly(Sodium 4-Styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters Using an Inertial Measurement Unit (IMU)-Based System. Sensors 2018, 19, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Xu, W.; Guan, N.; Ji, D.; Wei, Y.; Yi, W. Noninvasive and Continuous Blood Pressure Monitoring Using Wearable Body Sensor Networks. IEEE Intell. Syst. 2015, 30, 38–48. [Google Scholar] [CrossRef]
- Sekar, P.; Kumar, S. Blood Pressure Monitor Analog Front End; Cypress Semiconductor Corp: San Jose, CA, USA, 16 July 2011. [Google Scholar]
- Meidert, A.S.; Saugel, B. Techniques for Non-Invasive Monitoring of Arterial Blood Pressure. Front. Med. 2018, 4, 231. [Google Scholar] [CrossRef]
- Smulyan, H.; Safar, M.E. Blood Pressure Measurement: Retrospective and Prospective Views. Am. J. Hypertens. 2011, 24, 628–634. [Google Scholar] [CrossRef]
- Pahlm, O.; Wagner, G.S. Multimodal Cardiovascular Imaging: Principles and Clinical Applications; McGraw-Hill Medical: New York, NY, USA, 2011. [Google Scholar]
- Uemura, K.; Kawada, T.; Sugimachi, M. A Novel Minimally Occlusive Cuff Method Utilizing Ultrasound Vascular Imaging for Stress-Free Blood Pressure Measurement: A-Proof-of-Concept Study. IEEE Trans. Biomed. Eng. 2019, 66, 934–945. [Google Scholar] [CrossRef]
- Sone, S.; Hayase, T.; Funamoto, K.; Shirai, A. Simultaneous analysis system for blood pressure and flow using photoplethysmography and ultrasonic-measurement-integrated simulation. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1827–1830. [Google Scholar]
- Zakrzewski, A.M.; Anthony, B.W. Arterial blood pressure estimation using ultrasound: Clinical results on healthy volunteers and a medicated hypertensive volunteer. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, South Korea, 11–15 July 2017; pp. 2154–2157. [Google Scholar]
- Zakrzewski, A.M.; Anthony, B.W. Noninvasive Blood Pressure Estimation Using Ultrasound and Simple Finite Element Models. IEEE Trans. Biomed. Eng. 2018, 65, 2011–2022. [Google Scholar] [CrossRef]
- Kemmotsu, O.; Ueda, M.; Otsuka, H.; Yamamura, T.; Okamura, A.; Ishikawa, T.; Winter, D.C.; Eckerle, J.S. Blood Pressure Measurement by Arterial Tonometry in Controlled Hypotension. Anesth. Analg. 1991, 73, 54–58. [Google Scholar] [CrossRef]
- Shioya, K.; Dohi, T. Blood pressure measurement device based on the arterial tonometry method with micro triaxial force sensor. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 2389–2392. [Google Scholar]
- Blood Pressure. Available online: https://ib.bioninja.com.au/standard-level/topic-6-human-physiology/62-the-blood-system/blood-pressure.html (accessed on 3 February 2020).
- Abbas, A.K.; Bassam, R. Phonocardiography signal processing; Morgan & Claypool Publishers: San Rafael, CA, USA, 2009. [Google Scholar]
- Ismail, S.; Siddiqi, I.; Akram, U. Localization and classification of heart beats in phonocardiography signals—A comprehensive review. EURASIP J. Adv. Signal Process. 2018, 2018, 26. [Google Scholar] [CrossRef] [Green Version]
- Zalter, R.; Hodara, H.; Luisada, A.A. Phonocardiography: I. General principles and problems of standardization. Am. J. Cardiol. 1959, 4, 3–15. [Google Scholar] [CrossRef]
- Razavi, B. Design considerations for direct-conversion receivers. IEEE Trans. Circuits Syst. II Analog Digit. Signal Process. 1997, 44, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Lin, J. Digitally assisted low IF architecture for noncontact vital sign detection. In Proceedings of the 2015 IEEE MTT-S International Microwave Symposium, Phoenix, AZ, USA, 17–22 May 2015; pp. 1–4. [Google Scholar]
- Jensen, B.S.; Johansen, T.K.; Yan, L. An experimental vital signs detection radar using low-IF heterodyne architecture and single-sideband transmission. In Proceedings of the 2013 IEEE International Wireless Symposium (IWS), Beijing, China, 14–18 April 2013; pp. 1–4. [Google Scholar]
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler Radar-Based Non-Contact Health Monitoring for Obstructive Sleep Apnea Diagnosis: A Comprehensive Review. Big Data Cogn. Comput. 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Ichapurapu, R.; Jain, S.; Kakade, M.U.; Lie, D.Y.C.; Banister, R.E. A 2.4 GHz Non-Contact Biosensor System for Continuous Vital-Signs Monitoring on a Single PCB. In Proceedings of the IEEE 8th International Conference, Changsha, China, 20–23 October 2009; pp. 925–928. [Google Scholar]
- Lee, Y.S.; Pathirana, P.N.; Caelli, T.; Li, S. Further applications of Doppler radar for non-contact respiratory assessment. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3833–3836. [Google Scholar]
- Li, C.; Lin, J. Complex signal demodulation and random body movement cancellation techniques for non-contact vital sign detection. In Proceedings of the 2008 IEEE MTT-S International Microwave Symposium Digest, Atlanta GA, USA, 15–20 June 2008; pp. 567–570. [Google Scholar]
- Girbau, D.; Lázaro, A.; Ramos, Á.; Villarino, R. Remote Sensing of Vital Signs Using a Doppler Radar and Diversity to Overcome Null Detection. IEEE Sens. J. 2012, 12, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Rabiner, L.R.; Schafer, R.H.; Rader, C.M. The chirp z-transform algorithm and its applications. Bell Syst. Tech. J. 1969, 48, 1249–1292. [Google Scholar] [CrossRef]
- Noguchi, H.; Kubo, H.; Mori, T.; Sato, T.; Sanada, H. Signal Phase Estimation for Measurement of Respiration Waveform Using a Microwave Doppler Sensor. In Proceedings of the 35th Annual International Conference of the IEEE EMBS, Osaka, Japan, 3–7 July 2013; pp. 6740–6743. [Google Scholar]
- Rahman, M.S.; Jang, B.-J.; Kim, K.-D. A New Digital Signal Processor for Doppler Radar Cardiopulmonary Monitoring System. In Proceedings of the 5th International Conference on Electrical and Computer Engineering (ICECE 2008), Dhaka, Bangladesh, 20–22 December 2008; pp. 76–79. [Google Scholar]
- Tariq, A.; Shiraz, H.G. Doppler Radar Vital Signs Monitoring using Wavelet Transform. In Proceedings of the 2010 Loughborough Antennas & Propagation Conference, Loughborough, UK, 8–9 November 2010; pp. 293–296. [Google Scholar]
- Tran, V.P.; Al-Jumaily, A.A. Non-Contact Dual Pulse Doppler System based Respiratory and Heart Rates Estimation for CHF Patients. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 4202–4205. [Google Scholar]
- Chazal, P.D.; O’Hare, E.; Fox, N.; Heneghan, C. Assessment of Sleep/Wake Patterns Using a Non-Contact Biomotion Sensor. In Proceedings of the 30th Annual International IEEE EMBS Conference, Vancouver, BC, Canada, 20–24 August 2008; pp. 514–517. [Google Scholar]
- Chazal, P.D.; Fox, N.; O’Hare, E.; Heneghan, C.; Zaffaroni, A.; Boyle, P.; Smith, S.; O’Connell, C.; McNicholas, W.T. Sleep⁄wake measurement using a non-contact biomotion sensor. J. Sleep Res. 2011, 20, 356–366. [Google Scholar] [CrossRef]
- Massagram, W.; Hafner, N.; Lubecke, V.; Boric-Lubecke, O. Tidal Volume Measurement through Non-Contact Doppler Radar with DC Reconstruction. IEEE Sens. J. 2013, 13, 3397–3404. [Google Scholar] [CrossRef]
- Tran, V.P.; Al-Jumaily, A.A. Non-Contact Real-Time Estimation of Intrapulmonary Pressure and Tidal Volume for Chronic Heart Failure Patients. In Proceedings of the 38th Annual International Conference of the IEEE, Orlando, FA, USA, 16–20 August 2016; pp. 3564–3567. [Google Scholar]
- Lin, J.C.; Salinger, J. Microwave measurement of respiration. In Proceedings of the 1975 IEEE MTT-S International Microwave Symposium, Dig., Palo Alton, CA, USA, 12–14 May 1975; pp. 285–287. [Google Scholar]
- Diebold, S.; Ayhan, S.; Scherr, S.; Massler, H.; Tessmann, A.; Leuther, A.; Ambacher, O.; Zwick, T.; Kallfass, I. A W-Band MMIC Radar System for Remote Detection of Vital Signs. J. Infrared Millim. Terahertz Waves 2012, 33, 1250–1267. [Google Scholar] [CrossRef]
- Santra, A.; Ulaganathan, R.V.; Finke, T. Short-Range Millimetric-Wave Radar System for Occupancy Sensing Application. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Nosrati, M.; Shahsavari, S.; Tavassolian, N. Multi-Target Vital-Signs Monitoring Using a Dual-Beam Hybrid Doppler Radar. In Proceedings of the 2018 IEEE International Microwave Biomedical Conference (IMBioC), Philadelphia, PA, USA, 14–15 June 2018; pp. 58–60. [Google Scholar]
- Nosrati, M.; Shahsavari, S.; Lee, S.; Wang, H.; Tavassolian, N. A Concurrent Dual-Beam Phased-Array Doppler Radar Using MIMO Beamforming Techniques for Short-Range Vital-Signs Monitoring. IEEE Trans. Antennas Propag. 2019, 67, 2390–2404. [Google Scholar] [CrossRef]
- Lin, J.; Wu, W. Vital sign radars: Past, present, and future. In Proceedings of the WAMICON 2014, Tampa, FL, USA, 6 June 2014; pp. 1–4. [Google Scholar]
- Tang, M.-C.; Wang, F.-K.; Horng, T.-S. Single Self-Injection-Locked Radar With Two Antennas for Monitoring Vital Signs With Large Body Movement Cancellation. IEEE Trans. Microw. Theory Tech. 2017, 65, 5324–5333. [Google Scholar] [CrossRef]
- Mostafanezhad, I.; Yavari, E.; Boric-Lubecke, O.; Lubecke, V.M.; Mandic, D.P. Cancellation of Unwanted Doppler Radar Sensor Motion Using Empirical Mode Decomposition. IEEE Sens. J. 2013, 13, 1897–1904. [Google Scholar] [CrossRef]
- Kazemi, S.; Ghorbani, A.; Amindavar, H.; Li, C. Cyclostationary approach to Doppler radar heart and respiration rates monitoring with body motion cancelation using Radar Doppler System. Biomed. Signal Process. 2014, 13, 79–88. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, S.; Dong, X.; Peng, Z.; Zhang, W. Accurate Measurement in Doppler Radar Vital Sign Detection Based on Parameterized Demodulation. IEEE Trans. Microw. Theory Tech. 2017, 65, 4483–4492. [Google Scholar] [CrossRef]
- Fletcher, R.; Han, J. Low-cost differential front-end for Doppler radar vital sign monitoring. In Proceedings of the 2009 IEEE MTT-S International Microwave Symposium Digest, Boston, MA, USA, 7–12 June 2009; pp. 1325–1328. [Google Scholar]
- Bakhtiari, S.; Elmer, T.W.; Cox, N.M.; Gopalsami, N.; Raptis, A.C.; Liao, S.; Mikhelson, I.; Sahakian, A.V. Compact Millimeter-Wave Sensor for Remote Monitoring of Vital Signs. IEEE Trans. Instrum. Meas. 2012, 61, 830–841. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Chou, C.-C.; Lin, C.-C.; Yu, C.-H.; Huang, T.-H.; Chuang, H.-R. A 60-GHz CMOS direct-conversion Doppler radar RF sensor with clutter canceller for single-antenna noncontact human vital-signs detection. In Proceedings of the 2015 IEEE Radio Frequency Integrated Circuits Symposium (RFIC), Phoenix, AZ, USA, 17–19 May 2015; pp. 35–38. [Google Scholar]
- Lee, H.; Kim, B.-H.; Yook, J.-G. Path Loss Compensation Method for Multiple Target Vital Sign Detection with 24-GHz FMCW Radar. In Proceedings of the 2018 IEEE Asia-Pacific Conference on Antennas and Propagation (APCAP), Auckland, New Zealand, 5–8 August 2018; pp. 100–101. [Google Scholar]
- Thurn, K.; Ebelt, R.; Vossiek, M. Noise in Homodyne FMCW radar systems and its effects on ranging precision. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (MTT), Seattle, WA, USA, 2–7 June 2013; pp. 1–3. [Google Scholar]
- Wang, G.; Munoz-Ferreras, J.-M.; Gu, C.; Li, C.; Gomez-Garcia, R. Application of Linear-Frequency-Modulated Continuous-Wave (LFMCW) Radars for Tracking of Vital Signs. IEEE Trans. Microw. Theory Tech. 2014, 62, 1387–1399. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.-H.; Park, J.-K.; Yook, J.-G. A Novel Vital-Sign Sensing Algorithm for Multiple Subjects Based on 24-GHz FMCW Doppler Radar. Remote Sens. 2019, 11, 1237. [Google Scholar] [CrossRef] [Green Version]
- Tarassenko, L.; Villarroel, M.; Guazzi, A.; Jorge, J.; Clifton, D.; Pugh, C. Non-contact video-based vital sign monitoring using ambient light and auto-regressive models. Physiol. Meas. 2014, 35, 807. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shaker, G.; Almeida, J.C.M.D.; Morita, P.P.; Safavi-Naeini, S. Remote Monitoring of Human Vital Signs Using mm-Wave FMCW Radar. IEEE Access 2019, 7, 54958–54968. [Google Scholar] [CrossRef]
- Ahmad, A.; Roh, J.C.; Wang, D.; Dubey, A. Vital signs monitoring of multiple people using a FMCW millimeter-wave sensor. In Proceedings of the 2018 IEEE Radar Conference (RadarConf18), Oklahoma City, OK, USA, 23–27 April 2018; pp. 1450–1455. [Google Scholar]
- Peng, Z.; Munoz-Ferreras, J.M.; Tang, Y.; Liu, C.; Gomez-Garcia, R.; Ran, L.; Li, C. A Portable FMCW Interferometry Radar With Programmable Low-IF Architecture for Localization, ISAR Imaging, and Vital Sign Tracking. IEEE Trans. Microw. Theory Tech. 2017, 65, 1334–1344. [Google Scholar] [CrossRef]
- Lees, M. Digital beamforming calibration for FMCW radar. IEEE Trans. Aerosp. and Electron. Syst. 1989, 25, 281–284. [Google Scholar] [CrossRef]
- Fuchs, J.; Ward, K.; Tulin, M.; York, R. Simple techniques to correct for VCO nonlinearities in short range FMCW radars. In Proceedings of the 1996 IEEE MTT-S International Microwave Symposium Digest, San Francisco, CA, USA, 17–21 June 1996; pp. 1175–1178. [Google Scholar]
- Su, W.; Tang, M.; Arif, R.E.; Horng, T.; Wang, F. Stepped-Frequency Continuous-Wave Radar With Self-Injection-Locking Technology for Monitoring Multiple Human Vital Signs. IEEE Trans. Microw. Theory Tech. 2019, 67, 5396–5405. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S. Remote Detection of Human Vital Sign With Stepped-Frequency Continuous Wave Radar. IEEE J. STARS. 2014, 7, 775–782. [Google Scholar] [CrossRef]
- Zhang, Z.; Nian, Y.; Chen, J.; He, M. An Experimental Study to Optimize the Stepped-Frequency Continuous-Wave Radar Parameters for Noncontact Multi-target Vital Sign Monitoring. In Proceedings of the 2019 IEEE International Conference on Computational Electromagnetics (ICCEM), Shangai, China, 20–22 March 2019; pp. 1–4. [Google Scholar]
- Fathy, A.E.; Ren, L.; Nahar, S.; Kilic, O. Overview of human vital signs detection using radar techniques. In Proceedings of the 2017 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–14 July 2017; pp. 1229–1230. [Google Scholar]
- Ren, L.; Kong, L.; Foroughian, F.; Wang, H.; Theilmann, P.; Fathy, A.E. Comparison Study of Noncontact Vital Signs Detection Using a Doppler Stepped-Frequency Continuous-Wave Radar and Camera-Based Imaging Photoplethysmography. IEEE Trans. Microw. Theory Tech. 2017, 65, 3519–3529. [Google Scholar] [CrossRef]
- Wisland, D.T.; Granhaug, K.; Pleym, J.R.; Andersen, N.; Stoa, S.; Hjortland, H.A. Remote monitoring of vital signs using a CMOS UWB radar transceiver. In Proceedings of the 2016 14th IEEE International New Circuits and Systems Conference (NEWCAS), Van Couver, BC, USA, 26–29 June 2016; pp. 1–4. [Google Scholar]
- Host-Madsen, A.; Petrochilos, N.; Boric-Lubecke, O.; Lubecke, V.M.; Park, B.K.; Zhou, Q. Signal processing methods for Doppler radar heart rate monitoring. In Signal Processing Techniques for Knowledge Extraction and Information Fusion; Mandic, D., Ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Xu, Y.; Dai, S.; Wu, S.; Chen, J.; Fang, G. Vital Sign Detection Method Based on Multiple Higher Order Cumulant for Ultrawideband Radar. IEEE Trans. Geosci. Remote Sens. 2012, 50, 1254–1265. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Barrowes, B.E. Through-Wall Bio-Radiolocation With UWB Impulse Radar: Observation, Simulation and Signal Extraction. IEEE J. STARS. 2011, 4, 791–798. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, H.; Fang, G.; Ye, S.; Gulliver, T.A. An Improved Algorithm for Through-Wall Target Detection Using Ultra-Wideband Impulse Radar. IEEE Access 2017, 5, 22101–22118. [Google Scholar] [CrossRef]
- Ren, L.; Koo, Y.S.; Wang, Y.; Fathy, A.E. Noncontact heartbeat detection using UWB impulse Doppler radar. In Proceedings of the IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), San Diego, CA, USA, 25–28 January 2015; pp. 1–3. [Google Scholar]
- Ren, L.; Wang, H.; Naishadham, K.; Kilic, O.; Fathy, A.E. Phase-Based Methods for Heart Rate Detection Using UWB Impulse Doppler Radar. IEEE Trans. Microw. Theory Tech. 2016, 64, 3319–3331. [Google Scholar] [CrossRef]
- Liang, X.; Deng, J.; Zhang, H.; Gulliver, I.A. Ultra-Wideband Impulse Radar Through-Wall Vital Signs. Sci. Rep. 2018, 8, 13367. [Google Scholar] [CrossRef] [Green Version]
- Nezirovic, A.; Yarovoy, A.; Ligthart, L. Signal Processing for Improved Detection of Trapped Victims Using UWB Radar. IEEE Trans. Geosci. Remote Sens. 2010, 48, 2005–2014. [Google Scholar] [CrossRef]
- Hu, X.; Jin, T. Short-Range Vital Signs Sensing Based on EEMD and CWT Using IR-UWB Radar. Sensors 2016, 16, 2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, E.; Filippi, A.; Tomasin, S. ML Period Estimation With Application to Vital Sign Monitoring. IEEE Signal Process. Lett. 2010, 17, 905–908. [Google Scholar] [CrossRef]
- Liang, S.D. Sense-through-wall human detection based on UWB radar sensors. Signal Process. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Buyukhan, C.; Karamzadeh, S.; Orhan, M.; Caliskan, A.; Cilesiz, I. Experimental study of human respiratory detection using UWB GPR. In Proceedings of the 2017 4th International Conference on Electrical and Electronic Engineering (ICEEE), Ankara, Turkey, 8–10 April 2017; pp. 3143–3217. [Google Scholar]
- Mercuri, M.; Soh, P.J.; Pandey, G.; Karsmakers, P.; Vandenbosch, G.A.E.; Leroux, P.; Schreurs, D. Analysis of an Indoor Biomedical Radar-Based System for Health Monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Svecova, M.; Kocur, D.; Zetik, R.; Rovnakova, J. Target localization by a multistatic UWB radar. In Proceedings of the 20th International Conference Radioelektronika, Brno, Czech Republic, 19–21 April 2010; pp. 1–4. [Google Scholar]
- Koo, Y.S.; Ren, L.; Wang, Y.; Fathy, A.E. UWB MicroDoppler Radar for human Gait analysis, tracking more than one person, and vital sign detection of moving persons. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (MTT), Seattle, WA, USA, 2–7 June 2013; pp. 1–4. [Google Scholar]
- Chleicher, B.; Schumacher, H. Impulse generator targeting the European UWB mask. In Proceedings of the 2010 Topical Meeting on Silicon Monolithic Integrated Circuits in RF Systems (SiRF), New Orleans, LA, USA, 11–13 January 2010; pp. 21–24. [Google Scholar]
- Stauss, H.M. Heart rate variability. Am. J. Physiol. Integ. Regul. Comp. Physiol. 2003, 285, R927–R931. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, V.L.; Jankovic, M.M.; Lupsic, A.V.; Mihajlovic, V.R.; Popovic-Bozovic, J.S. High-Accuracy Real-Time Monitoring of Heart Rate Variability Using 24 GHz Continuous-Wave Doppler Radar. IEEE Access 2019, 7, 74721–74733. [Google Scholar] [CrossRef]
- Boric-Lubecke, O.; Massagram, W.; Lubecke, V.M.; Host-Madsen, A.; Jokanovic, B. Heart Rate Variability Assessment Using Doppler Radar with Linear Demodulation. In Proceedings of the 2008 38th European Microwave Conference, Amsterdam, The Netherlands, 27–31 October 2008; pp. 420–423. [Google Scholar]
- Al-Masri, E.; Momin, M. Detecting Heart Rate Variability using Millimeter-Wave Radar Technology. In Proceedings of the 2018 IEEE International Conference on Big Data (Big Data), Seattle, WA, USA, 10–13 December 2018; pp. 5282–5284. [Google Scholar]
- Han, Y.; Lauteslager, T.; Lande, T.S.; Constandinou, T.G. UWB Radar for Non-contact Heart Rate Variability Monitoring and Mental State Classification. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6578–6582. [Google Scholar]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [Green Version]
- Hey-Shipton, G.L.; Matthews, P.A.; Mcstay, J. The complex permittivity of human tissue at microwave frequencies. Phys. Med. Biol. 1982, 27, 1067–1071. [Google Scholar] [CrossRef]
- Chahat, N.; Zhadobov, M.; Augustine, R.; Sauleau, R. Human skin permittivity models for millimetre-wave range. Electron. Lett. 2011, 47, 427. [Google Scholar] [CrossRef]
- Amin, M.G. Radar for Indoor Monitoring: Detection, Classification, and Assessment; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Li, C.; Lin, J. Optimal Carrier Frequency of Non-contact Vital Sign Detectors. In Proceedings of the 2007 IEEE Radio and Wireless Symposium Long Beach, Long Beach, CA, USA, 9–11 January 2007; pp. 281–284. [Google Scholar]
- Tariq, A. Vital Signs Monitoring Using Doppler Radar and on-Body Antennas. Ph.D. thesis. 2012. Available online: https://etheses.bham.ac.uk/id/eprint/4332/1/Tariq13PhD.pdf (accessed on 4 March 2020).



















| Method | Vital Signs Detected | Minimum Number of Contacts | Accuracy | Long-Term Monitoring | Drawbacks | ||
|---|---|---|---|---|---|---|---|
| 1 | ECG | BR and HR | 3 | High | Yes | Expensive, MA effect | |
| 2 | PPG | BR and HR | 1 | High | Yes | MA, environmental effects | |
| 3 | Air-based sensing | Air component | BR | 1 | High | No | Environment effects |
| Air temperature | BR | 1 | High | No | - | ||
| Air humidity | BR | 1 | High | No | Environmental effects | ||
| 4 | Mechanical displacement sensing of chest | Strain-based | BR | 1 | High | Yes | Tightly attached probe |
| Impedance pneumography | BR | 1 | High | Yes | MA effect | ||
| 3D movement sensing | BR | 3 | Medium | Yes | Expensive | ||
| 5 | Blood pressure sensing | Non-invasive | HR and BP | 1 | Medium | Yes | Often requires physician |
| Invasive | HR and BP | 1 | High | No | Clinical uses only | ||
| 6 | PCG | HR | 1 | High | No | Surrounding sound effects | |
| Method | Vital Signs Detected | Multi-Subjects Detection | Range Estimation | Power Consumption |
|---|---|---|---|---|
| CW | BR and HR | No | No | Medium |
| FMCW | BR and HR | Yes | Yes | High |
| SFCW | BR and HR | Yes | Yes | Medium |
| UWB | BR and HR | Yes | Yes | Low |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebe, M.; Gadhafi, R.; Mohammad, B.; Sanduleanu, M.; Saleh, H.; Al-Qutayri, M. Human Vital Signs Detection Methods and Potential Using Radars: A Review. Sensors 2020, 20, 1454. https://doi.org/10.3390/s20051454
Kebe M, Gadhafi R, Mohammad B, Sanduleanu M, Saleh H, Al-Qutayri M. Human Vital Signs Detection Methods and Potential Using Radars: A Review. Sensors. 2020; 20(5):1454. https://doi.org/10.3390/s20051454
Chicago/Turabian StyleKebe, Mamady, Rida Gadhafi, Baker Mohammad, Mihai Sanduleanu, Hani Saleh, and Mahmoud Al-Qutayri. 2020. "Human Vital Signs Detection Methods and Potential Using Radars: A Review" Sensors 20, no. 5: 1454. https://doi.org/10.3390/s20051454





