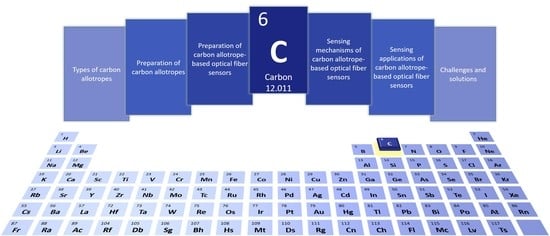
Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review
Abstract
:1. Introduction
2. Classifications of Carbon Allotropes
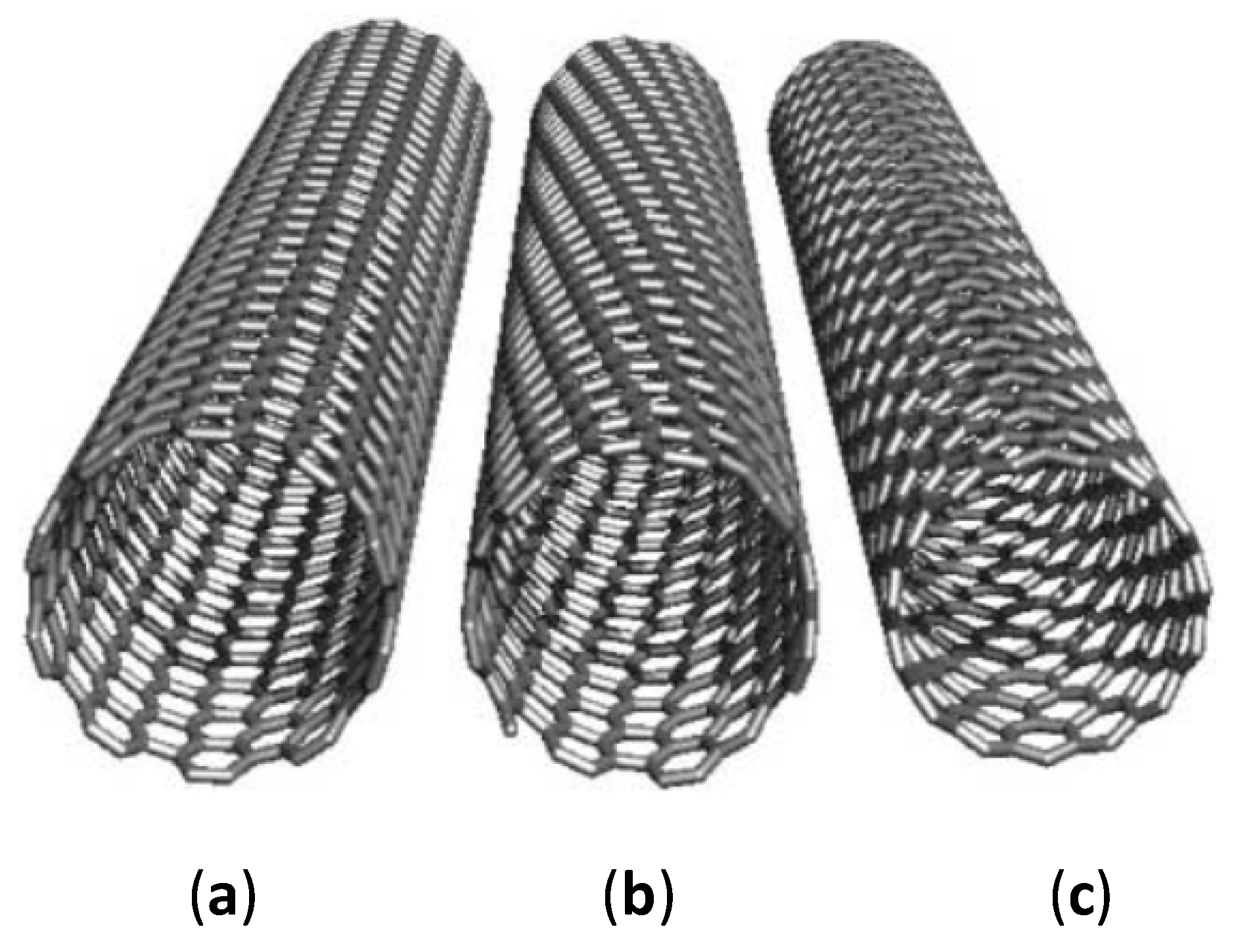
2.1. Carbon Nanotubes
2.2. Carbon Dots
2.3. Graphene
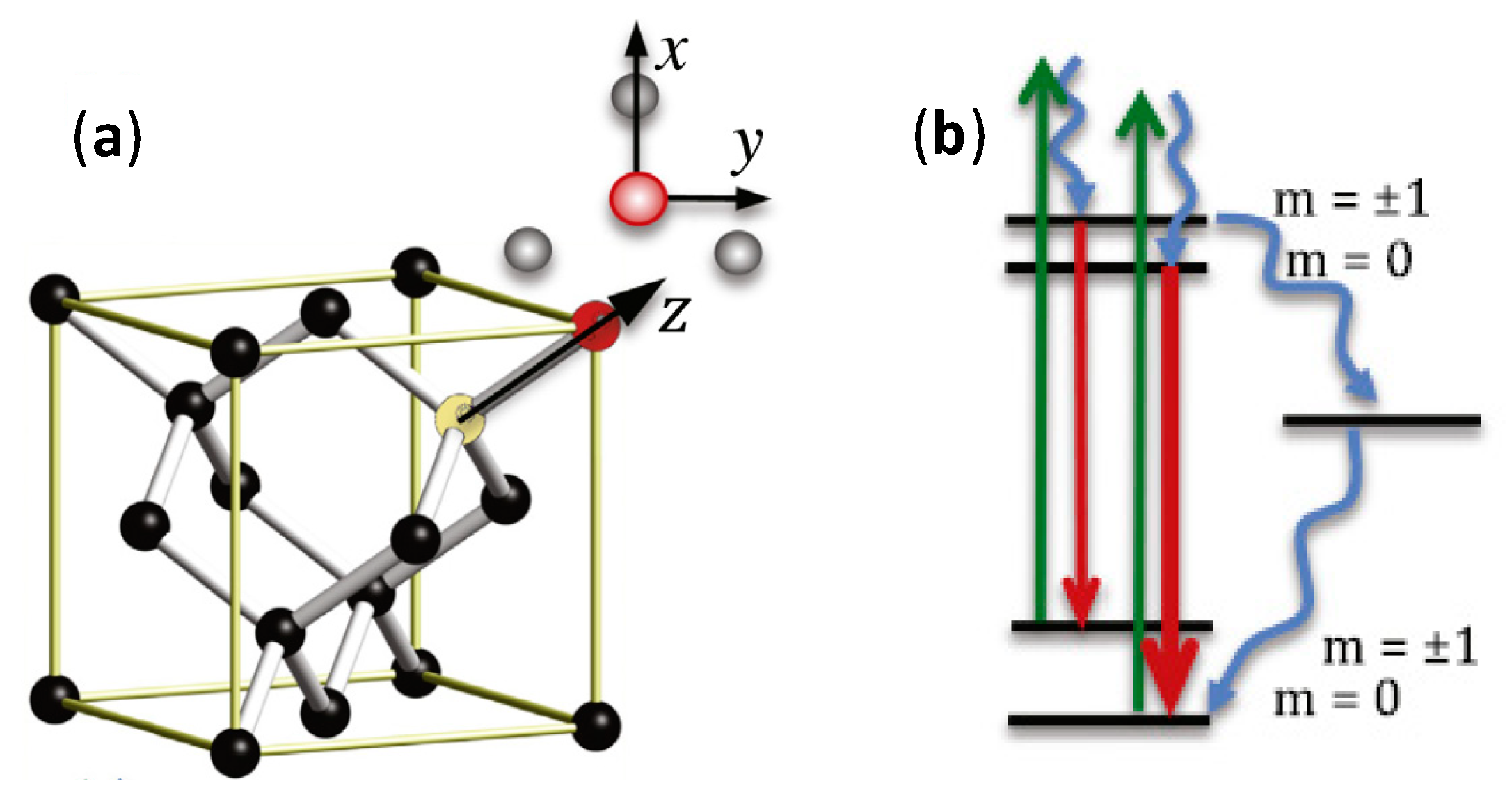
2.4. Nanodiamonds
3. Synthesis Approaches of Carbon Allotropes
3.1. Carbon Nanotubes
3.2. Carbon Dots
3.3. Graphene
3.4. Nanodiamonds
4. Preparation Techniques of Carbon Allotrope-Based Optical Fiber Sensors
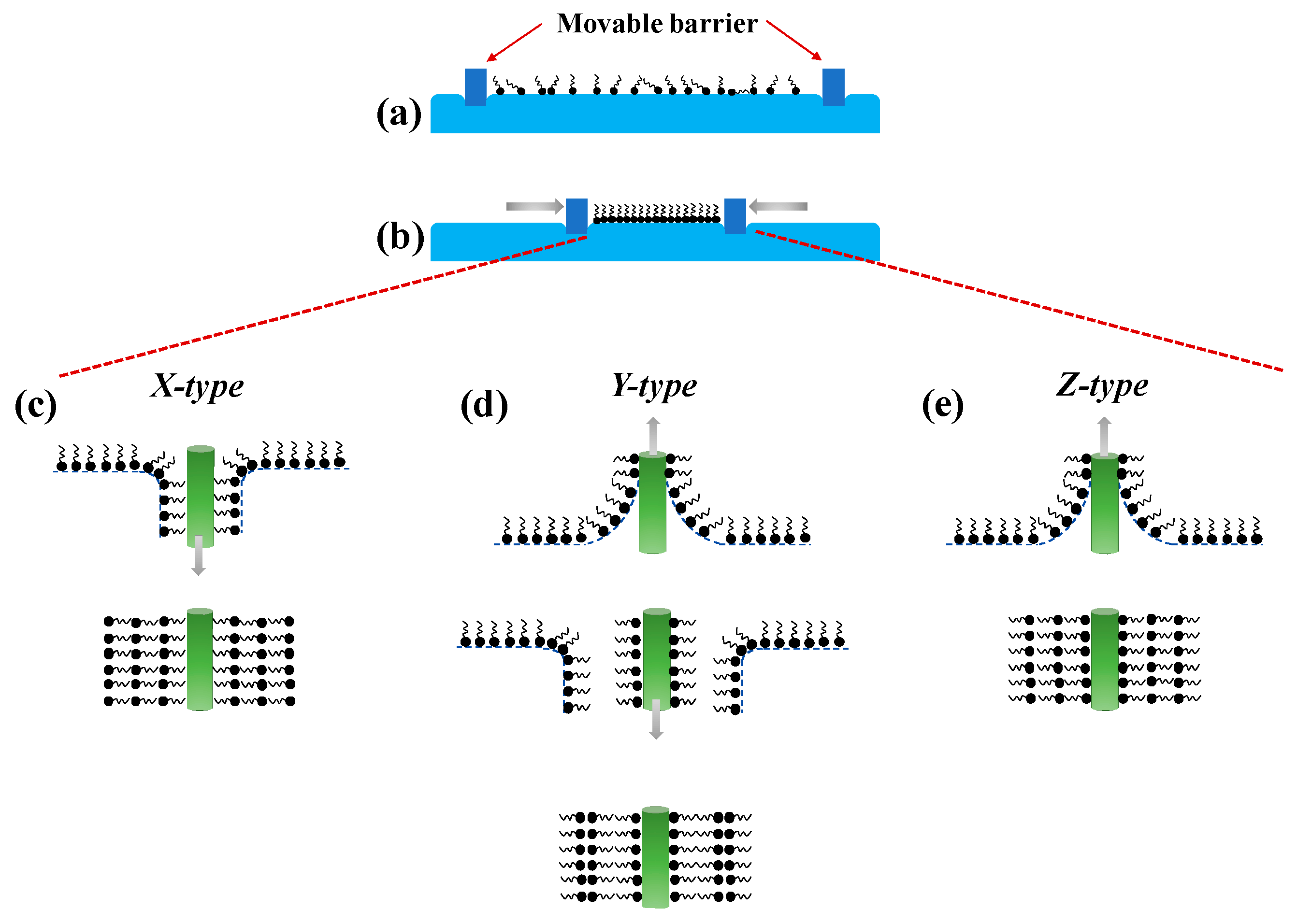
4.1. Langmuir-Blodgett (LB)
4.2. Layer-by-Layer Electrostatic Self-Assembly (LbL-ESA)
4.3. Chemical Vapor Deposition (CVD)
4.4. Optical Deposition
4.5. Crosslinking
4.6. Drop-Casting
5. Sensing Mechanisms of Carbon Allotrope-Based Optical Fiber Sensors
5.1. Thermo-Optic
5.2. Surface Plasmon Resonance
5.3. Fluorescence
5.4. Molecular Adsorption
6. Sensing Applications of Carbon Allotrope-Based Optical Fiber Sensors
6.1. Humidity
6.2. Temperature and Pressure
6.3. Other Physical Parameter Sensing Applications
6.4. Heavy Metal Ions
6.5. Alcohol
6.6. Ammonia and Volatile Organic Compounds
6.7. Other Biomolecule Sensing
7. Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Liao, G.; Jin, S.; Cao, D.; Wei, Q.; Lu, H.; Yu, J.; Cai, X.; Tan, S.; Xiao, Y.; et al. All-fiber-optic temperature sensor based on reduced graphene oxide. Laser Phys. Lett. 2014, 11, 035901. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.-T. Surface plasmon resonance biosensor based on graphene oxide/silver coated polymer cladding silica fiber. Sens. Actuators B Chem. 2018, 275, 332–338. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K.-T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications. Nano Micro Lett. 2018, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Chien, Y.-H.; Chan, K.K.; Yap, S.H.K.; Yong, K.-T. NIR-responsive nanomaterials and their applications; upconversion nanoparticles and carbon dots: A perspective. J. Chem. Technol. Biotechnol. 2018, 93, 1519–1528. [Google Scholar] [CrossRef]
- Grattan, K.T.V. Optical Fiber Sensor Technology Chemical and Environmental Sensing; Springer: Dordrecht, The Netherlands, 1999; pp. 15–112. [Google Scholar]
- Wu, Y.; Yao, B.; Yu, C.; Rao, Y. Optical Graphene Gas Sensors Based on Microfibers: A Review. Sensors 2018, 18, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, N.; Wang, S.; Jiang, L.; Jiang, Y.; Wang, P.; Zhang, L. Pressure and Temperature Sensor Based on Graphene Diaphragm and Fiber Bragg Gratings. IEEE Photonics Technol. Lett. 2018, 30, 431–434. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.-g.; Zhou, X.; Zhang, Y.-n. Review on the graphene based optical fiber chemical and biological sensors. Sens. Actuators B Chem. 2016, 231, 324–340. [Google Scholar] [CrossRef]
- Consales, M.; Cutolo, A.; Penza, M.; Aversa, P.; Giordano, M.; Cusano, A. Fiber optic chemical nanosensors based on engineered single-walled carbon nanotubes. J. Sens. 2008, 2008, 936074. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Niyogi, S.; Hamon, M.A.; Hu, H.; Zhao, B.; Bhowmik, P.; Sen, R.; Itkis, M.E.; Haddon, R.C. Chemistry of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1105–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahr, J.Y.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mota, J.P.; Rostam-Abadi, M.; Rood, M.J. Structural characterization of single-walled carbon nanotube bundles by experiment and molecular simulation. Langmuir 2005, 21, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhuang, R.C.; Zhang, J.; Liu, J.W.; Mäder, E. Glass fibers with carbon nanotube networks as multifunctional sensors. Adv. Funct. Mater. 2010, 20, 1885–1893. [Google Scholar] [CrossRef]
- Lee, R.S.; Kim, H.J.; Fischer, J.; Thess, A.; Smalley, R.E. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 1997, 388, 255–257. [Google Scholar] [CrossRef]
- Claye, A.S.; Fischer, J.E.; Huffman, C.B.; Rinzler, A.G.; Smalley, R.E. Solid-state electrochemistry of the Li single wall carbon nanotube system. J. Electrochem. Soc. 2000, 147, 2845–2852. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Kamaras, K.; Itkis, M.E.; Hu, H.; Zhao, B.; Haddon, R.C. Covalent Bond Formation to a Carbon Nanotube Metal. Science 2003, 301, 1501. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, Y.; Li, S.; Cui, H. Amino acids as the source for producing carbon nanodots: Microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem. Commun. 2012, 48, 9634–9636. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Chang, H.-T. Synthesis of high-quality carbon nanodots from hydrophilic compounds: Role of functional groups. Chem. Commun. 2012, 48, 3984–3986. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Castillo, A.; Ariza-Avidad, M.; Pritz, C.; Camprubí-Robles, M.; Fernández, B.; Ruedas-Rama, M.J.; Megia-Fernández, A.; Lapresta-Fernández, A.; Santoyo-Gonzalez, F.; Schrott-Fischer, A. Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources. Chem. Commun. 2013, 49, 1103–1105. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.-H.; Wu, G.-H.; Zhang, J.-R.; Zhu, J.-J. Hair fiber as a precursor for synthesizing of sulfur-and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe (III) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Shan, X.; Chai, L.; Ma, J.; Qian, Z.; Chen, J.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Li, X.; Wei, Z.; Du, X.; Wang, X. Boronic acid functionalized N-doped carbon quantum dots as fluorescent probe for selective and sensitive glucose determination. Mater. Res. Express 2014, 1, 025708. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv. Mater. 2015, 27, 7782–7787. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be 2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chan, K.K.; Anderson, T.; Kong, K.V.; Ng, B.K.; Yong, K.T. Advanced Near-Infrared Light-Responsive Nanomaterials as Therapeutic Platforms for Cancer Therapy. Adv. Ther. 2019, 2, 1800090. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; William, W.Y. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-dependent fluorescence in carbon dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-colour carbon dots: From energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Chan, K.K.; Xu, G.; Yin, M.; Lin, G.; Wang, X.; Lin, W.-J.; Birowosuto, M.D.; Zeng, S.; Ogi, T. Biodegradable Polymer-Coated Multifunctional Graphene Quantum Dots for Light-Triggered Synergetic Therapy of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2018, 11, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Barrow, C.J.; Yang, W. Molecularly engineered graphene surfaces for sensing applications: A review. Anal. Chim. Acta 2015, 859, 1–19. [Google Scholar] [CrossRef]
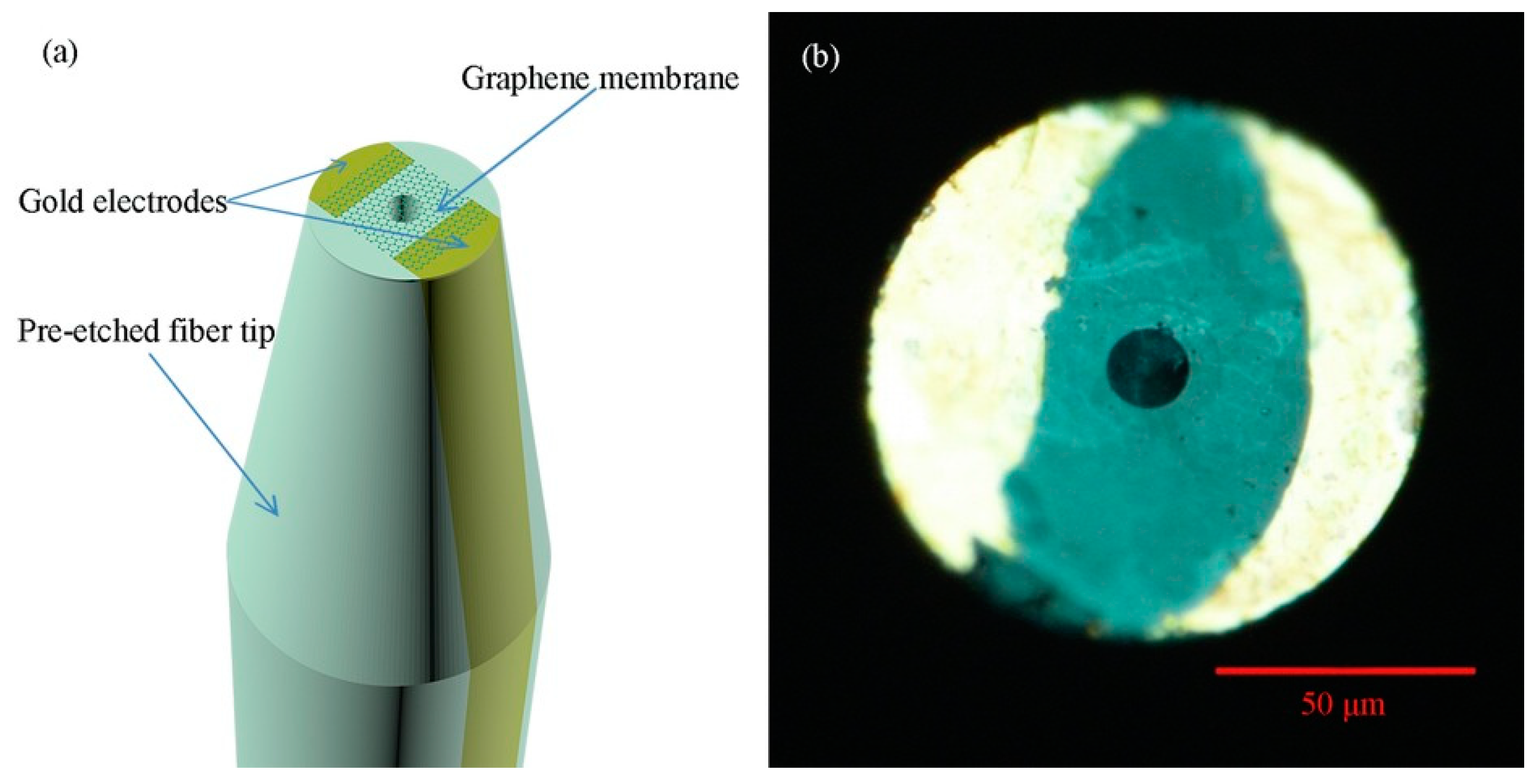
- Huang, Y.; Dong, X.; Shi, Y.; Li, C.M.; Li, L.-J.; Chen, P. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale 2010, 2, 1485–1488. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Choi, D.S.; Kim, Y.N.; Kim, H.; Yoon, D.H.; Ahn, S.-S.; Yang, J.-W.; Yang, W.S.; Seo, S. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can graphene be used as a substrate for Raman enhancement? Nano Lett. 2009, 10, 553–561. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhou, H.; Yang, H.; Chen, M.; Guo, Y.; Sun, L. Investigation of n-layer graphenes as substrates for Raman enhancement of crystal violet. J. Phys. Chem. C 2011, 115, 10019–10025. [Google Scholar] [CrossRef]
- Ju, L.; Geng, B.; Horng, J.; Girit, C.; Martin, M.; Hao, Z.; Bechtel, H.A.; Liang, X.; Zettl, A.; Shen, Y.R. Graphene plasmonics for tunable terahertz metamaterials. Nat. Nanotechnol. 2011, 6, 630. [Google Scholar] [CrossRef]
- Fei, Z.; Andreev, G.O.; Bao, W.; Zhang, L.M.; McLeod, A.S.; Wang, C.; Stewart, M.K.; Zhao, Z.; Dominguez, G.; Thiemens, M. Infrared nanoscopy of Dirac plasmons at the graphene–SiO2 interface. Nano Lett. 2011, 11, 4701–4705. [Google Scholar] [CrossRef] [Green Version]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.; Silveiro, I.n.; García de Abajo, F.J. Molecular sensing with tunable graphene plasmons. ACS Photonics 2015, 2, 876–882. [Google Scholar] [CrossRef]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, T.; Gurzadyan, G.G. The origin of fluorescence from graphene oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef] [Green Version]
- Hernaez, M.; Zamarreño, C.; Melendi-Espina, S.; Bird, L.; Mayes, A.; Arregui, F. Optical fibre sensors using graphene-based materials: A review. Sensors 2017, 17, 155. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Y.; Ding, L.; Duan, J.; Ebendorff-Heidepriem, H.; Monro, T.M. Integration of conductive reduced graphene oxide into microstructured optical fibres for optoelectronics applications. Sci. Rep. 2016, 6, 21682. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B: Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Shenderova, O.; Zhirnov, V.; Brenner, D. Carbon nanostructures. Crit. Rev. Solid State Mater. Sci. 2002, 27, 227–356. [Google Scholar] [CrossRef]
- Greiner, N.R.; Phillips, D.; Johnson, J.; Volk, F. Diamonds in detonation soot. Nature 1988, 333, 440–442. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent incorporation of aminated nanodiamond into an epoxy polymer network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ozawa, M.; Krueger, A. A general procedure to functionalize agglomerating nanoparticles demonstrated on nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, A.; Gour, S.; Mochalin, V.; Knoke, I.; Gogotsi, Y. Deaggregation of nanodiamond powders using salt-and sugar-assisted milling. ACS Appl. Mater. Interfaces 2010, 2, 3289–3294. [Google Scholar] [CrossRef]
- Morita, Y.; Takimoto, T.; Yamanaka, H.; Kumekawa, K.; Morino, S.; Aonuma, S.; Kimura, T.; Komatsu, N. A Facile and Scalable Process for Size-Controllable Separation of Nanodiamond Particles as Small as 4 nm. Small 2008, 4, 2154–2157. [Google Scholar] [CrossRef]
- Plakhotnik, T. Diamonds for quantum nano sensing. Curr. Opin. Solid State Mater. Sci. 2017, 21, 25–34. [Google Scholar] [CrossRef]
- Plakhotnik, T.; Aman, H.; Chang, H.-C. All-optical single-nanoparticle ratiometric thermometry with a noise floor of 0.3 K Hz− 1/2. Nanotechnology 2015, 26, 245501. [Google Scholar] [CrossRef]
- Plakhotnik, T.; Doherty, M.W.; Cole, J.H.; Chapman, R.; Manson, N.B. All-optical thermometry and thermal properties of the optically detected spin resonances of the NV–center in nanodiamond. Nano Lett. 2014, 14, 4989–4996. [Google Scholar] [CrossRef] [Green Version]
- Aman, H.; Plakhotnik, T. Accuracy in the measurement of magnetic fields using nitrogen-vacancy centers in nanodiamonds. Josa B 2016, 33, B19–B27. [Google Scholar] [CrossRef]
- Ando, Y.; Iijima, S. Preparation of carbon nanotubes by arc-discharge evaporation. Jpn. J. Appl. Phys. Part 2 Lett. 1993, 32, L107–L109. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T. A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 2009. [Google Scholar] [CrossRef]
- Mubarak, N.; Abdullah, E.; Jayakumar, N.; Sahu, J. An overview on methods for the production of carbon nanotubes. J. Ind. Eng. Chem. 2014, 20, 1186–1197. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Production and purification of carbon nanotubes. ChemInform 1997, 28, 139–162. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Gonçalves, H.; Jorge, P.A.; Fernandes, J.; da Silva, J.C.E. Hg (II) sensing based on functionalized carbon dots obtained by direct laser ablation. Sens. Actuators B Chem. 2010, 145, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2010, 47, 932–934. [Google Scholar] [CrossRef]
- Chang, M.M.F.; Ginjom, I.R.; Ngu-Schwemlein, M.; Ng, S.M. Synthesis of yellow fluorescent carbon dots and their application to the determination of chromium (III) with selectivity improved by pH tuning. Microchim. Acta 2016, 183, 1899–1907. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.-S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782. [Google Scholar] [CrossRef]
- Dutta, P.; Ghosh, T.; Kumar, H.; Jain, T.; Singh, Y. Hydrothermal and solvothermal synthesis of carbon dots from chitosan-ethanol system. Asian Chitin J 2015, 11, 1–4. [Google Scholar]
- Li, M.; Yu, C.; Hu, C.; Yang, W.; Zhao, C.; Wang, S.; Zhang, M.; Zhao, J.; Wang, X.; Qiu, J. Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield. Chem. Eng. J. 2017, 320, 570–575. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K. Solid State Carbon Dots-Based Sensor Using Optical Microfiber for Ferric Ion Detection. In Proceedings of the 2019 IEEE International Conference on Sensors and Nanotechnology, Penang, Malaysia, 24–25 July 2019; pp. 1–4. [Google Scholar]
- Chan, K.K.; Yang, C.; Chien, Y.-H.; Panwar, N.; Yong, K.-T. A Facile Synthesis of Label-Free Carbon Dots with Unique Selectivity-Tunable Characteristics for Ferric Ion Detection and Cellular Imaging Application. New J. Chem. Commun. 2019, 43, 4734–4744. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodie, B. Note sur un nouveau procédé pour la purification et la désagrégation du graphite. Ann. Chim. Phys. 1855, 45, 351–353. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Der Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Jang, J.W.; Cho, S.; Moon, G.h.; Ihm, K.; Kim, J.Y.; Youn, D.H.; Lee, S.; Lee, Y.H.; Choi, W.; Lee, K.H. Photocatalytic Synthesis of Pure and Water-Dispersible Graphene Monosheets. Chem. A Eur. J. 2012, 18, 2762–2767. [Google Scholar] [CrossRef]
- Sutter, P.W.; Flege, J.-I.; Sutter, E.A. Epitaxial graphene on ruthenium. Nat. Mater. 2008, 7, 406–411. [Google Scholar] [CrossRef]
- Dedkov, Y.S.; Fonin, M.; Rüdiger, U.; Laubschat, C. Rashba effect in the graphene/Ni (111) system. Phys. Rev. Lett. 2008, 100, 107602. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, W.; Chen, S.; Wee, A.T.S. Bottom-up growth of epitaxial graphene on 6H-SiC (0001). ACS Nano 2008, 2, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- First, P.N.; de Heer, W.A.; Seyller, T.; Berger, C.; Stroscio, J.A.; Moon, J.-S. Epitaxial graphenes on silicon carbide. Mrs Bull. 2010, 35, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Zangwill, A.; Vvedensky, D.D. Novel growth mechanism of epitaxial graphene on metals. Nano Lett. 2011, 11, 2092–2095. [Google Scholar] [CrossRef] [Green Version]
- Dolmatov, V.Y. Detonation synthesis ultradispersed diamonds: Properties and applications. Russ. Chem. Rev. 2001, 70, 607–626. [Google Scholar] [CrossRef]
- Shenderova, O.A.; Gruen, D.M. Ultrananocrystalline Diamond: Synthesis, Properties and Applications; William Andrew: Norwich, NY, USA, 2012; pp. 79–114. [Google Scholar]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Gruen, D.M.; Shenderova, O.A.; Vul, A.Y. Synthesis, Properties and Applications of Ultrananocrystalline Diamond. In Proceedings of the NATO ARW on Synthesis, Properties and Applications of Ultrananocrystalline Diamond, St. Petersburg, Russia, 7–10 June 2004; Springer Science & Business Media: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Krüger, A.; Kataoka, F.; Ozawa, M.a.a.; Fujino, T.; Suzuki, Y.; Aleksenskii, A.E.; Vul, A.Y.; Ōsawa, E. Unusually tight aggregation in detonation nanodiamond: Identification and disintegration. Carbon 2005, 43, 1722–1730. [Google Scholar] [CrossRef]
- Ozawa, M.; Inaguma, M.; Takahashi, M.; Kataoka, F.; Krueger, A.; Ōsawa, E. Preparation and behavior of brownish, clear nanodiamond colloids. Adv. Mater. 2007, 19, 1201–1206. [Google Scholar] [CrossRef]
- Amans, D.; Chenus, A.-C.; Ledoux, G.; Dujardin, C.; Reynaud, C.; Sublemontier, O.; Masenelli-Varlot, K.; Guillois, O. Nanodiamond synthesis by pulsed laser ablation in liquids. Diam. Relat. Mater. 2009, 18, 177–180. [Google Scholar] [CrossRef]
- Bai, P.; Hu, S.; Zhang, T.; Sun, J.; Cao, S. Effect of laser pulse parameters on the size and fluorescence of nanodiamonds formed upon pulsed-laser irradiation. Mater. Res. Bull. 2010, 45, 826–829. [Google Scholar] [CrossRef]
- Kumar, A.; Lin, P.A.; Xue, A.; Hao, B.; Yap, Y.K.; Sankaran, R.M. Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour. Nat. Commun. 2013, 4, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petty, M.C. Langmuir-Blodgett films an introduction; Cambridge University Press: Cambridge, UK, 1996; pp. 39–41. [Google Scholar]
- Rees, N.D.; James, S.W.; Tatam, R.P.; Ashwell, G.J. Optical fiber long-period gratings with Langmuir–Blodgett thin-film overlays. Opt. Lett. 2002, 27, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Peterson, I.R. Langmuir-Blodgett films. J. Phys. D Appl. Phys. 1990, 23, 379–395. [Google Scholar] [CrossRef]
- Smietana, M.; Bock, W.J.; Szmidt, J.; Pickrell, G.R. Nanocoating Enhanced Optical Fiber Sensors. In Advances in Materials Science for Environmental and Nuclear Technology; The American Ceramic Society: Pittsburgh, PA, USA, 2010; pp. 275–286. [Google Scholar]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Paul, P.K.; Hansda, C.; Hussain, S.A. Layer-by-Layer Electrostatic Self-assembly Method: A Facile Approach of Preparing Nanoscale Molecular Thin Films. Invertis J. Sci. Technol. 2014, 7, 104–113. [Google Scholar]
- Gowri, A.; Sai, V.V.R. Development of LSPR based U-bent plastic optical fiber sensors. Sens. Actuators B Chem. 2016, 230, 536–543. [Google Scholar] [CrossRef]
- Lindgren, E.B.; Derbenev, I.N.; Khachatourian, A.; Chan, H.-K.; Stace, A.J.; Besley, E. Electrostatic Self-Assembly: Understanding the Significance of the Solvent. J. Chem. Theory Comput. 2018, 14, 905–915. [Google Scholar] [CrossRef]
- Verveniotis, E.; Kromka, A.; Ledinský, M.; Čermák, J.; Rezek, B. Guided assembly of nanoparticles on electrostatically charged nanocrystalline diamond thin films. Nanoscale Res. Lett. 2011, 6, 144. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, H.M.R.; Duarte, A.J.; Davis, F.; Higson, S.P.J.; Esteves da Silva, J.C.G. Layer-by-layer immobilization of carbon dots fluorescent nanomaterials on single optical fiber. Anal. Chim. Acta 2012, 735, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberto, N.; Vigário, C.; Duarte, D.; Almeida, N.A.F.; Gonçalves, G.; Pinto, J.L.; Marques, P.A.A.P.; Nogueira, R.; Neto, V. Characterization of Graphene Oxide Coatings onto Optical Fibers for Sensing Applications. Mater. Today Proc. 2015, 2, 171–177. [Google Scholar] [CrossRef]
- Hernaez, M.; Mayes, A.G.; Melendi-Espina, S. Graphene Oxide in Lossy Mode Resonance-Based Optical Fiber Sensors for Ethanol Detection. Sensors 2018, 18, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2010; pp. 314–363. [Google Scholar]
- Yao, B.; Wu, Y.; Wang, Z.; Cheng, Y.; Rao, Y.; Gong, Y.; Chen, Y.; Li, Y. Demonstration of complex refractive index of graphene waveguide by microfiber-based Mach–Zehnder interferometer. Opt. Express 2013, 21, 29818–29826. [Google Scholar] [CrossRef]
- Jeschkowski, U.; Niederwald, H.; Möhl, W.; Beier, W.; Disam, J.; Gohlke, D.; Lübbers, K. Coating Technologies. In Thin Films on Glass; Bach, H., Krause, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 51–98. [Google Scholar]
- Kashiwagi, K.; Yamashita, S.; Set, S.Y. In-situ monitoring of optical deposition of carbon nanotubes onto fiber end. Opt. Express 2009, 17, 5711–5715. [Google Scholar] [CrossRef]
- Malagnino, N.; Pesce, G.; Sasso, A.; Arimondo, E. Measurements of trapping efficiency and stiffness in optical tweezers. Opt. Commun. 2002, 214, 15–24. [Google Scholar] [CrossRef]
- Jonáš, A.; Zemánek, P. Light at work: The use of optical forces for particle manipulation, sorting, and analysis. Electrophoresis 2008, 29, 4813–4851. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Yamashita, S. Deposition of carbon nanotubes around microfiber via evanascent light. Opt. Express 2009, 17, 18364–18370. [Google Scholar] [CrossRef]
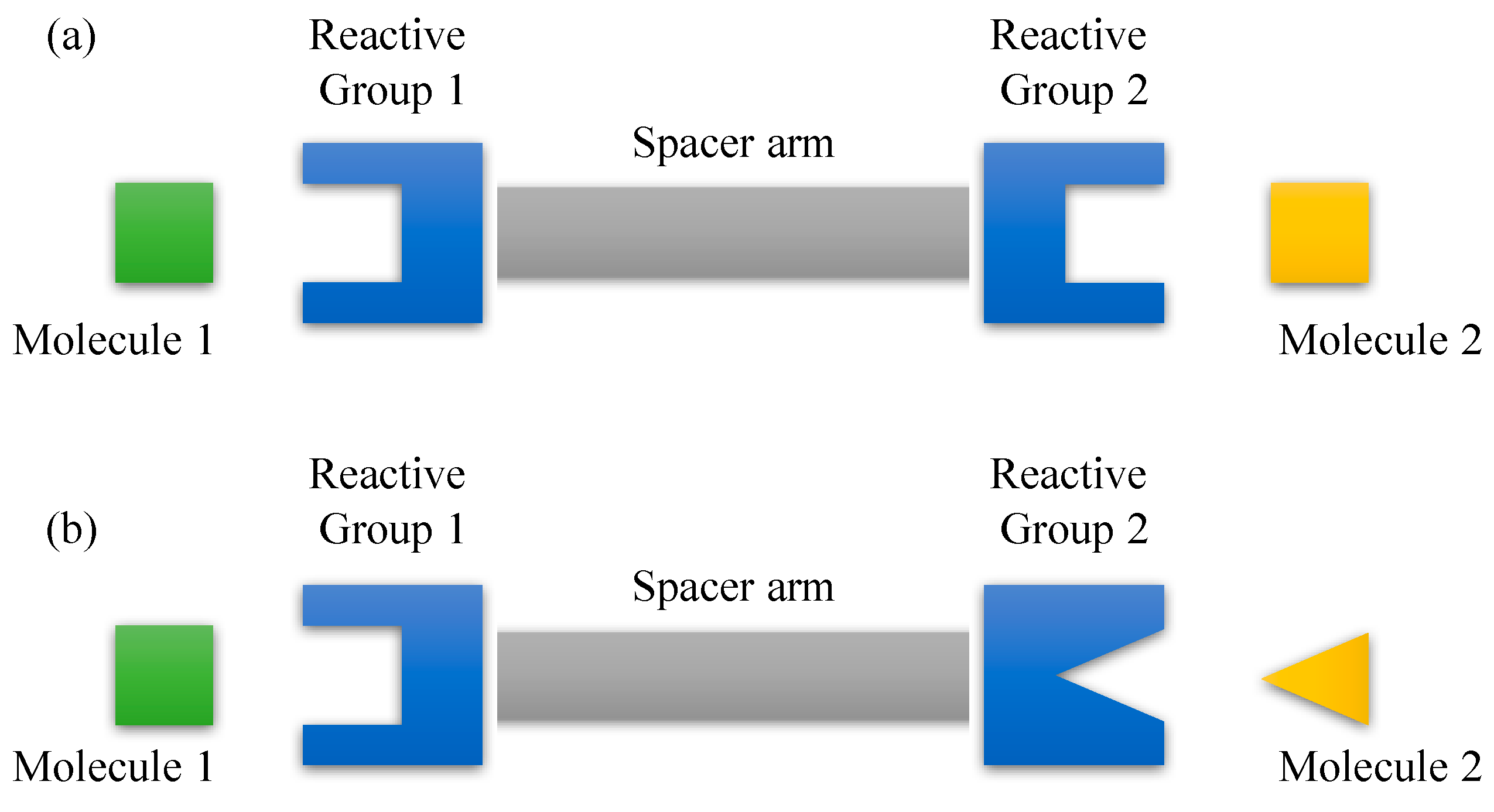
- Hermanson, G.T. Chapter 3 - The Reactions of Bioconjugation. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 229–258. [Google Scholar]
- Hartman, F.C.; Wold, F. Bifunctional Reagents. Cross-Linking of Pancreatic Ribonuclease with a Diimido Ester1. J. Am. Chem. Soc. 1966, 88, 3890–3891. [Google Scholar] [CrossRef]
- Avrameas, S. Coupling of enzymes to proteins with glutaraldehyde: Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 1969, 6, 43–52. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 4 - Zero-Length Crosslinkers. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 259–273. [Google Scholar]
- Yap, S.H.K.; Chan, K.K.; Chien, Y.; Yong, K. Factors Influencing Metal Binding Efficiency at Solid/Liquid Interface: An Investigation for the Prediction of Heavy Metal Ion Sensing Performance. In Proceedings of the 2019 IEEE International Conference on Sensors and Nanotechnology, Penang, Malaysia, 24–25 July 2019. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chan, K.K.; Zhang, G.; Tjin, S.C.; Yong, K.-T. Carbon Dot-functionalized Interferometric Optical Fiber Sensor for Detection of Ferric Ions in Biological Samples. ACS Appl. Mater. Interfaces 2019, 11, 28546–28553. [Google Scholar] [CrossRef] [PubMed]
- Shabaneh, A.A.; Girei, S.H.; Arasu, P.T.; Rashid, S.A.; Yunusa, Z.; Mahdi, M.A.; Paiman, S.; Ahmad, M.Z.; Yaacob, M.H. Reflectance Response of Optical Fiber Coated With Carbon Nanotubes for Aqueous Ethanol Sensing. IEEE Photonics J. 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cai, X.; Tan, S.; Yu, J.; Lu, H.; Luo, Y.; Liao, G.; Li, S.; Tang, J.; et al. Reduced graphene oxide for fiber-optic humidity sensing. Opt. Express 2014, 22, 31555–31567. [Google Scholar] [CrossRef] [PubMed]
- Azzuhri, S.R.A.; Amiri, I.S.; Zulkhairi, A.S.; Salim, M.A.M.; Razak, M.Z.A.; Khyasudeen, M.F.; Ahmad, H.; Zakaria, R.; Yupapin, P. Application of graphene oxide based Microfiber-Knot resonator for relative humidity sensing. Results Phys. 2018, 9, 1572–1577. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chan, C.L.; Gau, C.; Liu, C.; Shiau, S.H.; Ting, J. Nano Temperature Sensor Using Selective Lateral Growth of Carbon Nanotube Between Electrodes. IEEE Trans. Nanotechnol. 2007, 6, 63–69. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.L.; Byun, K.M. Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt. Express 2011, 19, 458–466. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, B.D. On the sensitivity and signal to noise ratio of a step-index fiber optic surface plasmon resonance sensor with bimetallic layers. Opt. Commun. 2005, 245, 159–169. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.-Q.; Meng, X.-M.; Coquet, P.; Yong, K.-T. Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Prabowo, B.; Purwidyantri, A.; Liu, K.-C. Surface plasmon resonance optical sensor: A review on light source technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Okamoto, A. Hybridization-Sensitive On–Off DNA Probe: Application of the Exciton Coupling Effect to Effective Fluorescence Quenching. Chem. Asian J. 2008, 3, 958–968. [Google Scholar] [CrossRef]
- Ji, W.B.; Tan, Y.C.; Lin, B.; Tjin, S.C.; Chow, K.K. Nonadiabatically Tapered Microfiber Sensor With Ultrashort Waist. IEEE Photonics Technol. Lett. 2014, 26, 2303–2306. [Google Scholar] [CrossRef]
- Ji, W.B.; Liu, H.H.; Tjin, S.C.; Chow, K.K.; Lim, A. Ultrahigh Sensitivity Refractive Index Sensor Based on Optical Microfiber. IEEE Photonics Technol. Lett. 2012, 24, 1872–1874. [Google Scholar] [CrossRef]
- An, G.; Li, S.; Cheng, T.; Yan, X.; Zhang, X.; Zhou, X.; Yuan, Z. Ultra-stable D-shaped Optical Fiber Refractive Index Sensor with Graphene-Gold Deposited Platform. Plasmonics 2019, 14, 155–163. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, M.; Ding, J.; Wu, J.; Zhu, Y.; Li, H.; Wang, Q.; Yang, C. A high sensitivity D-type surface plasmon resonance optical fiber refractive index sensor with graphene coated silver nano-columns. Opt. Fiber Technol. 2019, 48, 34–39. [Google Scholar] [CrossRef]
- Sridevi, S.; Vasu, K.S.; Jayaraman, N.; Asokan, S.; Sood, A.K. Optical bio-sensing devices based on etched fiber Bragg gratings coated with carbon nanotubes and graphene oxide along with a specific dendrimer. Sens. Actuators B Chem. 2014, 195, 150–155. [Google Scholar] [CrossRef]
- Shivananju, B.N.; Yamdagni, S.; Fazuldeen, R.; Kumar, A.K.S.; Nithin, S.P.; Varma, M.M.; Asokan, S. Highly Sensitive Carbon Nanotubes Coated Etched Fiber Bragg Grating Sensor for Humidity Sensing. IEEE Sens. J. 2014, 14, 2615–2619. [Google Scholar] [CrossRef]
- Mohamed, H.; Irawati, N.; Ahmad, F.; Ibrahim, M.H.; Ambran, S.; Rahman, M.A.A.; Harun, S.W. Optical humidity sensor based on tapered fiber with multi-walled carbon nanotubes slurry. Indones. J. Electr. Eng. Comput. Sci. 2017, 6, 97–103. [Google Scholar] [CrossRef]
- Isa, N.M.; Irawati, N.; Harun, S.W.; Ahmad, F.; Rahman, H.A.; Yusoff, M.H.M. Multi-walled carbon nanotubes doped Poly(Methyl MethAcrylate) microfiber for relative humidity sensing. Sens. Actuators A Phys. 2018, 272, 274–280. [Google Scholar] [CrossRef]
- Ma, Q.F.; Tou, Z.Q.; Ni, K.; Lim, Y.Y.; Lin, Y.F.; Wang, Y.R.; Zhou, M.H.; Shi, F.F.; Niu, L.; Dong, X.Y.; et al. Carbon-nanotube/Polyvinyl alcohol coated thin core fiber sensor for humidity measurement. Sens. Actuators B Chem. 2018, 257, 800–806. [Google Scholar] [CrossRef]
- Gao, R.; Lu, D.-f.; Cheng, J.; Jiang, Y.; Jiang, L.; Qi, Z.-m. Humidity sensor based on power leakage at resonance wavelengths of a hollow core fiber coated with reduced graphene oxide. Sens. Actuators B Chem. 2016, 222, 618–624. [Google Scholar] [CrossRef]
- Xing, Z.; Zheng, Y.; Yan, Z.; Feng, Y.; Xiao, Y.; Yu, J.; Guan, H.; Luo, Y.; Wang, Z.; Zhong, Y.; et al. High-sensitivity humidity sensing of microfiber coated with three-dimensional graphene network. Sens. Actuators B Chem. 2019, 281, 953–959. [Google Scholar] [CrossRef]
- Ahmad, H.; Rahman, M.T.; Sakeh, S.N.A.; Razak, M.Z.A.; Zulkifli, M.Z. Humidity sensor based on microfiber resonator with reduced graphene oxide. Optik 2016, 127, 3158–3161. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, W.; Li, Z.; Chen, G.; Chen, L.; Zhou, J.; Lin, H.; Guan, J.; Fang, W.; Liu, X.; et al. High-performance fibre-optic humidity sensor based on a side-polished fibre wavelength selectively coupled with graphene oxide film. Sens. Actuators B Chem. 2018, 255, 57–69. [Google Scholar] [CrossRef]
- Chu, R.; Guan, C.; Bo, Y.; Shi, J.; Zhu, Z.; Li, P.; Yang, J.; Yuan, L. All-optical graphene-oxide humidity sensor based on a side-polished symmetrical twin-core fiber Michelson interferometer. Sens. Actuators B Chem. 2019, 284, 623–627. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Lou, W.; Shentu, F. Polarization-dependent humidity sensor based on an in-fiber Mach-Zehnder interferometer coated with graphene oxide. Sens. Actuators B Chem. 2016, 234, 503–509. [Google Scholar] [CrossRef]
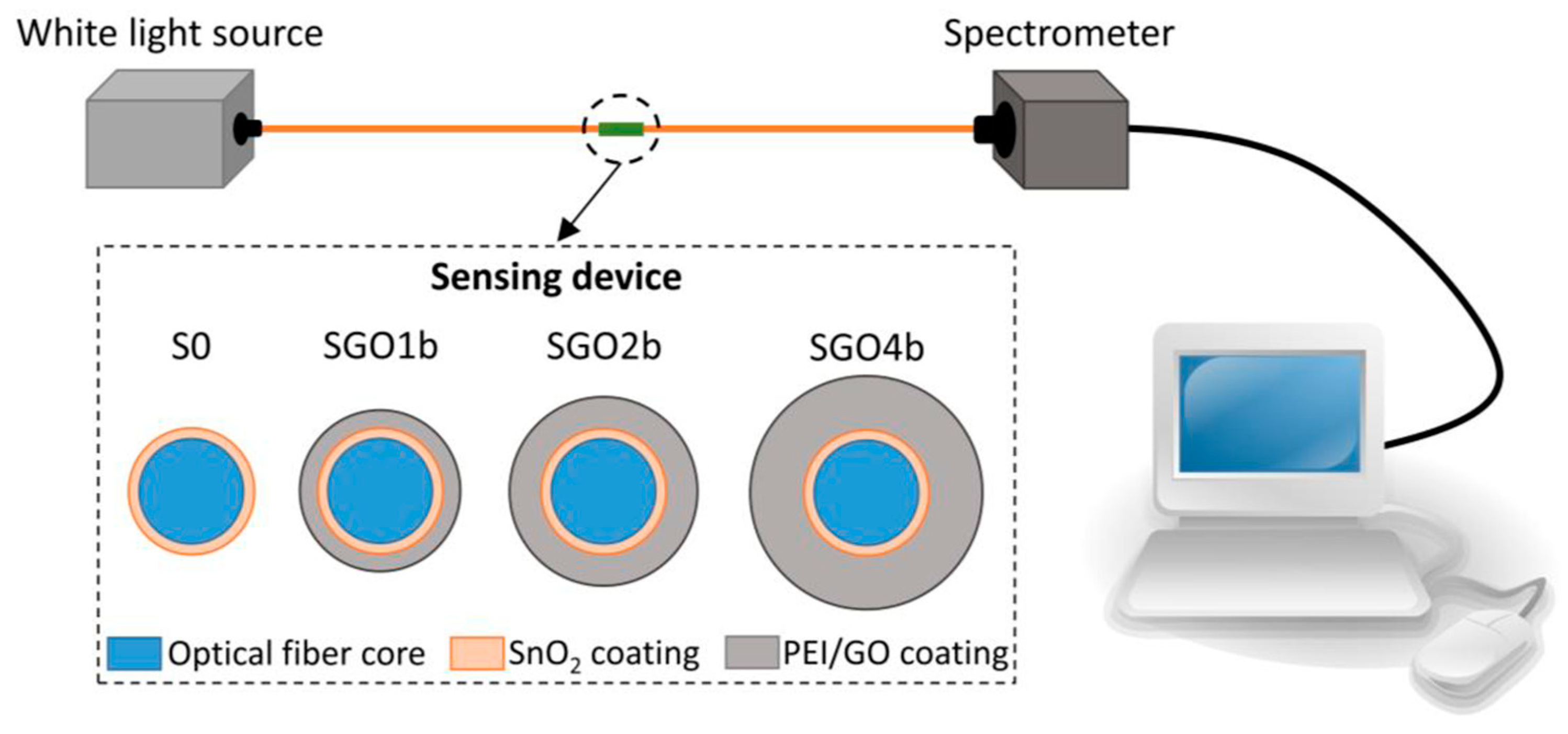
- Hernaez, M.; Acevedo, B.; Mayes, A.G.; Melendi-Espina, S. High-performance optical fiber humidity sensor based on lossy mode resonance using a nanostructured polyethylenimine and graphene oxide coating. Sens. Actuators B Chem. 2019, 286, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, C.; Lou, W.; Shentu, F.; Zhong, C.; Dong, X.; Tong, L. Fiber optic relative humidity sensor based on the tilted fiber Bragg grating coated with graphene oxide. Appl. Phys. Lett. 2016, 109, 031107. [Google Scholar] [CrossRef]
- Jiang, B.; Bi, Z.; Hao, Z.; Yuan, Q.; Feng, D.; Zhou, K.; Zhang, L.; Gan, X.; Zhao, J. Graphene oxide-deposited tilted fiber grating for ultrafast humidity sensing and human breath monitoring. Sens. Actuators B Chem. 2019, 293, 336–341. [Google Scholar] [CrossRef]
- Liu, S.; Meng, H.; Deng, S.; Wei, Z.; Wang, F.; Tan, C. Fiber Humidity Sensor Based on a Graphene-Coated Core-Offset Mach–Zehnder Interferometer. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Mohamed, H.; Hussin, N.; Ahmad, F.; Ambran, S.; Harun, S.W. Optical based relative humidity sensor using tapered optical fiber coated with graphene oxide. In Proceedings of the AIP Conference, Melville, NY, USA, 15–17 August 2016; p. 050006. [Google Scholar] [CrossRef]
- Weimin, L.; Youqing, W.; Changyu, S.; Fengying, S. An optical fiber humidity sensor based on Mach-Zehnder interferometer coated with a composite film of graphene oxide and polyvinyl alcohol. In Proceedings of the 15th International Conference on Optical Communications and Networks (ICOCN), Hangzhou, China, 24–27 September 2016. [Google Scholar]
- Falkovsky, L.A. Optical properties of graphene. In Proceedings of the the International Conference on Theoretical Physics ‘DUBNA-NANO2008’, Moscow, Russia, 7–11 July 2008; IOP Publishing: Bristol, UK; p. 012004. [Google Scholar] [CrossRef]
- Vasu, K.S.; Asokan, S.; Sood, A.K. Enhanced strain and temperature sensing by reduced graphene oxide coated etched fiber Bragg gratings. Opt. Lett. 2016, 41, 2604–2607. [Google Scholar] [CrossRef]
- Chu, R.; Guan, C.; Bo, Y.; Shi, J.; Zhu, Z.; Li, P.; Yang, J.; Yuan, L. Temperature Sensor in Suspended Core Hollow Fiber Covered With Reduced Graphene Oxide. IEEE Photonics Technol. Lett. 2019, 31, 553–556. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, X.; Jia, W.; Xu, Z.; Luo, H.; Liu, D.; Zhang, L. Graphene-Assisted Microfiber for Optical-Power-Based Temperature Sensor. IEEE Photonics Technol. Lett. 2016, 28, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, D.; Wang, R.; Zhu, J.; Ren, Z. PDMS-assisted graphene microfiber ring resonator for temperature sensor. Opt. Quantum Electron. 2018, 50, 132. [Google Scholar] [CrossRef]
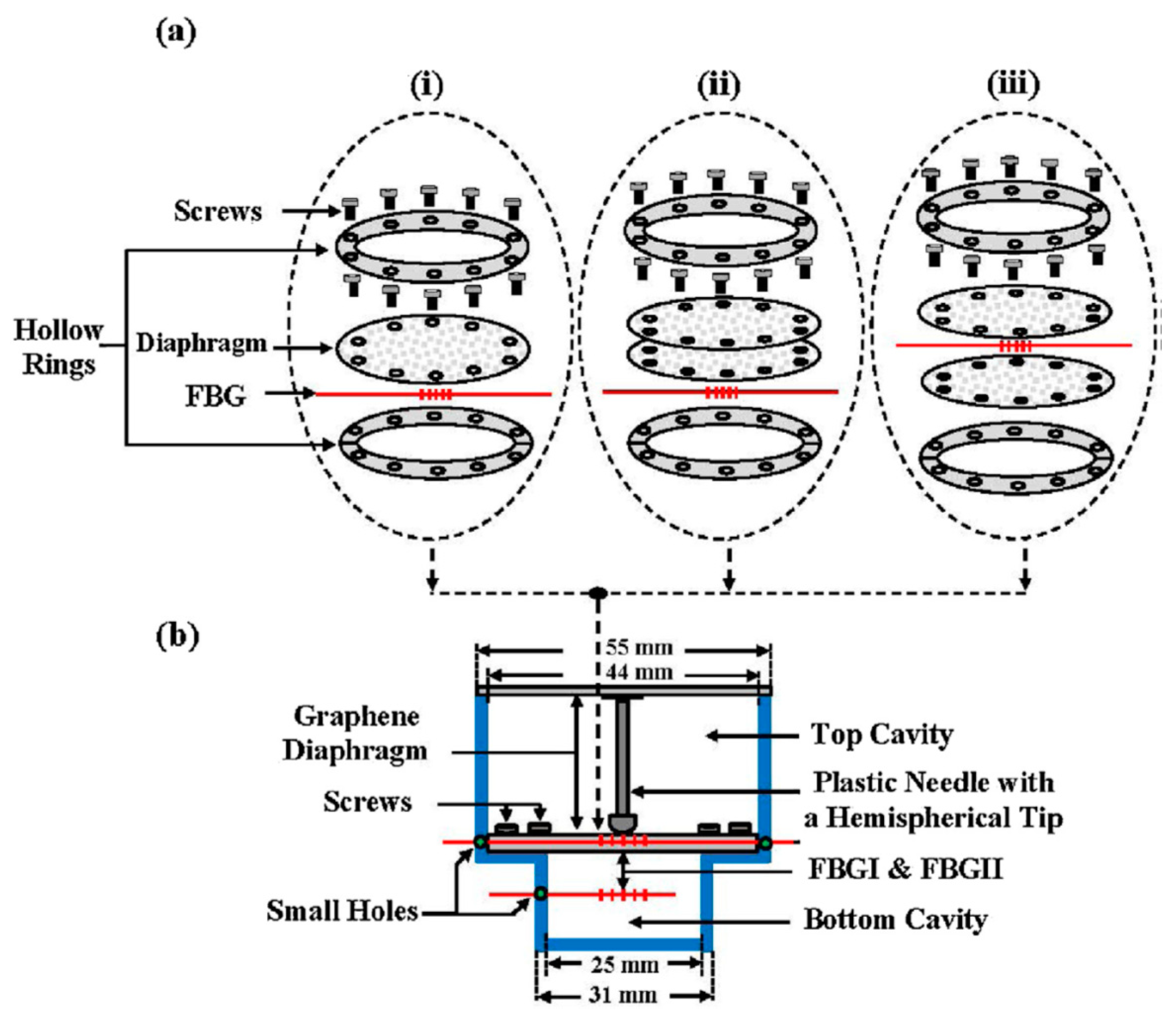
- Ameen, O.F.; Younus, M.H.; Aziz, M.S.; Azmi, A.I.; Raja Ibrahim, R.K.; Ghoshal, S.K. Graphene diaphragm integrated FBG sensors for simultaneous measurement of water level and temperature. Sens. Actuators A Phys. 2016, 252, 225–232. [Google Scholar] [CrossRef]
- Cui, Q.; Thakur, P.; Rablau, C.; Avrutsky, I.; Cheng, M.M. Miniature Optical Fiber Pressure Sensor With Exfoliated Graphene Diaphragm. IEEE Sens. J. 2019, 19, 5621–5631. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Yang, X.; Shi, J.; Zhao, J.; Zhang, C. Analysis of Hollow Fiber Temperature Sensor Filled with Graphene-Ag Composite Nanowire and Liquid. Sensors 2016, 16, 1656. [Google Scholar] [CrossRef] [Green Version]
- Jasmi, F.; Azeman, N.H.; Bakar, A.A.A.; Zan, M.S.D.; Badri, K.H.; Su’ait, M.S. Ionic Conductive Polyurethane-Graphene Nanocomposite for Performance Enhancement of Optical Fiber Bragg Grating Temperature Sensor. IEEE Access 2018, 6, 47355–47363. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Peng, X.; Fan, S. Analyzing the temperature sensitivity of Fabry-Perot sensor using multilayer graphene diaphragm. Opt. Express 2015, 23, 27494–27502. [Google Scholar] [CrossRef]
- Li, L.; Feng, Z.; Qiao, X.; Yang, H.; Wang, R.; Su, D.; Wang, Y.; Bao, W.; Li, J.; Shao, Z.; et al. Ultrahigh Sensitive Temperature Sensor Based on Fabry–Pérot Interference Assisted by a Graphene Diaphragm. IEEE Sens. J. 2015, 15, 505–509. [Google Scholar] [CrossRef]
- Li, C.; Xiao, J.; Guo, T.; Fan, S.; Jin, W. Interference characteristics in a Fabry–Perot cavity with graphene membrane for optical fiber pressure sensors. Microsyst. Technol. 2015, 21, 2297–2306. [Google Scholar] [CrossRef]
- Li, C.; Xiao, J.; Guo, T.; Fan, S.; Jin, W. Effects of graphene membrane parameters on diaphragm-type optical fibre pressure sensing characteristics. Mater. Res. Innov. 2015, 19, S5-17–S5-23. [Google Scholar] [CrossRef]
- Ma, J.; Jin, W.; Ho, H.L.; Dai, J.Y. High-sensitivity fiber-tip pressure sensor with graphene diaphragm. Opt. Lett. 2012, 37, 2493–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.-C.; Yan, S.-C.; Chen, J.-H.; Cui, G.-X.; Xu, F.; Lu, Y.-Q. Miniature optical fiber current sensor based on a graphene membrane. Laser Photonics Rev. 2015, 9, 517–522. [Google Scholar] [CrossRef]
- Wang, D.; Fan, S.; Jin, W. Graphene diaphragm analysis for pressure or acoustic sensor applications. Microsyst. Technol. 2015, 21, 117–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Liu, Z.; Duan, Z.; Cui, W.; Han, J.; Gu, Y.; Wu, Z.; Jing, Z.; Sun, C.; et al. Fiber-optic anemometer based on single-walled carbon nanotube coated tilted fiber Bragg grating. Opt. Express 2017, 25, 24521–24530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, B.; Zhang, X.; Hu, N.; Li, K.; Chiavaioli, F.; Gui, X.; Guo, T. Plasmonic Fiber-Optic Photothermal Anemometers With Carbon Nanotube Coatings. J. Lightwave Technol. 2019, 37, 3373–3380. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Zhang, Y.; Jing, Z.; Peng, W. Low-Power-Consumption Fiber-Optic Anemometer Based on Long-Period Grating With SWCNT Coating. IEEE Sens. J. 2019, 19, 2592–2597. [Google Scholar] [CrossRef]
- Ruan, Y.; Simpson, D.A.; Jeske, J.; Ebendorff-Heidepriem, H.; Lau, D.W.M.; Ji, H.; Johnson, B.C.; Ohshima, T.; Afshar, V.S.; Hollenberg, L.; et al. Magnetically sensitive nanodiamond-doped tellurite glass fibers. Sci. Rep. 2018, 8, 1268. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, A.M.; Nakonieczna, P.; Mrózek, M.; Sycz, K.; Kruk, A.; Ficek, M.; Głowacki, M.; Bogdanowicz, R.; Gawlik, W. Optical Magnetometry Based on Nanodiamonds with Nitrogen-Vacancy Color Centers. Materials 2019, 12, 2951. [Google Scholar] [CrossRef] [Green Version]
- Rondin, L.; Tetienne, J.P.; Hingant, T.; Roch, J.F.; Maletinsky, P.; Jacques, V. Magnetometry with nitrogen-vacancy defects in diamond. Rep. Prog. Phys. 2014, 77, 056503. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Y.; Simpson, D.A.; Jeske, J.; Ebendorff-Heidepriem, H.; Lau, D.W.; Ji, H.; Johnson, B.C.; Ohshima, T.; Hollenberg, L.; Greentree, A.D. Remote nanodiamond magnetometry. arXiv 2016, arXiv:1602.06611. [Google Scholar]
- Bai, D.; Capelli, M.; Huynh, H.; Ebendorff-Heidepriem, H.; Foster, S.; Greentree, A.D.; Gibson, B.C. Hybrid Diamond-Glass Optical Fibres for Magnetic Sensing. In Proceedings of the 26th International Conference on Optical Fiber Sensors, Lausanne, Switzerland, 24–28 September 2018. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chien, Y.-H.; Tan, R.; bin Shaik Alauddin, A.R.; Ji, W.B.; Tjin, S.C.; Yong, K.-T. An Advanced Hand-Held Microfiber-Based Sensor for Ultrasensitive Lead Ion Detection. ACS Sens. 2018, 3, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, Y.; Jin, J.; Xiong, Z.; Li, D.; Yao, J.; Liu, Y. Highly selective, rapid-functioning and sensitive fluorescent test paper based on graphene quantum dots for on-line detection of metal ions. Anal. Methods 2018, 10, 1163–1171. [Google Scholar] [CrossRef]
- Ji, W.B.; Yap, S.H.K.; Panwar, N.; Zhang, L.L.; Lin, B.; Yong, K.T.; Tjin, S.C.; Ng, W.J.; Majid, M.B.A. Detection of low-concentration heavy metal ions using optical microfiber sensor. Sens. Actuators B Chem. 2016, 237, 142–149. [Google Scholar] [CrossRef]
- Abdulkhaleq Alwahib, A.; Fawzi Alhasan, S.; Yaacob, M.H.; Lim, H.N.; Adzir Mahdi, M. Surface plasmon resonance sensor based on D-shaped optical fiber using fiberbench rotating wave plate for sensing pb ions. Optik 2020, 202, 163724. [Google Scholar] [CrossRef]
- Yao, B.C.; Wu, Y.; Yu, C.B.; He, J.R.; Rao, Y.J.; Gong, Y.; Fu, F.; Chen, Y.F.; Li, Y.R. Partially reduced graphene oxide based FRET on fiber-optic interferometer for biochemical detection. Sci. Rep. 2016, 6, 23706. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, X.; Yang, J.; Maa, H.; Zu, P.; So, P.L.; Chan, C.C. Detection of Ni2+ with optical fiber Mach-Zehnder interferometer coated with chitosan/MWCNT/PAA. In Proceedings of the 16th International Conference on Optical Communications and Networks (ICOCN), Wuzhen, China, 7–10 August 2017. [Google Scholar] [CrossRef]
- Gonçalves, H.M.R.; Duarte, A.J.; Esteves da Silva, J.C.G. Optical fiber sensor for Hg(II) based on carbon dots. Biosens. Bioelectron. 2010, 26, 1302–1306. [Google Scholar] [CrossRef] [Green Version]
- Girei, S.H.; Shabaneh, A.A.; Ngee-Lim, H.; Hamidon, M.N.; Mahdi, M.A.; Yaacob, M.H. Tapered optical fiber coated with graphene based nanomaterials for measurement of ethanol concentrations in water. Opt. Rev. 2015, 22, 385–392. [Google Scholar] [CrossRef]
- Aziz, A.; Lim, H.N.; Girei, S.H.; Yaacob, M.H.; Mahdi, M.A.; Huang, N.M.; Pandikumar, A. Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B Chem. 2015, 206, 119–125. [Google Scholar] [CrossRef]
- Shabaneh, A.; Girei, S.; Arasu, P.; Mahdi, M.; Rashid, S.; Paiman, S.; Yaacob, M. Dynamic Response of Tapered Optical Multimode Fiber Coated with Carbon Nanotubes for Ethanol Sensing Application. Sensors 2015, 15, 10452–10464. [Google Scholar] [CrossRef]
- Khalaf, A.L.; Shabaneh, A.A.A.; Yaacob, M.H. Chapter 10 - Carbon Nanotubes and Graphene Oxide Applications in Optochemical Sensors. In Synthesis, Technology and Applications of Carbon Nanomaterials; Rashid, S.A., Raja Othman, R.N.I., Hussein, M.Z., Eds.; Elsevier: Selangor, Malaysia, 2019; pp. 223–246. [Google Scholar]
- Gao, S.S.; Qiu, H.W.; Zhang, C.; Jiang, S.Z.; Li, Z.; Liu, X.Y.; Yue, W.W.; Yang, C.; Huo, Y.Y.; Feng, D.J.; et al. Absorbance response of a graphene oxide coated U-bent optical fiber sensor for aqueous ethanol detection. RSC Adv. 2016, 6, 15808–15815. [Google Scholar] [CrossRef]
- Girei, S.H.; Shabaneh, A.A.; Lim, H.M.; Huang, N.H.; Mahdi, M.A.; Yaacob, M.H. Absorbance response of graphene oxide coated on tapered multimode optical fiber towards liquid ethanol. J. Eur. Opt. Soc. Rapid Publ. 2015, 10, 15019. [Google Scholar] [CrossRef]
- Arasu, P.T.; Noor, A.S.; Shabaneh, A.A.; Yaacob, M.H.; Lim, H.N.; Mahdi, M.A. Fiber Bragg grating assisted surface plasmon resonance sensor with graphene oxide sensing layer. Opt. Commun. 2016, 380, 260–266. [Google Scholar] [CrossRef]
- Khalaf, A.L.; Arasu, P.T.; Lim, H.N.; Paiman, S.; Yusof, N.A.; Mahdi, M.A.; Yaacob, M.H. Modified plastic optical fiber with CNT and graphene oxide nanostructured coatings for ethanol liquid sensing. Opt. Express 2017, 25, 5509–5520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavinkumar, T.; Manivannan, S. Uniform decoration of silver nanoparticle on exfoliated graphene oxide sheets and its ammonia gas detection. Ceram. Int. 2016, 42, 1769–1776. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Manivannan, S. Synthesis, Characterization and Gas Sensing Properties of Graphene Oxide-Multiwalled Carbon Nanotube Composite. J. Mater. Sci. Technol. 2016, 32, 626–632. [Google Scholar] [CrossRef]
- Consales, M.C.A.; Penza, M.; Aversa, P.; Veneri, P.D.; Giordano, M.; Cusano, A. SWCNT nano-composite optical sensors for VOC and gas trace detection. Sens. Actuators B Chem. 2009, 138, 351–361. [Google Scholar] [CrossRef]
- Zhang, J.Y.D.; Ding, E.J.; Xu, S.C.; Li, Z.H.; Wang, X.X.; Song, F. Sensitization of an optical fiber methane sensor with graphene. Opt. Fiber Technol. 2017, 37, 26–29. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Sastikumar, D.; Manivannan, S. Effect of functional groups on dielectric, optical gas sensing properties of graphene oxide and reduced graphene oxide at room temperature. RSC Adv. 2015, 5, 10816–10825. [Google Scholar] [CrossRef]
- Manivannan, S.; Shobin, L.; Saranya, A.; Renganathan, B.; Sastikumar, D.; Park, K.C. Carbon nanotubes coated fiber optic ammonia gas sensor. In Proceedings of the Integrated Optics: Devices, Materials, and Technologies XV, San Francisco, CA, USA, 24–26 January 2011; p. 79410M. [Google Scholar] [CrossRef]
- Manivannan, S.; Saranya, A.M.; Renganathan, B.; Sastikumar, D.; Gobi, G.; Park, K.C. Single-walled carbon nanotubes wrapped poly-methyl methacrylate fiber optic sensor for ammonia, ethanol and methanol vapors at room temperature. Sens. Actuators B Chem. 2012, 171–172, 634–638. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.-Y.; Wen, G.-F.; Han, Z.-X. Graphene-based optical fiber ammonia gas sensor. Instrum. Sci. Technol. 2018, 46, 12–27. [Google Scholar] [CrossRef]
- Khalaf, A.L.; Mohamad, F.S.; Rahman, N.A.; Lim, H.N.; Paiman, S.; Yusof, N.A.; Mahdi, M.A.; Yaacob, M.H. Room temperature ammonia sensor using side-polished optical fiber coated with graphene/polyaniline nanocomposite. Opt. Mater. Express 2017, 7, 1858–1870. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, B.C.; Zhang, A.Q.; Cao, X.L.; Wang, Z.G.; Rao, Y.J.; Gong, Y.; Zhang, W.; Chen, Y.F.; Chiang, K.S. Graphene-based D-shaped fiber multicore mode interferometer for chemical gas sensing. Opt. Lett. 2014, 39, 6030–6033. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, B.; Zhang, A.; Rao, Y.; Wang, Z.; Cheng, Y.; Gong, Y.; Zhang, W.; Chen, Y.; Chiang, K.S. Graphene-coated microfiber Bragg grating for high-sensitivity gas sensing. Opt. Lett. 2014, 39, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Pawar, D.; Rao, B.V.B.; Kale, S.N. Fe3O4-decorated graphene assembled porous carbon nanocomposite for ammonia sensing: Study using an optical fiber Fabry–Perot interferometer. Analyst 2018, 143, 1890–1898. [Google Scholar] [CrossRef]
- Yao, B.W.Y.; Cheng, Y.; Zhang, A.; Gong, Y.; Rao, Y.-J.; Wang, Z.; Chen, Y. All-optical Mach–Zehnder interferometric NH3 gas sensor based on graphene/microfiber hybrid waveguide. Sens. Actuators B Chem. 2014, 194, 142–148. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. Surface Plasmon Resonance-Based Fiber Optic Methane Gas Sensor Utilizing Graphene-Carbon Nanotubes-Poly(Methyl Methacrylate) Hybrid Nanocomposite. Plasmonics 2015, 10, 1147–1157. [Google Scholar] [CrossRef]
- Yang, J.; Che, X.; Shen, R.; Wang, C.; Li, X.; Chen, W. High-sensitivity photonic crystal fiber long-period grating methane sensor with cryptophane-A-6Me absorbed on a PAA-CNTs/PAH nanofilm. Opt. Express 2017, 25, 20258–20267. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Sastikumar, D.; Manivannan, S. Reduced graphene oxide coated optical fiber for methanol and ethanol vapor detection at room temperature. In .Proceedings of the SPIE/COS Photonics, Beijing, China, 9–11 October 2014; p. 92700U. [Google Scholar]
- Shobin, L.R.; Renganathan, B.; Sastikumar, D.; Park, K.C.; Manivannan, S. Pure and Iso-Butyl Methyl Ketone Treated Multi-Walled Carbon Nanotubes for Ethanol and Methanol Vapor Sensing. IEEE Sens. J. 2014, 14, 1238–1243. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, J.; Shun, L.; Tan, S.; Cai, X.; Luo, Y.; Zhang, J.; Dong, H.; Lu, H.; Guan, H.; et al. Reduced graphene oxide for fiber-optic toluene gas sensing. Opt. Express 2016, 24, 28290–28302. [Google Scholar] [CrossRef]
- Qiu, H.W.X.; Xu, S.C.; Jiang, S.Z.; Li, Z.; Chen, P.X.; Gao, S.S.; Zhang, C.; Feng, D.J. A novel graphene-based tapered optical fiber sensor for glucose detection. Appl. Surf. Sci. 2015, 329, 390–395. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Zhang, C.; Gao, S.; Li, Z.; Qiu, H.; Li, C.; Yang, C.; Liu, M.; Liu, Y. A novel U-bent plastic optical fibre local surface plasmon resonance sensor based on a graphene and silver nanoparticle hybrid structure. J. Phys. D Appl. Phys. 2017, 50, 165105. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, J. Graphene based chalcogenide fiber-optic evanescent wave sensor for detection of hemoglobin in human blood. Opt. Fiber Technol. 2018, 41, 125–130. [Google Scholar] [CrossRef]
- Batumalay, M. Tapered plastic optical fiber coated with single wall carbon nanotubes polyethylene oxide composite for measurement of uric acid concentration. Sens. Rev. 2014, 34, 75–79. [Google Scholar] [CrossRef]
- Hu, W.H.Y.; Chen, C.; Liu, Y.; Guo, T.; Guan, B.-O. Highly sensitive detection of dopamine using a graphene functionalized plasmonic fiber-optic sensor with aptamer conformational amplification. Sens. Actuators B Chem. 2018, 264, 440–447. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Ham, S.W.; Hong, H.P.; Kim, J.H.; Min, S.J.; Min, N.K. Effect of Oxygen Plasma Treatment on Carbon Nanotube-Based Sensors. J. Nanosci. Nanotechnol. 2014, 14, 8476–8481. [Google Scholar] [CrossRef]
- Pandit, S.; Banerjee, T.; Srivastava, I.; Nie, S.; Pan, D. Machine Learning-Assisted Array-Based Biomolecular Sensing Using Surface-Functionalized Carbon Dots. ACS Sens. 2019, 4, 2730–2737. [Google Scholar] [CrossRef]
- Feder, K.S.; Westbrook, P.S.; Ging, J.; Reyes, P.I.; Carver, G.E. In-fiber spectrometer using tilted fiber gratings. IEEE Photonics Technol. Lett. 2003, 15, 933–935. [Google Scholar] [CrossRef]
- Wagener, J.L.; Strasser, T.A.; Pedrazzani, J.R.; DeMarco, J.; DiGiovanni, D. Fiber grating optical spectrum analyzer tap. In Proceedings of the 11th International Conference on Integrated Optics and Optical Fibre Communications, and 23rd European Conference on Optical Communications, Edinburgh, UK, 22–25 September 1997; pp. 65–68. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef] [Green Version]
- Bo, L.; Xiao, C.; Hualin, C.; Mohammad, M.A.; Xiangguang, T.; Luqi, T.; Yi, Y.; Tianling, R. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 021001. [Google Scholar] [CrossRef]
- Go, D.B.; Atashbar, M.Z.; Ramshani, Z.; Chang, H.-C. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Kang, T.-G.; Kim, B.-H.; Lee, H.-J.; Choi, H.H.; Yook, J.-G. Real-time Humidity Sensor Based on Microwave Resonator Coupled with PEDOT:PSS Conducting Polymer Film. Sci. Rep. 2018, 8, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyfors, E. Industrial Microwave Sensors—A Review. Subsurf. Sens. Technol. Appl. 2000, 1, 23–43. [Google Scholar] [CrossRef]
- Bruno, T.; Svoronos, P. CRC Handbook of Basic Tables for Chemical Analysis; CRC Press: Boca Raton, FL, USA, 2010; p. 887. [Google Scholar]

| Type of Carbon Allotrope | Properties |
|---|---|
| Carbon Nanotubes |
|
| Carbon Dots |
|
| Graphene |
|
| Nanodiamonds |
|
| Types of Carbon Allotropes | Synthesis Strategy | Advantages | Disadvantages |
|---|---|---|---|
| Carbon nanotubes | Arc-discharge |
|
|
| Chemical vapor deposition |
|
| |
| Laser ablation |
|
| |
| Carbon dots | Optical |
|
|
| Chemical |
|
| |
| Thermal |
|
| |
| Graphene | Mechanical exfoliation |
|
|
| Liquid exfoliation |
|
| |
| Chemical treatment |
|
| |
| Nanodiamonds | Detonation |
|
|
| Pulsed laser |
|
|
| Sensing Material | Optical Fiber | Linear Detection Range (%) | Sensitivity (% RH−1) | Ref. |
|---|---|---|---|---|
| CNT | Etched FBG | 20–90 | 31 pm | [147] |
| CNTs doped PMMA | Optical microfiber | 45–80 | 0.3341 dBm | [149] |
| MWCNTs slurry | Optical microfiber | 45–80 | 5.17 μW | [148] |
| CNT/PVA | Thin core fiber | 70–86 | 0.4573 dB | [150] |
| rGO | Hollow core fiber | 60–90 | 0.229 dB | [151] |
| rGO | Side polished fiber | 70–95 | 0.31 dB | [134] |
| rGO | Microfiber resonator | 30–50 | 0.0537 nm | [153] |
| rGO/PS | Optical microfiber | 50.5–70.6 79.5–85.0 | 0.224 dB 4.118 dB | [152] |
| GO | Side polished fiber | 32–85 85–97.6 | 0.145 nm 0.915 nm | [154] |
| GO | Side polished twin core fiber | 40–75 | 2.720 nm | [155] |
| GO | Polarization maintaining fiber | 60–77 | 0.349 dB | [156] |
| GO/PEI | Multimode fiber | 20–70 70–90 | 0.317 nm 0.311 nm | [157] |
| GO | Tilted FBG | 10–80 | 0.129 dB | [158] |
| GO | Tilted FBG | 30–80 | 0.027 dB 18.5 pm | [159] |
| GO | Single mode fiber | 30–60 | 0.104 dB 0.0272 nm | [160] |
| GO/PVA | Optical microfiber | 40–60 | 0.0606 dBm | [161] |
| GO/PVA | Waist enlarged taper SMF | 25–80 | 0.193 dB | [162] |
| Sensing Material | Optical Fiber | Linear Detection Range (° C) | Sensitivity (° C−1) | Ref. |
|---|---|---|---|---|
| rGO | Side polished fiber | −7.8–77 | 0.134 dB | [1] |
| rGO | Etched FBG | −100–300 | 33 pm | [164] |
| rGO | Suspended core hollow fiber | 30–80 | 179.4 pm | [165] |
| Graphene | Optical microfiber | 30–80 | 0.1018 dB | [166] |
| Graphene/PDMS | Microfiber ring resonator | 30–60 | 0.544 dB | [167] |
| Graphene/Ag | Hollow core fiber | 22–47 | 9.44 nm | [170] |
| PU/Graphene | FBG | 25–60 | 6 pm | [171] |
| Graphene diaphragm | Fabry-Perot interferometer | 20–60 | 352 nm | [172] |
| Graphene diaphragm | Fabry-Perot interferometer | 500–510 1000–1008 | 1.56 nm 1.87 nm | [173] |
| Graphene diaphragm | FBG | 27–77 | 13.31 pm | [168] |
| Graphene diaphragm | FBG | 20–100 | 306.2 nm | [7] |
| Sensing Material | Optical Fiber | Linear Detection Range (kPa) | Sensitivity (nm kPa−1) | Ref. |
|---|---|---|---|---|
| Graphene diaphragm | Fabry-Perot interferometer | 0–3.5 | 1096 | [174] |
| Graphene diaphragm | Fabry-Perot interferometer | 0–2.5 | 80 | [175] |
| Graphene diaphragm | Fabry-Perot interferometer | 0–13 | 65.71 | [169] |
| Graphene diaphragm | Fabry-Perot interferometer | 0–5 | 39.4 | [176] |
| Graphene diaphragm | FBG | 0–2 | 501.4 | [7] |
| Graphene diaphragm | FBG | 0–9.81 | 0.25 | [168] |
| Sensing Material | Optical Fiber | Detection Range (%) | LOD* (%) | Sensitivity | Ref. |
|---|---|---|---|---|---|
| Graphene | Tapered MMF | 0–40 | - | 0.829 a.u. / % | [194] |
| GO | Plastic cladding silica fiber | 10–80 | 5 | - | [198] |
| GO | Tapered MMF | 5–40 | 5 | - | [199] |
| GO | FBG | 0–80 | 10 | - | [200] |
| GO | Tapered MMF | 0–40 | - | 1.330 a.u. / % | [194] |
| SnO2/GO/PEI | MMF | 0–100 | 20 | - | [119] |
| Ag/rGO | MMF | 0–100 | 1 | - | [195] |
| CNTs | Tapered tip fiber | 5–80 | - | 0.144 a.u. / % | [196] |
| CNTs | POF | 20–100 | 0.2 | - | [201] |
| CNTs | MMF | 5–80 | 0.02 | - | [133] |
| Target Molecule | Sensing Material | Optical Fiber | Detection Range | LOD | Sensitivity* | Ref. |
|---|---|---|---|---|---|---|
| NH3 | GO-MWCNTs | MMF | 0–500 ppm | – | 0.41 cts ppm−1 | [202] |
| GO | MMF | 0–500 ppm | – | −0.32 cts ppm−1 | [206] | |
| rGO | MMF | 0–500 ppm | – | 0.08 cts ppm−1 | [202] | |
| Ag/GO | MMF | 0–500 ppm | – | 0.17 cts ppm−1 | [202] | |
| MWCNTs | MMF | 0–500 ppm | – | 0.31 cts ppm−1 | [207] | |
| SWCNTs | MMF | 50–500 ppm | – | 0.22 cts ppm−1 | [208] | |
| Graphene | Optical microfiber | 0–300 ppm | – | 0.015 nm/ppm | [209] | |
| Graphene/PANI | Side polished fiber | 0–1% | – | 132.8 a.u./% | [210] | |
| Graphene | D-shaped fiber | 0–1000 ppm | 0.04 ppm | – | [211] | |
| Graphene | Microfiber Bragg grating | 0–100 ppm | 0.2 ppm | – | [212] | |
| Fe3O4 - graphene | SMF | 1.5–150 ppm | 7 ppb | – | [213] | |
| Graphene | Microfiber hybrid waveguide | 0–360 ppm | 0.3 ppm | – | [214] | |
| CH4 | Graphene/CNTs | MMF | 10–100 ppm | – | 0.3 nm/ppm | [215] |
| PAA-NTs/PAH | PCF-LPG | 0–3.5% | 0.18% | 1.078 nm/% | [216] | |
| SnO2/Graphene | Side polished fiber | 0–55% | – | 200 a.u./% | [205] | |
| Ethanol | GO-MWCNTs | MMF | 0–500 ppm | – | 0.36 cts ppm−1 | [203] |
| GO | MMF | 0–500 ppm | – | -0.26 cts ppm−1 | [206] | |
| rGO | MMF | 0–500 ppm | – | 0.065 cts ppm−1 | [217] | |
| MWCNTs | MMF | 0–500 ppm | – | 0.52 cts ppm−1 | [218] | |
| SWCNTs | MMF | 50–500 ppm | – | 0.20 cts ppm−1 | [208] | |
| CdA/SWCNTs | Fabry-Perot interferometer | – | – | 0.5 10−3 / ppm | [204] | |
| Methanol | GO-MWCNTs | MMF | 0–500 ppm | – | 0.23 cts ppm−1 | [203] |
| GO | MMF | 0–500 ppm | – | −0.20 cts ppm−1 | [206] | |
| rGO | MMF | 0–500 ppm | – | 0.038 cts ppm−1 | [217] | |
| MWCNTs | MMF | 0 – 500 ppm | – | 0.14 cts ppm−1 | [218] | |
| SWCNTs | MMF | 50–500 ppm | – | 0.01 cts ppm−1 | [208] | |
| Toluene | rGO | Side polished fiber | 50–200 ppm | 79 ppm | – | [219] |
| CdA/SWCNTs | Fabry-Perot interferometer | – | – | 1.3 10−3 / ppm | [204] | |
| Xylene | Graphene | Microfiber Bragg grating | 0–100 ppm | 0.5 ppm | – | [212] |
| CdA/SWCNTs | Fabry-Perot interferometer | – | – | 3.2 10−3 / ppm | [204] |
| Sensing Methods | Advantages | Limitations |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, S.H.K.; Chan, K.K.; Tjin, S.C.; Yong, K.-T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors 2020, 20, 2046. https://doi.org/10.3390/s20072046
Yap SHK, Chan KK, Tjin SC, Yong K-T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors. 2020; 20(7):2046. https://doi.org/10.3390/s20072046
Chicago/Turabian StyleYap, Stephanie Hui Kit, Kok Ken Chan, Swee Chuan Tjin, and Ken-Tye Yong. 2020. "Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review" Sensors 20, no. 7: 2046. https://doi.org/10.3390/s20072046
APA StyleYap, S. H. K., Chan, K. K., Tjin, S. C., & Yong, K. -T. (2020). Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors, 20(7), 2046. https://doi.org/10.3390/s20072046