A Rapid and Nondestructive Approach for the Classification of Different-Age Citri Reticulatae Pericarpium Using Portable Near Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. CRP Sample
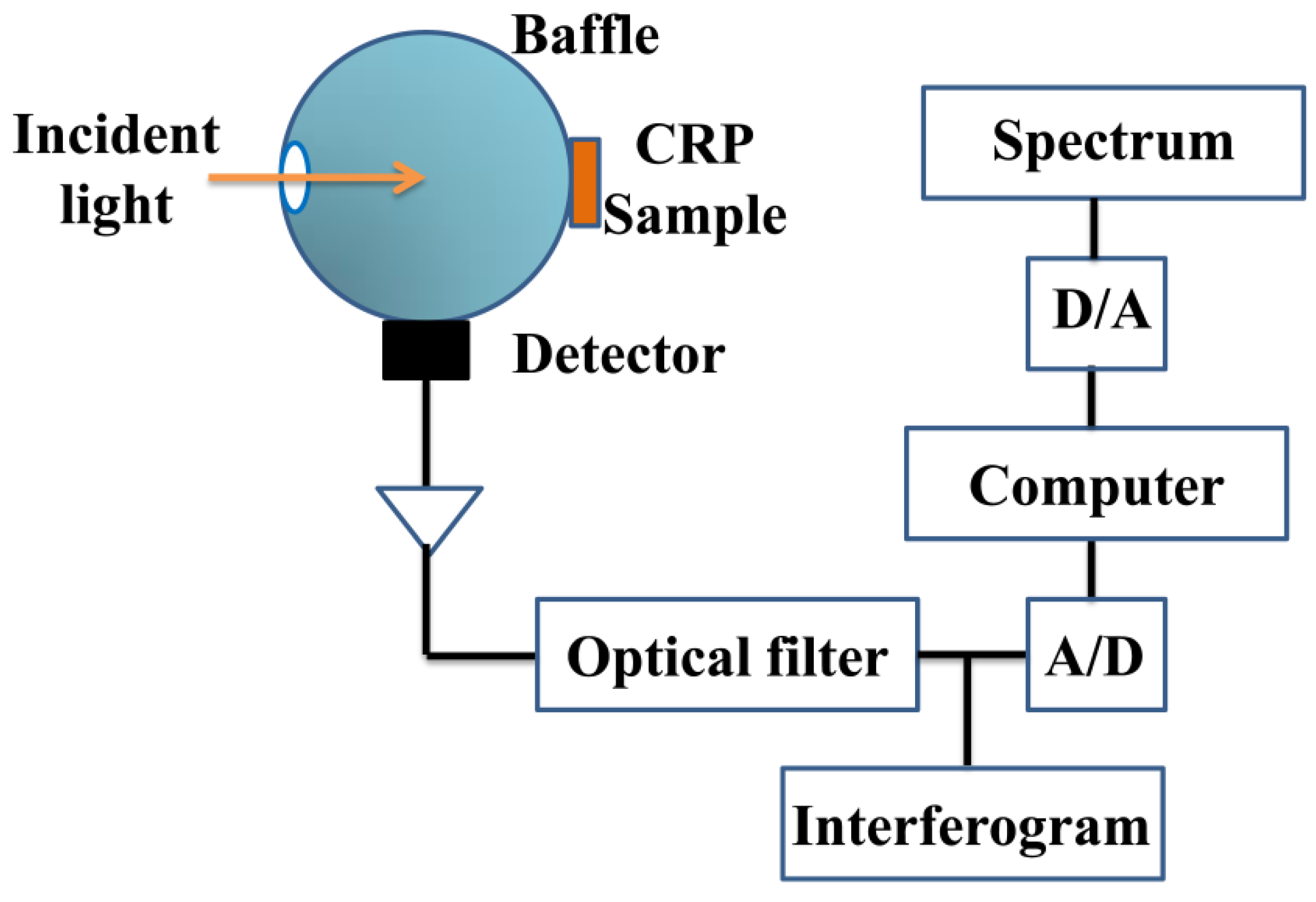
2.2. Instrumentation and Measurements
2.3. Data Analysis
3. Results and Discussion
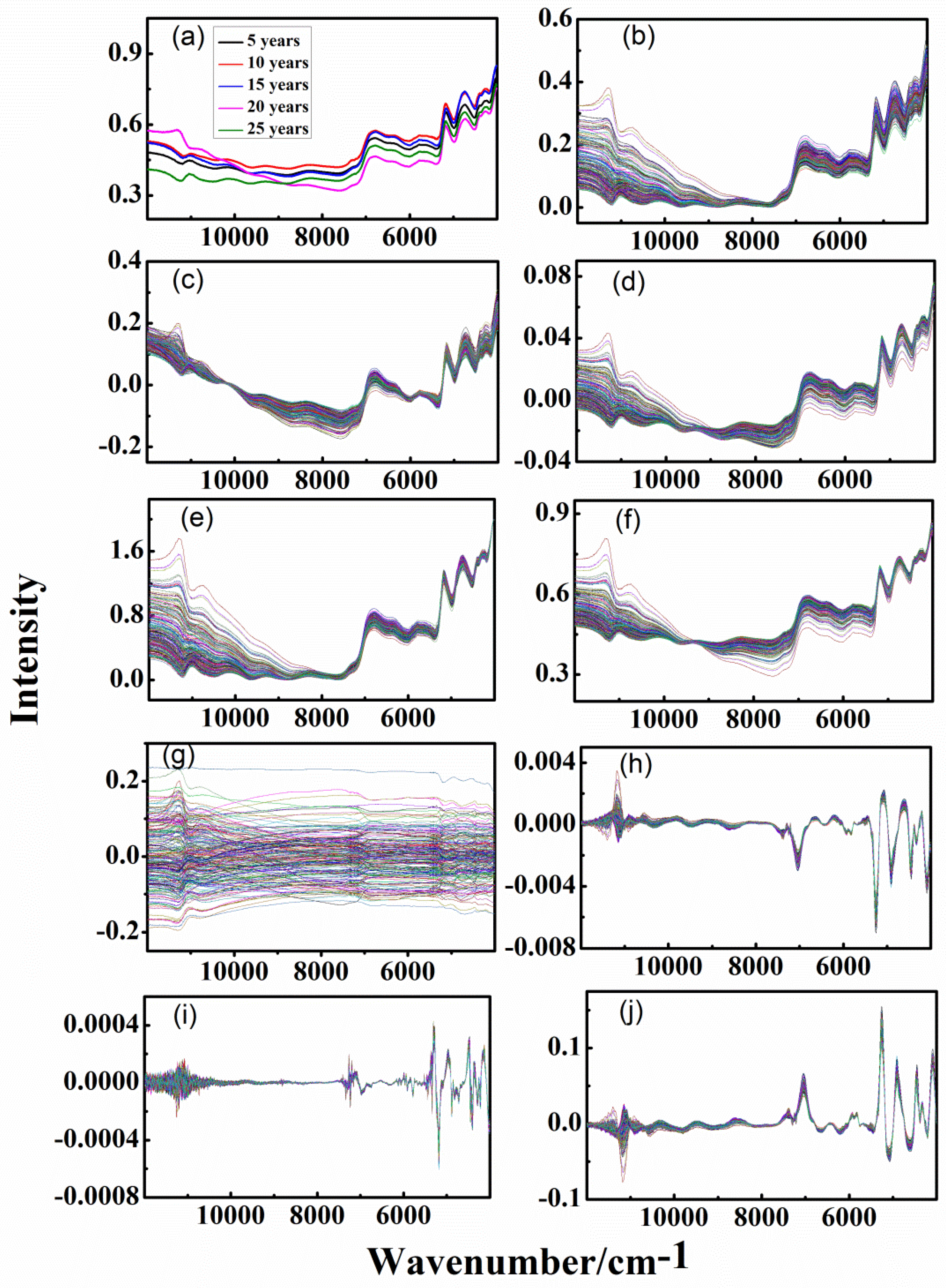
3.1. Spectra of Different-age CRPs with Single Pretreatment Techniques
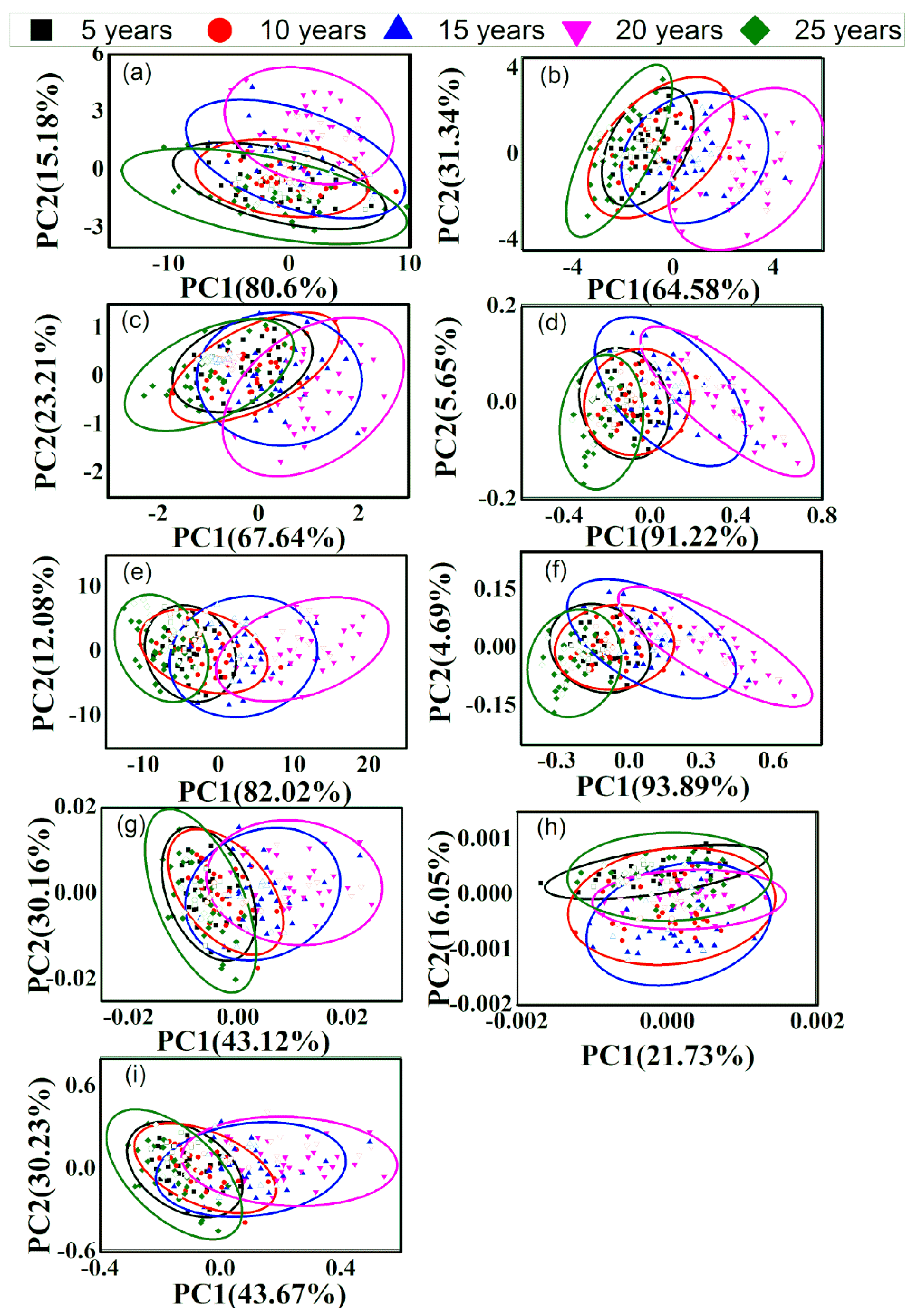
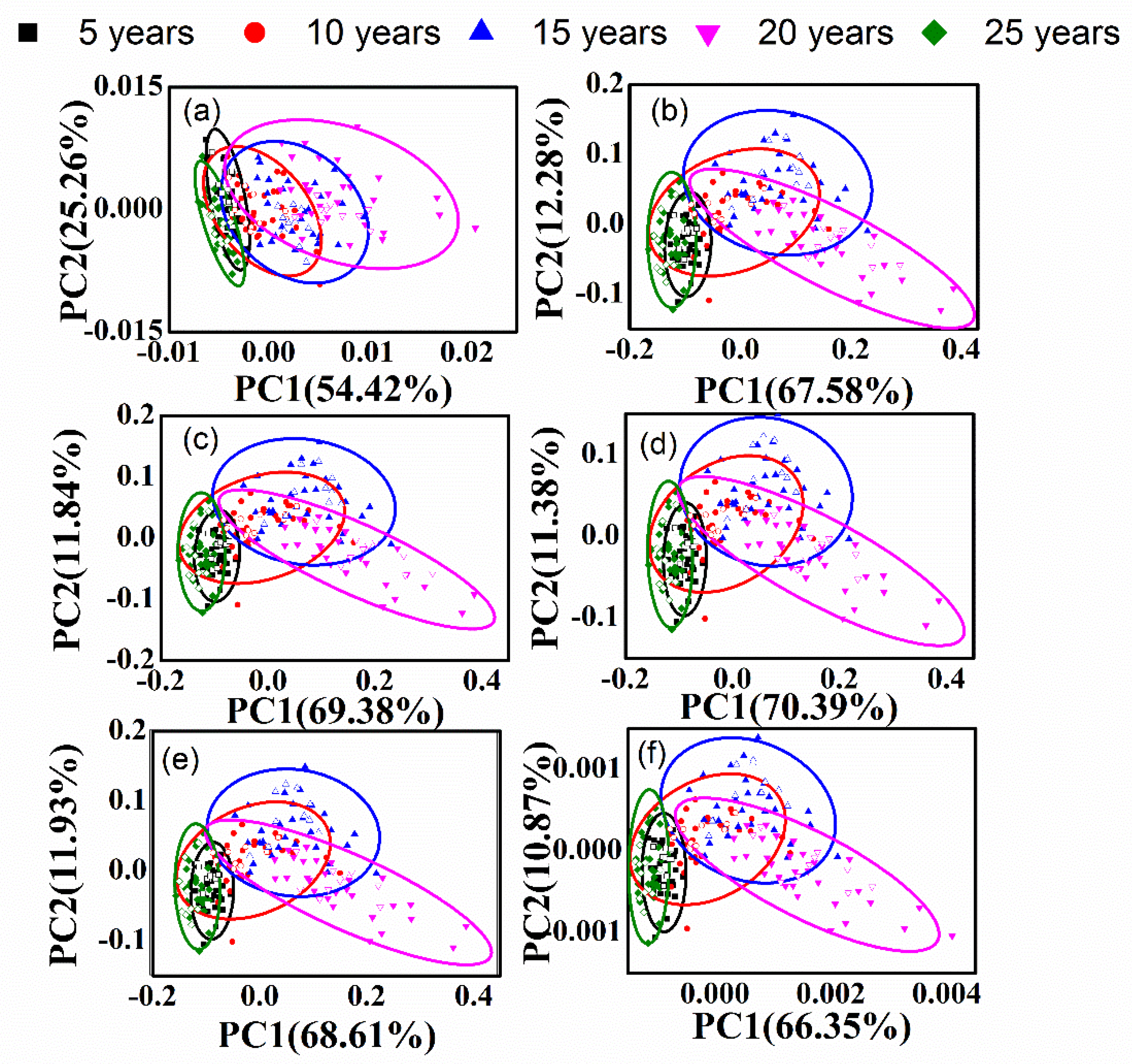
3.2. PCA of Different-Age CRPs with Single Pretreatment Techniques
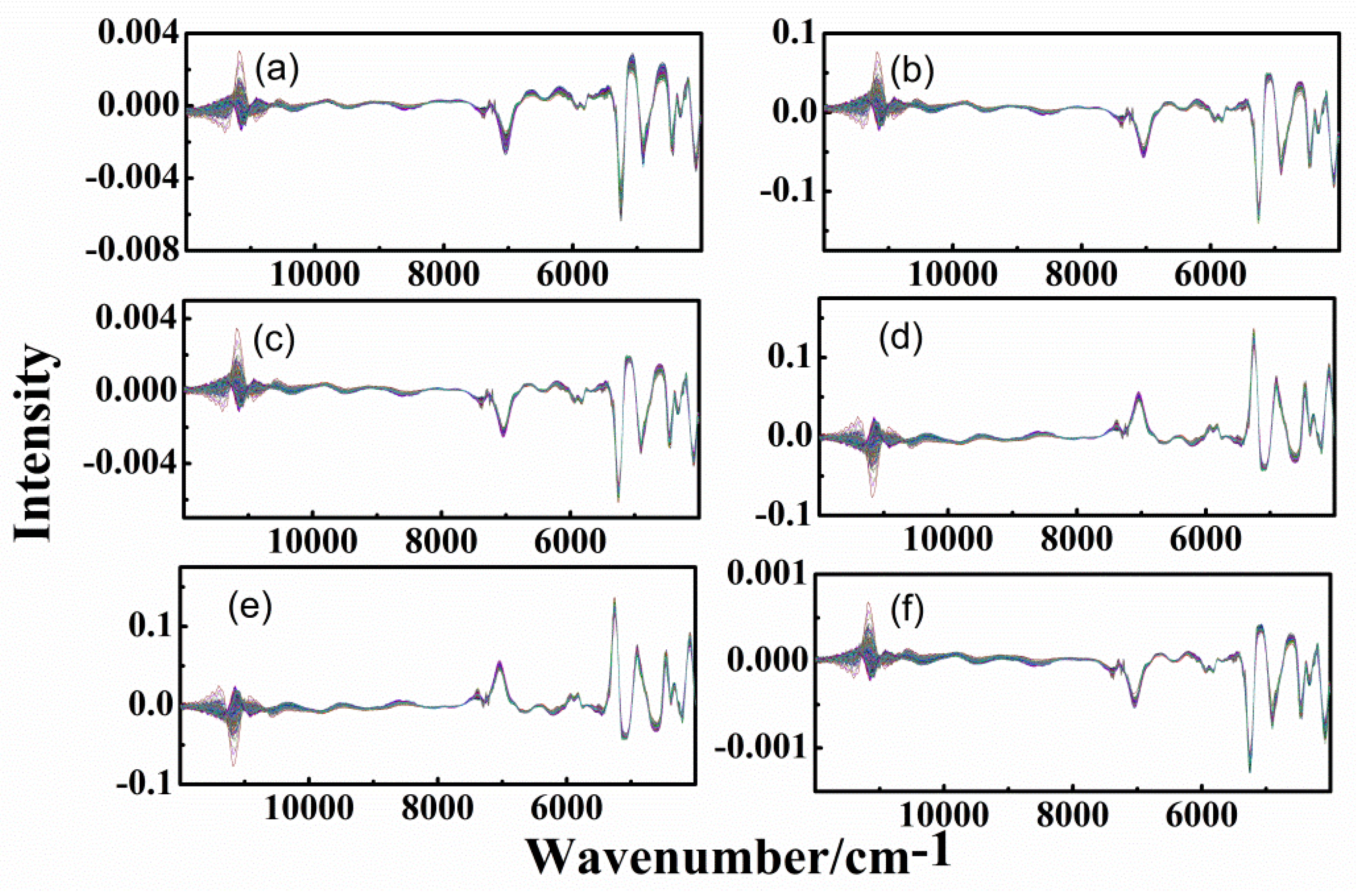
3.3. Spectra of Different-Age CRPs with Combined Pretreatment Techniques
3.4. PCA of Different-Age CRPs with Combined Pretreatment Techniques
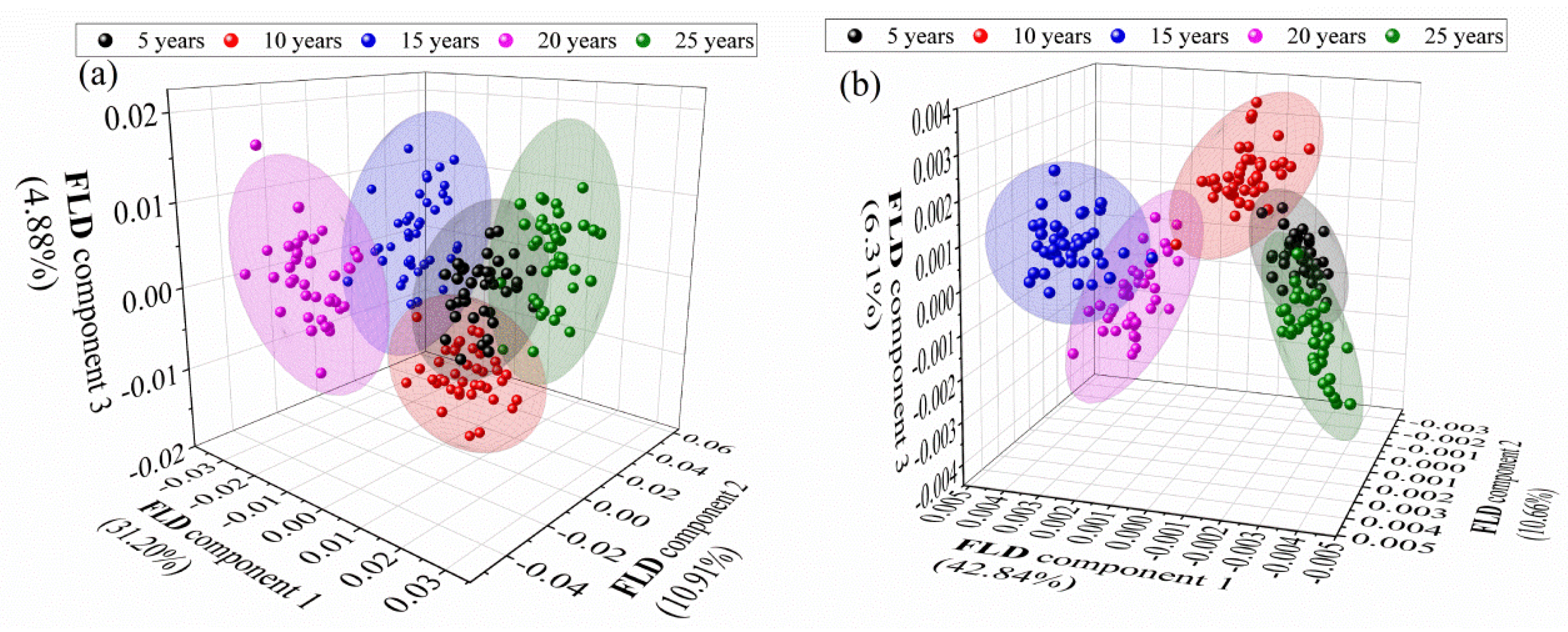
3.5. FLD of Different-Age CRPs with Pretreatment Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aires, A.; Carvalho, R.; Matos, M.; Carnide, V.; Silva, A.P.; Gonçalves, B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in central Portugal. J. Food Biochem. 2017, 41, e12414. [Google Scholar] [CrossRef]
- Shi, Q.R.; Guo, T.T.; Yin, T.J.; Wang, Z.Q.; Li, C.H.; Sun, X.; Guo, Y.M.; Yuan, W.H. Classification of Pericarpium Citri Reticulatae of different ages by using a voltammetric electronic tongue system. Int. J. Electrochem. Sci. 2018, 13, 11359–11374. [Google Scholar] [CrossRef]
- Luo, M.X.; Luo, H.J.; Hu, P.J.; Yang, Y.T.; Wu, B.; Zheng, G.D. Evaluation of chemical components in Citri Reticulatae Pericarpium of different cultivars collected from different regions by GC-MS and HPLC. Food Sci. Nutr. 2018, 6, 400–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Z.; Guan, X.M.; Gao, Z.; Lan, H.C.; Yin, Q.; Chu, C.; Yang, D.P.; Liu, E.H.; Zhou, P. A simple method to discriminate Guangchenpi and Chenpi by high performance thin-layer chromatography and high-performance liquid chromatography based on analysis of dimethyl anthranilate. J. Chromatogr. B 2019, 1126–1127, 121736. [Google Scholar] [CrossRef]
- Lv, W.S.; Lin, T.; Ren, Z.Y.; Jiang, Y.Q.; Zhang, J.; Bi, F.J.; Gu, L.H.; Hou, H.C.; He, J.N. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, W.; Huang, K.E.; Li, D.X.; Chen, W.; Yu, X.Q.; Ke, X.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as well as the Citrus reticulata ‘Chachi’ within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharm. Biomed. 2019, 171, 218–231. [Google Scholar]
- Yu, H.Y.; Niu, X.Y.; Lin, H.J.; Ying, Y.B.; Li, B.B.; Pan, X.X. A feasibility study on on-line determination of rice wine composition by Vis-NIR spectroscopy and least-squares support vector machines. Food Chem. 2009, 113, 291–296. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, S.; Zhao, G. Rapid determination of total protein and wet gluten in commercial wheat flour using siSVR-NIR. Food Chem. 2017, 221, 1939–1946. [Google Scholar] [CrossRef]
- Xiao, H.; Feng, L.; Song, D.; Tu, K.; Peng, J.; Pan, L.Q. Grading and sorting of grape berries using visible-near infrared spectroscopy on the basis of multiple inner quality parameters. Sensors 2019, 19, 2600. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.Y.; Sun, Y.M.; Cai, C.Y.; He, Y.; Nie, P.C. Rapid determination of chlorogenic acid, luteoloside and 3,5-o-dicaffeoylquinic acid in chrysanthemum using near-infrared spectroscopy. Sensors 2019, 19, 1981. [Google Scholar] [CrossRef] [Green Version]
- Tardaguila, J.; Fernández-Novales, J.; Gutiérrez, S.; Paz Diago, M. Non-destructive assessment of grapevine water status in the field using a portable NIR spectrophotometer. J. Sci. Food Agric. 2017, 97, 3772–3780. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.E.; O’Shea, M.G.; Johnson, R.A.; Kokot, S. Near-infrared spectroscopy for the prediction of disease ratings for fiji leaf gall in sugarcane clones. Appl. Spectrosc. 2009, 63, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Jenal, A.; Bareth, G.; Bolten, A.; Kneer, C.; Weber, I.; Bongartz, J. Development of a VNIR/SWIR multispectral imaging system for vegetation monitoring with unmanned aerial vehicles. Sensors 2019, 19, 5507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Du, G.R.; Cai, W.S.; Shao, X.G. Rapid and nondestructive analysis of pharmaceutical products using near-infrared diffuse reflectance spectroscopy. J. Pharm. Biomed. 2012, 70, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Sandak, J.; Sandak, A.; Zitek, A.; Hintestoisser, B.; Picchi, G. Development of Low-Cost Portable Spectrometers for Detection of Wood Defects. Sensors 2020, 20, 545. [Google Scholar] [CrossRef] [Green Version]
- Rinnan, A.; Berg, F.V.D.; Engelsen, S.B. Review of the Most Common pre-Processing Techniques for Near-Infrared Spectra. Trac Trend Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Bian, X.H.; Li, S.J.; Shao, X.G.; Liu, P. Variable space boosting partial least squares for multivariate calibration of near-infrared spectroscopy. Chemometr. Intell. Lab. 2016, 158, 174–179. [Google Scholar] [CrossRef]
- Han, X.; Huang, Z.X.; Chen, X.D.; Li, Q.F.; Xu, K.X.; Chen, D. On-line multi-component analysis of gases for mud logging industry using data driven Raman spectroscopy. Fuel 2017, 207, 146–153. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ying, Y.B.; Fu, X.P. Study on predicting sugar content and valid acidity of apples by near infrared diffuse reflectance technique. Spectrosc. Spect. Anal. 2005, 25, 1793–1796. [Google Scholar]
- Geladi, P.; Macdougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, J.S. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Lister, S.J.; Sanderson, R.; Barnes, R.J. The link between multiplicative scatter correction (MSC) and standard normal variate (SNV) transformations of NIR spectra. J. Near Infrared Spec. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Helland, S.I.; Naes, T.; Isaksson, T. Related versions of the multiplicative scatter correction method for preprocessing spectroscopic data. Chemometr. Intell. Lab. 1995, 29, 233–241. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wang, S.S.; Cai, R.; Meng, Y.; Xie, X.; Zhao, W.J. Rapid discrimination and quantification of alkaloids in Corydalis Tuber by near-infrared spectroscopy. J. Pharm. Biomed. 2012, 59, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Shao, X.G.; Leung, A.K.M.; Chau, F.T. Wavelet: A new trend in chemistry. Acc. Chem. Res. 2003, 36, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.H.; Wang, K.Y.; Tan, E.R.; Diwu, P.Y.; Zhang, F.; Guo, Y.G. A selective ensemble preprocessing strategy for near-infrared spectral quantitative analysis of complex samples. Chemometr. Intell. Lab. 2020, 197, 103916. [Google Scholar] [CrossRef]
- Yun, Y.H.; Li, H.D.; Deng, B.C.; Cao, D.S. An overview of variable selection methods in multivariate analysis of near-infrared spectra. Trac Trend Anal. Chem. 2019, 113, 102–115. [Google Scholar] [CrossRef]
- Li, P.; Du, G.R.; Ma, Y.J.; Zhou, J.; Jiang, L.W. A novel multivariate calibration method based on variable adaptive boosting partial least squares algorithm. Chemometr. Intell. Lab. 2018, 176, 157–161. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemometr. Intell. Lab. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chapter four-chemometrics applied to plant spectral analysis. Compr. Anal. Chem. 2018, 80, 69–104. [Google Scholar]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Yan, S.; Lai, X.X.; Du, G.R.; Xiang, Y.H. Identification of aminoglycoside antibiotics in milk matrix with a colorimetric sensor array and pattern recognition methods. Anal. Chim. Acta 2018, 1034, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.L.B.; Brito, L.R.; Honorato, F.A.; Pontes, M.J.C.; Pontes, L.F.B.L. Classification of cereal bars using near infrared spectroscopy and linear discriminant analysis. Food Res. Int. 2013, 51, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mei, M.H.; Ni, Y.N.; Kokot, S. Combined NIR/MIR analysis: A novel method for the classification of complex substances such as illicium verum Hook. F. and its adulterants. Spectrochim. Acta A 2014, 130, 539–545. [Google Scholar] [CrossRef]





| Dataset | Pretreatment Method | 5 years (%) | 10 years (%) | 15 years (%) | 20 years (%) | 25 years (%) | Whole Data (%) |
|---|---|---|---|---|---|---|---|
| Outer skin data | Raw | 100 | 100 | 100 | 100 | 100 | 100 |
| DT | 100 | 100 | 100 | 100 | 100 | 100 | |
| de-bias | 100 | 100 | 100 | 100 | 90 | 98 | |
| SNV | 100 | 100 | 100 | 100 | 100 | 100 | |
| MinMax | 100 | 100 | 100 | 100 | 100 | 100 | |
| MSC | 100 | 100 | 100 | 100 | 100 | 100 | |
| 1st | 100 | 100 | 100 | 100 | 90 | 98 | |
| 2nd | 70 | 100 | 80 | 90 | 90 | 86 | |
| CWT | 90 | 100 | 100 | 100 | 100 | 98 | |
| 1st-DT | 80 | 100 | 100 | 100 | 100 | 96 | |
| 1st-SNV | 90 | 100 | 100 | 100 | 90 | 96 | |
| 1st-MSC | 100 | 100 | 100 | 100 | 90 | 98 | |
| CWT-SNV | 90 | 100 | 100 | 100 | 90 | 96 | |
| CWT-MSC | 90 | 100 | 100 | 100 | 100 | 98 | |
| CWT-SNV | 100 | 100 | 100 | 100 | 90 | 98 | |
| Inner capsule data | Raw | 90 | 100 | 100 | 100 | 100 | 98 |
| DT | 100 | 100 | 100 | 100 | 100 | 100 | |
| de-bias | 100 | 100 | 100 | 100 | 100 | 100 | |
| SNV | 100 | 100 | 100 | 100 | 100 | 100 | |
| MinMax | 100 | 100 | 100 | 100 | 100 | 100 | |
| MSC | 100 | 100 | 100 | 100 | 100 | 100 | |
| 1st | 80 | 100 | 100 | 100 | 90 | 94 | |
| 2nd | 50 | 100 | 90 | 100 | 60 | 80 | |
| CWT | 90 | 100 | 100 | 100 | 90 | 96 | |
| 1st-DT | 80 | 100 | 100 | 100 | 100 | 96 | |
| 1st-SNV | 90 | 100 | 100 | 100 | 90 | 96 | |
| 1st-MSC | 90 | 100 | 100 | 100 | 90 | 96 | |
| CWT-SNV | 90 | 100 | 100 | 100 | 90 | 96 | |
| CWT-MSC | 90 | 100 | 100 | 100 | 90 | 96 | |
| CWT-SNV | 90 | 100 | 100 | 100 | 100 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, X.; Li, S.; Du, G.; Jiang, L.; Liu, X.; Ding, S.; Shan, Y. A Rapid and Nondestructive Approach for the Classification of Different-Age Citri Reticulatae Pericarpium Using Portable Near Infrared Spectroscopy. Sensors 2020, 20, 1586. https://doi.org/10.3390/s20061586
Li P, Zhang X, Li S, Du G, Jiang L, Liu X, Ding S, Shan Y. A Rapid and Nondestructive Approach for the Classification of Different-Age Citri Reticulatae Pericarpium Using Portable Near Infrared Spectroscopy. Sensors. 2020; 20(6):1586. https://doi.org/10.3390/s20061586
Chicago/Turabian StyleLi, Pao, Xinxin Zhang, Shangke Li, Guorong Du, Liwen Jiang, Xia Liu, Shenghua Ding, and Yang Shan. 2020. "A Rapid and Nondestructive Approach for the Classification of Different-Age Citri Reticulatae Pericarpium Using Portable Near Infrared Spectroscopy" Sensors 20, no. 6: 1586. https://doi.org/10.3390/s20061586






