Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA
Abstract
1. Introduction
2. Experimental Section
2.1. Apparatus
2.2. Reagents
2.3. Synthesis of g-C3N4 (GCN)
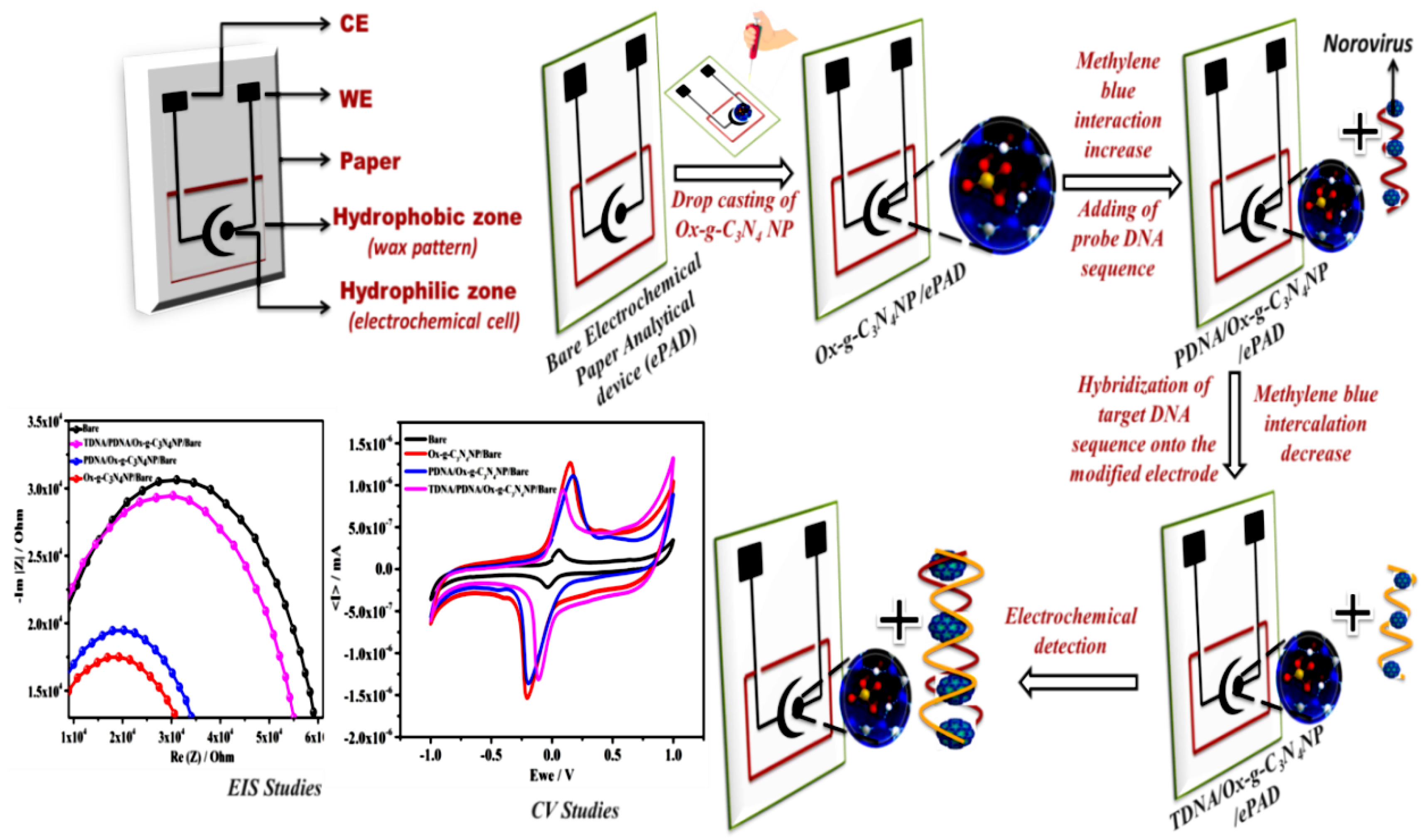
2.4. Fabrication of ePADs Grafted with Ox-g-C3N4 Nanosheets
2.5. Fabrication of PDNA/Ox-g-C3N4/ePAD
2.6. Hybridization of Target DNA
3. Results and Discussions
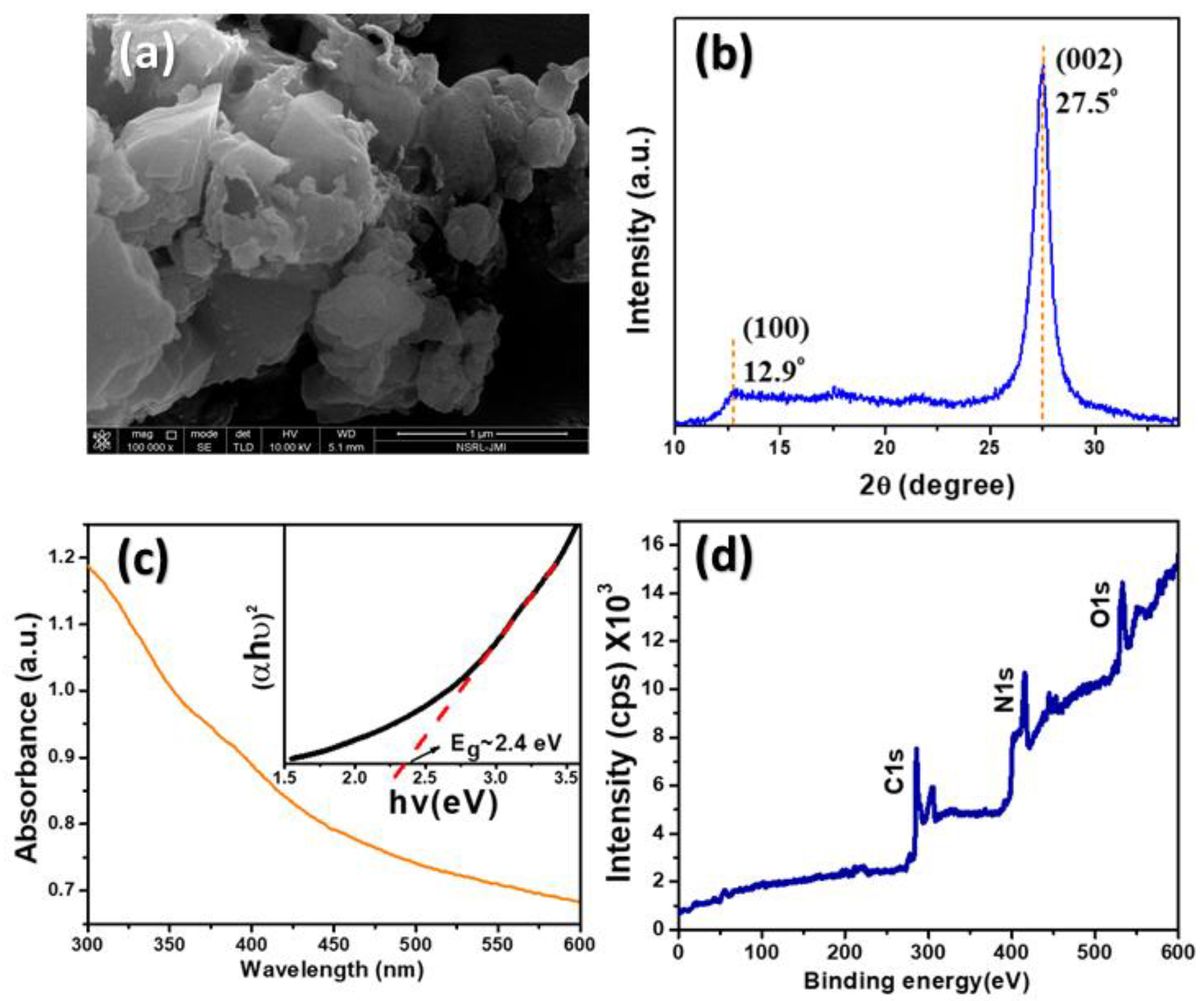
3.1. Ox-g-C3N4 Nanosheets Characterization
3.2. Electrochemical Response of Ox-g-C3N4 Modified Electrodes
3.3. Analytical Performance of the Developed Biosensor
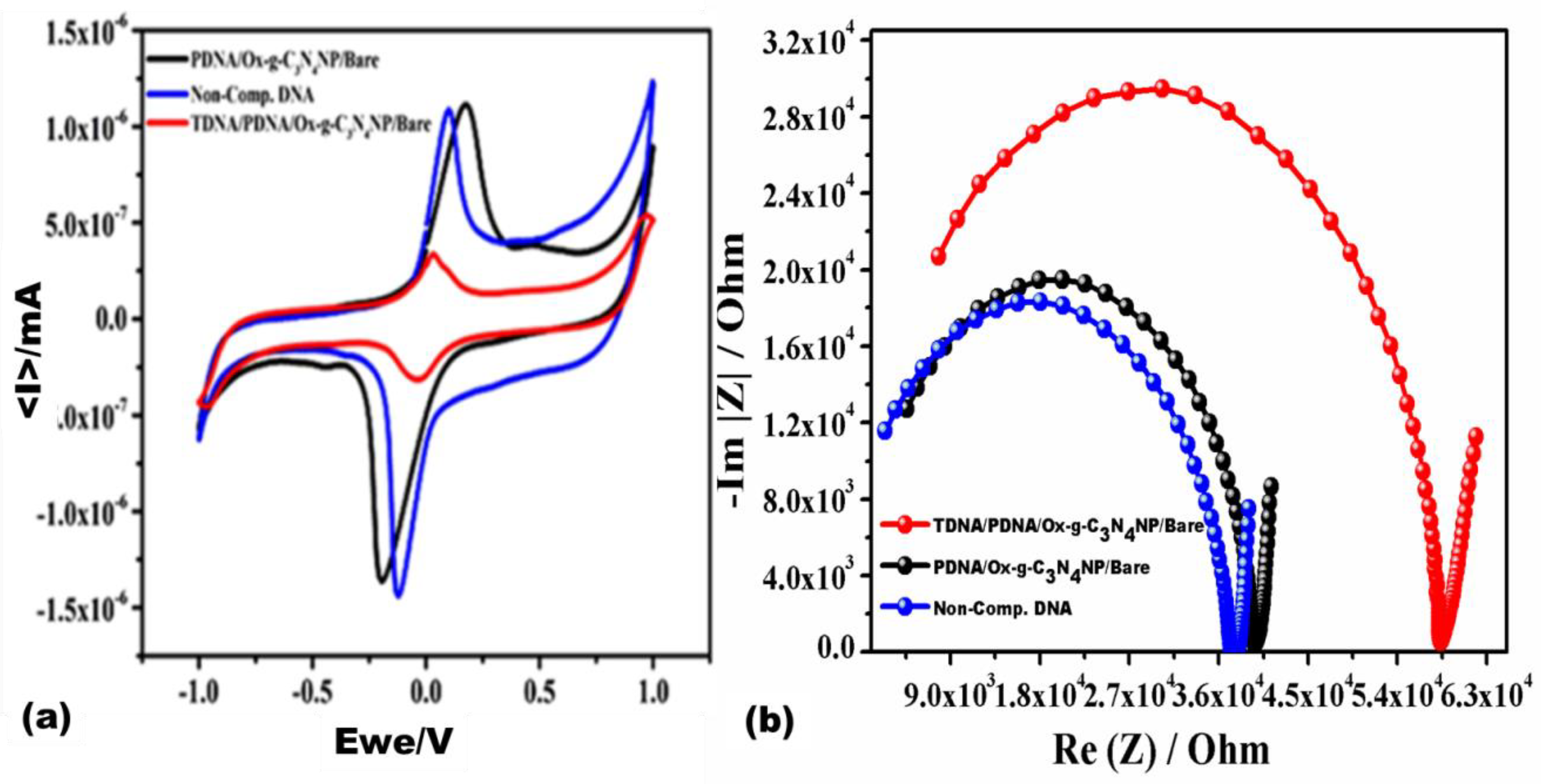
3.4. Selectivity of the Developed Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Grazia, S.; Bonura, F.; Cappa, V.; Muli, S.L.; Pepe, A.; Urone, N.; Giammanco, G.M. Performance evaluation of a newly developed molecular assay for the accurate diagnosis of gastroenteritis associated with norovirus of genogroup II. Arch. Virol. 2018, 163, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Rockx, B.; de Wit, M.; Vennema, H.; Vinjé, J.; de Bruin, E.; van Duynhoven, Y.; Koopmans, M. Natural history of human calicivirus infection: A prospective cohort study. Clin. Infect. Dis. 2002, 35, 246–253. [Google Scholar] [CrossRef]
- Vinjé, J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Haibo, L.; Wengfeng, S. Emergence of GI. 6 Outbreaks in a High School in Fangshan District, Beijing, China. Sci. J. Public. Health 2018, 6, 106. [Google Scholar] [CrossRef]
- Bert, F.; Scaioli, G.; Gualano, M.R.; Passi, S.; Specchia, M.L.; Cadeddu, C.; Viglianchino, C.; Siliquini, R. Norovirus outbreaks on commercial cruise ships: A systematic review and new targets for the public health agenda. Food Environ. Virol. 2014, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.D.; Goulter, R.M.; Jaykus, L.A. Human norovirus as a foodborne pathogen: Challenges and developments. Ann. Rev. Food Sci. Technol. 2015, 6, 411–433. [Google Scholar] [CrossRef]
- Mounts, A.W.; Ando, T.; Koopmans, M.; Bresee, J.S.; Noel, J.; Glass, R.I. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J. Infect. Dis. 2000, 18, S284–S287. [Google Scholar] [CrossRef]
- Rodriguez-Lazaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.J.; Bosch, A. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 2012, 36, 786–814. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Miller, S.E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 2009, 22, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Takao, S.; Kuwayama, M.; Shimazu, Y.; Miyazaki, K. Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 2006, 44, 1376–1381. [Google Scholar] [CrossRef]
- de Cal, I.W.; Revilla, A.; Del Alamo, J.M.; Roman, E.; Moreno, S.; Sanchez-Fauquier, A. Evaluation of two commercial enzyme immunoassays for the detection of norovirus in faecal samples from hospitalised children with sporadic acute gastroenteritis. Clin. Microbiol. Infect. 2007, 13, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Costantini, V.; Grenz, L.; Fritzinger, A.; Lewis, D.; Biggs, C.; Hale, A.; Vinjé, J. Diagnostic accuracy and analytical sensitivity of IDEIA Norovirus assay for routine screening of human norovirus. J. Clin. Microbiol. 2010, 48, 2770–2778. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hu, Q.; Xiong, Q.; Zhang, F.; He, P. An ultrasensitive scanning electrochemical microscopy (SECM)-based DNA biosensing platform amplified with the long self-assembled DNA concatemers. Electrochim. Act. 2016, 192, 127–132. [Google Scholar] [CrossRef]
- Narang, J.; Mishra, A.; Pilloton, R.; VV, A.; Wadhwa, S.; Pundir, C.S.; Khanuja, M. Development of MoSe2 nano-urchins as a sensing platform for a selective bio-capturing of Escherichia coli shiga toxin DNA. Biosensors 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Talan, A.; Mishra, A.; Eremin, S.A.; Narang, J.; Kumar, A.; Gandhi, S. Ultrasensitive electrochemical immuno-sensing platform based on gold nanoparticles triggering chlorpyrifos detection in fruits and vegetables. Biosens. Bioelectron. 2018, 105, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2014, 87, 230–249. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, P.X.; Wang, A.J.; Luo, X.; Xue, Y.; Zhang, L.; Feng, J.J. A novel electrochemical immunosensor for highly sensitive detection of prostate-specific antigen using 3D open-structured PtCu nanoframes for signal amplification. Biosens. Bioelectron. 2019, 126, 187–192. [Google Scholar] [CrossRef]
- Du, A.; Sanvito, S.; Li, Z.; Wang, D.; Jiao, Y.; Liao, T.; Smith, S.C. Hybrid graphene and graphitic carbon nitride nanocomposite: Gap opening, electron–hole puddle, interfacial charge transfer, and enhanced visible light response. J. Americ. Chem. Soc. 2012, 134, 4393–4397. [Google Scholar] [CrossRef]
- Wu, J.H.; Shao, F.Q.; Han, S.Y.; Bai, S.; Feng, J.J.; Li, Z.; Wang, A.J. Shape-controlled synthesis of well-dispersed platinum nanocubes supported on graphitic carbon nitride as advanced visible-light-driven catalyst for efficient photoreduction of hexavalent chromium. J. Colloid Interface Sci. 2019, 535, 41–49. [Google Scholar] [CrossRef]
- Chen, H.Y.; Jin, M.X.; Zhang, L.; Wang, A.J.; Yuan, J.; Zhang, Q.L.; Feng, J.J. One-pot aqueous synthesis of two-dimensional porous bimetallic PtPd alloyed nanosheets as highly active and durable electrocatalyst for boosting oxygen reduction and hydrogen evolution. J. Colloid Interface Sci. 2019, 543, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.J.; Zhang, L.; Feng, J.J.; Zhang, Q.L.; Huang, H.; Wang, A.J. Graphene-encapsulated cobalt nanoparticles embedded in porous nitrogen-doped graphitic carbon nanosheets as efficient electrocatalysts for oxygen reduction reaction. J. Colloid Interface Sci. 2019, 522, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Ning, J.; He, B.; Li, Z.; Zheng, C.; Zhong, Y.; Hu, Y. Unusual formation of tetragonal microstructures from nitrogen-doped carbon nanocapsules with cobalt nanocores as a bi-functional oxygen electrocatalyst. J. Mater. Chem. A 2017, 5, 2271–2279. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Simple and large scale construction of MoS 2-gC 3 N 4 heterostructures using mechanochemistry for high performance electrochemical supercapacitor and visible light photocatalytic applications. Sci. Rep. 2017, 7, 43055. [Google Scholar] [CrossRef]
- Hassan, M.; Walter, M.; Moseler, M. Interactions of polymers with reduced graphene oxide: Van der Waals binding energies of benzene on graphene with defects. Phys. Chem. Chem. Phys. 2014, 16, 33–37. [Google Scholar] [CrossRef]
- Cui, J.; Liang, S.; Wang, X.; Zhang, J.M. Structural, electronic and optical properties of a hybrid triazine-based graphitic carbon nitride and graphene nanocomposite. Phys. Chem. Chem. Phys. 2015, 17, 23613–23618. [Google Scholar] [CrossRef]
- Antiochia, R.; Tortolini, C.; Tasca, F.; Gorton, L.; Bollella, P. Graphene and 2D-Like Nanomaterials: Different Biofunctionalization Pathways for Electrochemical Biosensor Development. In Graphene Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–35. [Google Scholar]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mat. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Singhal, C.; Pundir, C.S.; Narang, J. A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Khanuja, M. A comparative photocatalytic study of pure and acid-etched template free graphitic C3N4 on different dyes: An investigation on the influence of surface modifications. Mater. Chem. Phys. 2020, 243, 122402. [Google Scholar] [CrossRef]
- Singh, S.; Khanuja, M. Pronounced light trapping effect and enhanced photo-electrochemical property of type (II) aligned graphitic- C3N4 with embedded 1-D ZnO nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 443, 012033. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, M.W.; Park, C.Y.; Choi, C.S.; Kailasa, S.K.; Park, J.P.; Park, T.J. Development of a rapid and sensitive electrochemical biosensor for detection of human norovirus via novel specific binding peptides. Biosens. Bioelectron. 2019, 123, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.A.; Kwon, J.; Kim, D.; Yang, S. A rapid, sensitive and selective electrochemical biosensor with concanavalin A for the preemptive detection of norovirus. Biosens. Bioelectron. 2015, 64, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Neethirajan, S. Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchimic. Act. 2017, 184, 4545–4552. [Google Scholar] [CrossRef]
- Heo, N.S.; Oh, S.Y.; Ryu, M.Y.; Baek, S.H.; Park, T.J.; Choi, C.; Park, J.P. Affinity Peptide-guided Plasmonic Biosensor for Detection of Noroviral Protein and Human Norovirus. Biotechnol. Bioprocess Eng. 2019, 24, 318–325. [Google Scholar] [CrossRef]
- Hwang, H.J.; Ryu, M.Y.; Park, C.Y.; Ahn, J.; Park, H.G.; Choi, C.; Park, J.P. High sensitive and selective electrochemical biosensor: Label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017, 87, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Yakes, B.J.; Papafragkou, E.; Conrad, S.M.; Neill, J.D.; Ridpath, J.F.; Burkhardt, W., III; DeGrasse, S.L. Surface plasmon resonance biosensor for detection of feline calicivirus, a surrogate for norovirus. Int. J. Food Microbiol. 2013, 162, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Lee, J.; Suzuki, T.; Hara, T.; Abe, F.; Park, E.Y. Ultrasensitive detection of norovirus using a magnetofluoroimmunoassay based on synergic properties of gold/magnetic nanoparticle hybrid nanocomposites and quantum dots. Sens. Actuators B Chem. 2019, 296, 126672. [Google Scholar] [CrossRef]
- Ashiba, H.; Sugiyama, Y.; Wang, X.; Shirato, H.; Higo-Moriguchi, K.; Taniguchi, K.; Fujimaki, M. Detection of norovirus virus-like particles using a surface plasmon resonance-assisted fluoroimmunosensor optimized for quantum dot fluorescent labels. Biosens. Bioelectron. 2017, 93, 260–266. [Google Scholar] [CrossRef] [PubMed]


| Working Electrode | Temperature (°C) | Limit of Detection (LOD) | Linear Range | Response Time (minutes) | References |
|---|---|---|---|---|---|
| Screen Printed Gold electrode | - | 1.7 copies/mL | 0–105 copies/mL | 30 | [33] |
| Gold electrode | - | 35 copies/mL | 102–106 copies/mL | 60 | [34] |
| Paper-based electrode | - | 4.4 ng/mL–3.3 ng/mL | 13 ng/mL–13 µg/mL | - | [35] |
| Localized surface plasmonic resonance (LSPR) | 25 | 9.9 copies/mL | 0.001–100 µg/mL | 10 | [36] |
| Gold electrode | - | 7.8 copies/mL | - | - | [37] |
| CM3 Sensor chip | 25 | 104 TCID50 FCV/ML | 15 | [38] | |
| LSPR | 84 copies/mL | 1 pg/mL to 5 ng/mL | - | [39] | |
| V-trench sensor chip | - | 0.01 ng/mL | 0–100 ng/mL | 1 | [40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.; Killa, M.; Yadav, N.; Mishra, A.; Mathur, A.; Kumar, A.; Khanuja, M.; Narang, J.; Pilloton, R. Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA. Sensors 2020, 20, 2070. https://doi.org/10.3390/s20072070
Rana A, Killa M, Yadav N, Mishra A, Mathur A, Kumar A, Khanuja M, Narang J, Pilloton R. Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA. Sensors. 2020; 20(7):2070. https://doi.org/10.3390/s20072070
Chicago/Turabian StyleRana, Aditya, Manjari Killa, Neelam Yadav, Annu Mishra, Ashish Mathur, Arun Kumar, Manika Khanuja, Jagriti Narang, and Roberto Pilloton. 2020. "Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA" Sensors 20, no. 7: 2070. https://doi.org/10.3390/s20072070
APA StyleRana, A., Killa, M., Yadav, N., Mishra, A., Mathur, A., Kumar, A., Khanuja, M., Narang, J., & Pilloton, R. (2020). Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA. Sensors, 20(7), 2070. https://doi.org/10.3390/s20072070







